3.1 Introduction
Man’s friendship with metals dates from prehistoric times. Initially, man became familiar with those metals which were occurring free in nature, like gold and copper. Gold was found at the burial sites. Then man came across copper and this was the first metal man started using extensively. This is the reason why this period was called the Copper Age. This was followed by the Bronze Age and the Iron Age.
3.2 Metals and Non-Metals
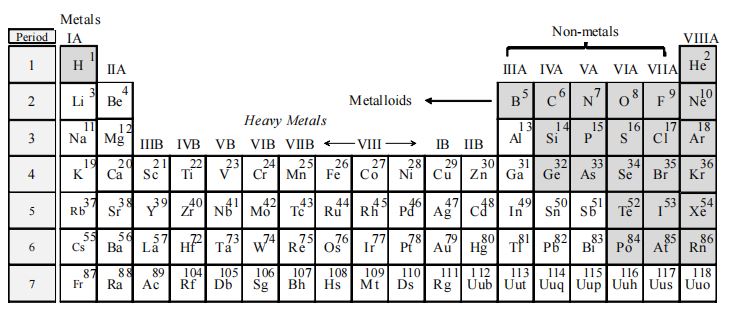
There are more than 100 elements known today. On the basis of their general characteristics, they are broadly divided into two major groups: (i) Metals and (ii) Non-metals.
There is yet another group of elements which shows characteristics of both metals and non-metals. Such elements are called metalloids. Among the total elements discovered, approximately 80% of them are metals and the remaining are either non-metals or metalloids. Position in the periodic table: In the periodic table, metals are placed on the left hand side and in the centre, whereas non-metals are placed on the right hand side with the exception of hydrogen, which is placed with metals on the extreme left. The metalloids form a border diagonal line, separating metals from non-metals, as shown below:
3.3 Physical Properties of Metals and Non-metals
|
Physical property |
Metals |
Non-metals |
|
1. State
|
All metals are solids at room temperature. Eg : Iron Exception : Mercury and Gallium (liquids) |
Generally gases at ordinary temperature. Eg: chlorine. Exception : Bromine is a liquid. Iodine is a solid. |
|
2. Lustre (ability to reflect light) |
Lustrous and can be polished. |
Possess no metallic luster and cannot be polished. Exception : Iodine and diamond |
|
3. Hardness |
Generally hard Exception : Sodium, potassium, mercury and lead |
Generally soft Exception : Diamond (hardest substance known) |
|
4. Density |
Generally have high density Exception : Sodium, potassium |
Generally have low density. |
|
5. Melting and boiling point |
Generally have high M.P. and B.P. Exception : Sodium, potassium, gallium, mercury |
Generally have low M.P. and B.P. Exception : Carbon, boron and silicon. |
|
6. Sonority (ability to produce sound) |
They are sonorous (make sound when struck). |
Non-sonorous. |
|
7. Malleability (Ability to mould into shapes and sheets without breaking) |
Generally malleable. Eg: Silver and gold-highly malleable. |
Non-malleable. |
|
8. Ductility (ability to be drawn into a thin wire) |
Generally ductile. |
Non-ductile. |
|
9. Conductivity |
Good conductors of heat and electricity. Exception : Tungsten |
Poor conductors of heat and electricity.
|
|
10. Solubility
|
Usually metals are insoluble in water or other solvents. But if a metal dissolves, it does by reacting chemically with the solution. Eg : Na with water |
Non-metals are soluble event without chemical reaction and reobtained by evaporation. Eg: Sulphur in carbon disulphide |
|
11. Alloy formation (A mixture made by combining two or more metals)
|
Forms alloys. Eg: Brass, bronze etc. |
Generally do not form alloys. But carbon, phosphorous, sulphur etc, can be present in some alloys. |
|
|
|
|
3.4 Chemical Properties of Metals
The atoms of the metals have usually 1, 2 or 3 electrons in their outermost shells. These outermost electrons are loosely held by their nuclei. Therefore, the metal atoms can easily lose their outermost electrons to from positively charged ions. For example, sodium metal can lose outermost one electron to form positively charged ions, . After losing the outermost electron, it gets stable electronic configuration of the noble gas (Ne: 2, 8 ), Similarly, magnesium can lose two outermost electrons to form Mg2+ ions and aluminium can lose its three outermost electrons to form ion.
The metal atoms lose electrons and form positively charged ions, therefore, the metals are called electropositive elements.
Some of the important chemical properties of metals are discussed below:
I. Reaction with oxygen
Metals react with oxygen to form oxides. These oxides are basic in nature. For example, sodium metal reacts with oxygen of the air and form sodium oxide.
Sodium oxide reacts with water to form an alkali called sodium hydroxide. Therefore, sodium oxide is a basic oxide.
Due to the formation of sodium hydroxide (which is an alkali), the solution of sodium oxide in water turns red litmus blue (common property of all alkaline solutions).
When metal oxides are dissolved in water, they give alkaline solutions.
Similarly, magnesium is a metal and it reacts with oxygen to form magnesium oxide. However, magnesium is less reactive than sodium and therefore, heat is required for the reaction.
Thus, when a metal combines with oxygen, it loses its valence electrons and forms positively charged metal ions. We can say that oxidation of metal takes palace.
Note:
All metals do not react with oxygen with equal ease. The reactivity of oxygen depends upon the nature of the metal. Some metals react with oxygen even at room temperature, some react on on heating while still others react only on strong heating.
Examples
(A) Reaction with Na, K, Ca
Metals like sodium, potassium and calcium react with oxygen even at room temperature to form their oxides.
Sodium Oxygen Sodium oxide
Potassium Oxygen Potassium oxide
Calcium Oxygen Calcium oxide
(B) Reaction with Mg and Zn
Metals like magnesium and zinc do not react with oxygen at room temperature. They burn in air only on strong heating to form corresponding oxides.
Magnesium Oxygen Magnesium oxide
(C) Reaction with Fe and Cu
Metals like iron and copper do not burn in air even on strong heating. However, they react with oxygen only on prolonged heating.
II. Reaction with water
Metals react with water to form metal oxide or metals hydroxide and hydrogen. The reactivity of metals towards water depends upon the nature of the metals. Some metals react even with cold water, some react with water only on heating while there are some metals do not react even with steam. For example,
A) Reaction with Na and K
Sodium and potassium metals react vigorously with cold water to form respective hydroxide and hydrogen gas is liberated.
The reaction between sodium and water is so violent that the hydrogen evolved catches fire.
B) Reaction with Ca
Calcium reacts with cold water to form calcium hydroxide and hydrogen gas. The reaction is less violent.
C) Reaction with Mg
Magnesium reacts very slowly with cold water but reacts rapidly with hot boiling water forming magnesium oxide and hydrogen.
D) Reaction with Zn and Al
Metals like zinc and aluminum react only with steam to form their corresponding oxides and hydrogen.
E) Reaction with Fe
Iron metal does not react with water under ordinary conditions. The reactions occurs only when steam is passed over red hot iron and the products are iron (II, III) oxide and hydrogen.
F) Reaction with other Metals
Metals like copper, silver and gold do not react with water even under strong conditions. The order of reactivities of different metals with water is:
Na > Mg > Zn > Fe > Cu
Reactivity with water decreases
III. Reaction with dilute acids
Many metals react with dilute acids and liberate hydrogen gas. Only less reactive metals such as copper, silver, gold etc. do liberate hydrogen from dilute acids. The reactions of metals with dilute hydrochloric acid (HCl) and dilute sulphuric acid () are similar.
With dil. HCl, they given metal chlorides and hydrogen whereas with dil. H2SO4, they give metal sulphates and hydrogen.
Dilute nitric acid () is an oxidising agent which oxidises metals, but does not produce hydrogen.
The reactivity of different metals is different with the same acid. For example:
A) Reaction with Na, Mg, Ca
Sodium, magnesium and calcium react violently with dilute hydrochloric acid (HCI) or dilute sulphuric acid () liberating hydrogen gas and corresponding metal salt.
Similarly,
Similarly,
Reaction with Fe
Iron react slowly with dilute or dil. and therefore, it is less reactive than zinc and aluminum.
C) Reaction with Cu
Copper does not react with dil. HCl or dil. .
Therefore copper is even less reactive that iron.
The order of reactivity of different metals with dilute acid:
Na > Mg > Al > Zn > Fe > Cu
Reactivity with dil. acids decreases from sodium to copper.
IV. Reactions of metals with salt solutions
When a more reactive metal is placed in a salt solution of less reactive metal, then the more reactive metal displaces the less reactive metal from its salt solution. For example, we will take a solution of copper sulphate (blue coloured solution) and put a strip of zinc metal in the solution. It is observed that the blue colour of copper sulphate fades gradually and copper metals are deposited on the zinc strip. this means that the following reaction occurs:
Here, zinc displaces copper from its salt solution.
However, if we take zinc sulphate solution and put a string of copper metal in this solution, no reaction occurs.
This means that copper cannot displace zinc metal from its solution. Thus, we can conclude that zinc is more reactive than copper. However, if we put gold or platinum strip in the copper sulphate solution, then copper is not displaced by gold or platinum. Thus, gold and platinum are less reactive than copper.
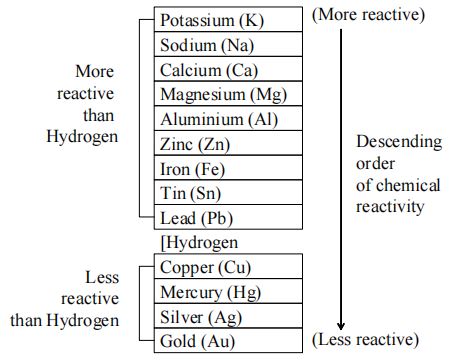
V. Reactivity Series
A) Introduction
We have learnt that some metals are chemically very reactive while others are less reactive or do not react at all.
On the basis of reactivity of different metals with oxygen, water acids as well as displacement reactions, the metals have been arranged in the decreasing order of their reactivities.
The arrangement of metals in order of decreasing reactivities is called reactivity series or activity series of metals.
The activity series of some common metals is given in Table. In this table, the most reactive metal is placed at the top whereas the least reactive metal is placed at the bottom. As we go down the series the chemical reactivity of metals decreases.
B) Reasons for Different Reactivities
In the activity series of metals, the basis of reactivity is the tendency of metals to lose electrons. If a metals can lose electrons easily to form positive ions, it will react readily with other substances. Therefore, it will be a reactive metal. On the other hand, if a metal loses electrons less rapidly to form a positive ion, it will react slowly with the other substances. Therefore, such a metal will be less reactive. For example, alkali metals such as sodium and potassium lose electrons very readily to from alkali metal ions, therefore, they are very reactive.
C) Displacement of Hydrogen from Acids by Metals
All metals above hydrogen in the reactivity series (i.e. more active than hydrogen) like zinc, magnesium, nickel can liberate hydrogen from acids like HCl, . These metals have greater tendency to lose electrons than hydrogen. Therefore, the ions in the acids will accept electrons and give hydrogen gas as:
The metals which are below hydrogen in the reactivity series (i.e. less reactive than hydrogen) like copper, silver, gold cannot liberate hydrogen form acids like HCl, etc. These metals have lesser tendency to lose electrons than hydrogen. Therefore, they cannot lose electrons to ions.
D) Reactivity Series and Displacement Reactions
The reactivity series can also explain displacement reactions. In general, a more reactive metal (placed higher in the activity series) can displace the less reactive metal from its solution. For example, zinc displaces copper from its solution.
E) Usefulness of Activity Series
The activity series is very useful and it gives the following information:
(i) The metal which is higher in the activity series is more reactive than the other. Lithium is the most reactive and platinum is the least reactive.
(ii) The metals which have been placed above hydrogen are more reactive than hydrogen and these can displace hydrogen from its compounds like water and acids to liberate hydrogen gas.
(iii) The metals which are placed below hydrogen are less reactive than hydrogen and these cannot displace hydrogen from its compounds like water and acids.
(iv) A more reactive metal (placed higher in the activity series) can displace the less reactive metal from its solution.
(v) Metals at the top of the series are very reactive and, therefore, they do not occur free in nature. The metals at the bottom of the series are least reactive and, therefore, they normally occur free in nature. For example, gold, present in the reactivity series is found in Free State in nature.
3.5 How do Metals and Non-Metals React
I. Reaction with oxygen
All things that we see around are formed of one or more of the 120 odd known elements. From the time we wake up and till we go to sleep, we are dependent every second on one or the other compounds formed by the combination of these elements. There are more than trillions of compounds found over earth. Do you know the number of elements responsible for the formation of these many number of compounds? You will be surprised to know that it is only 84 elements. Is it not astounding to know that atoms of these 84 elements form these trillions of compounds and help in human existence? What then is the chemical activity in which these atoms are involved to form huge number of compounds? What is the cause for this chemical activity?
II. Secret of inertness of inert gases
All the elements of VIIIA group are called noble gases.
The noble gases are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) & Radon (Rn).
The following are the special features of noble gases:
i) They do not combine with themselves or their elements.
ii) They can exist independently. Thus, they are chemically inert.
Owing to their inert nature, they are also called inert gases.
Let us observe the number of electrons present in their outer shell.
|
Noble gas |
Symbol |
Atomic number |
Electronic configuration |
|
Helium Neon Argon Krypton Xenon Redon |
He Ne Ar Kr Xe Rn |
2 10 18 36 54 86 |
2 2, 8 2, 8, 8 2, 8, 18, 8 2, 8, 18, 18, 8 2, 8, 18, 32, 18, 8 |
Observation: Except helium, all other elements have 8 electrons in their outermost shell.
Note: The configuration in which the outermost shell contains 2 & 8 electrons is known as Duplet and Octet configuration respectively.
Conclusion: The secret of inertness of inert gases is the stable duplet or octet configuration.
III. Why do atoms combine?
To answer this, let us first recall the reason for non-reactivity of noble gases. We know that inert gases are stable due to their stable Octet/Duplet configuration. It is to be known that except the noble gases, all other elements have 1-7 electrons in their outermost shell. All the atoms except inert gases are unstable. They have a tendency to combine with themselves or with the atoms of different elements so as to acquire stable duplet/octet configuration. Hence, the reason for the combination of atoms is to attain stability.
It is the same reason, a reason for the cause of a chemical reaction also?
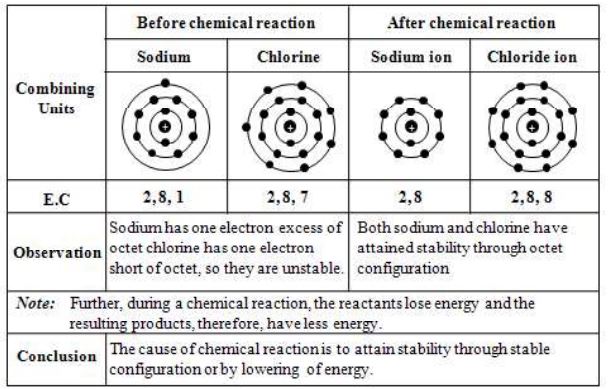
Consider the reaction between sodium and chlorine. During this reaction, sodium loses one electron and chlorine gains one electron to form sodium chloride.
IV. Electron theory of valency
A number of attempts were made to explain formation of chemical bonds in terms of electrons, but it was only in 1916, Kossel and Lewis’ succeeded independently in giving a satisfactory explanation.
They proposed a theory, based on electronic concept of atoms, known as electron theory of valency.
The secret of stability of atoms
Atoms with eight electrons in the outermost shell (two in the case of Helium) are chemically more stable.
Cause of chemical reaction
The cause for chemical reaction is to attain stability. An atom achieves this by acquiring the octet configuration (inert gas configuration) in its outermost shell.
Type of electrons
The electrons present in the outermost shell of an atom are responsible for chemical reaction. The outermost shell is called valence shell and hence, the electrons present in it are called valence electrons. The number of electrons taking part in a chemical reaction is called valency of that element.
Attainment of Inert gas configuration
The atoms of various elements achieve the nearest inert gas configuration, either by transfer (losing or gaining) or by sharing of electrons with another atom. This transfer or sharing of electrons results in the development of an attractive force between the atoms, which holds the atoms together by a bond.
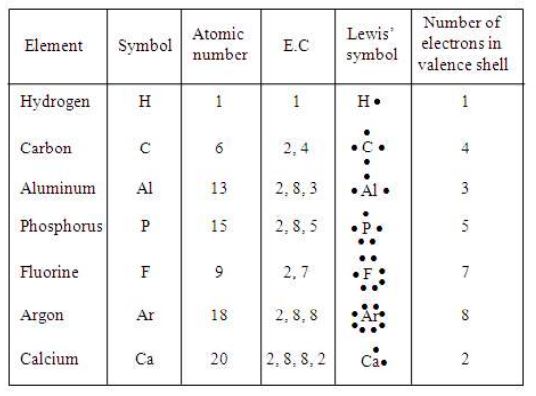
V. Lewis’ dot symbols
In the formation of any molecule, only the electrons present in the outermost shell are shown. The reason for not showing the inner shell electrons is that they are well-protected and do not involve in chemical reaction. Therefore, valence electrons are considered for the formation of the chemical bonds. G.N. Lewis introduced simple symbols called Lewis’ symbols to denote the valence electrons in an atom.
Note
While representing Lewis’ symbol of an element, we show the valence electron in the form of dots round symbols of the element.
Example:
The electronic configuration of chlorine is 2, 8, 7. Thus, there are seven valence electrons.
The Lewis symbol of chlorine atom is
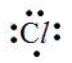
VI. Ionic bond
The chemical bond formed between two atoms by the transfer of one or more electrons from the valence shell of electropositive element (metal) to valence shell of electronegative element (non-metal) is called ionic bond. Oppositely charged ions attracted to each other and a bond between them is formed. The bond existing between oppositely charged ions is called ionic bond (or) electrovalent bond.
(i) Formation of Sodium Chloride
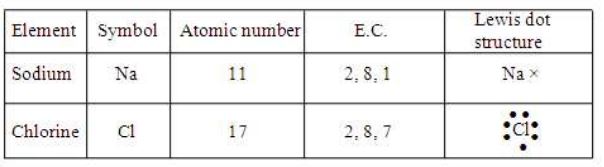
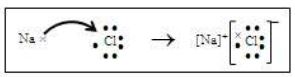
Sodium has one electron excess of octet and chlorine has one electron short of octet. To obtain stability, sodium should lose one electron and chlorine should gain one electron. When sodium combines with chlorine, sodium donates one electron to chlorine After transfer of electron, both form ions and attain stability.
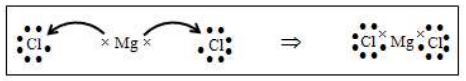
(ii) Formation of Magnesium Chloride
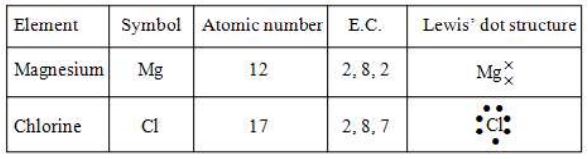
Magnesium atom has two electrons excess of octet and each chlorine atom is one electron short of octet. When magnesium combines with two chlorine atoms, each chlorine atom accepts one electron from magnesium atom; one Mg atom can combine with 2 chlorine atoms.
VII. Properties of ionic compounds
(i) Physical state
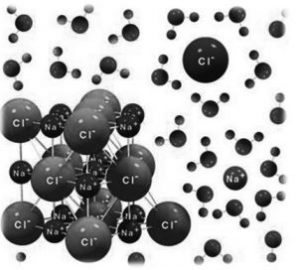
Most of the Ionic compounds are hard crystalline, solids.
Reason: This is due to strong electrostatic force of attraction between cation and anions of ionic compounds
Though ionic compounds are hard, they are brittle in nature. Why?
The behaviour of ionic compounds is much like glass, which breaks into many pieces on falling.
Normally, the alignment is such that the oppositely charged ions are next to each other as shown:
Owing to the impact on failing, the alignment is disturbed such that the ions with similar charge come next to each other.
Since the charges repel each other, the crystal breaks along the line of force.
(ii) Melting and Boiling point
All the ionic compounds have high melting and boiling points.
Reason: Melting and boiling of ionic compounds involve breaking of the lattice structure and setting the ions free.
In a lattice, there are strong electrostatic forces between oppositely charged ions. Therefore, a considerable amount of energy is required to break these forces. Hence, the melting and boiling points of ionic compounds are high.
(iii) Solubility
Ionic compounds are soluble in polar solvents like water.
Reason: Polar solvent like water overcome the forces of attraction between the ions in the crystal. The resulting ions become mobile and disperse in all directions in polar solvent.
v) Electrical conductivity
Ionic solids are bad conductors of electricity
Reason: In ionic solids, the opposite ions are held by a strong electrostatic force of attraction which makes the ions immobile. Hence, conductivity is not possible.
Ionic solutions are good conductors of electricity.
Reason: In fused or aqueous state, ionic compounds possess free or mobile ions. Hence, they are good conductors.
E) Reactivity
Ionic compounds react rapidly either in fused state or in their aqueous solutions due to the presences of mobile ions.
Reason: This is due to the presences of mobile ions.
3.6 Metallurgy
I. Occurrence of Metals
All metals are present in the earth’s crust either in the free state or in the form of their compounds. Aluminum is the most abundant metal in the earth’s crust. The second most abundant metal is iron and third one is calcium.
Native and Combined State of Metals
Metals occur in the crust of earth in the following two states –
(i) Native state or free state: A metal is said to occur in a free or a native state when it is found in the crust of the earth in the elementary or uncombined form.
The metals which are very uncreative (lying at the bottom of activity series) are found in the free state. These have no tendency to react with oxygen and are not attacked by moisture, carbon dioxide of air or other non-metals. Silver, copper, gold and platinum are some examples of such metals.
(ii) Combined state : A metal is said to occur in a combined state if it is found in nature in the form of its compounds. e.g. Sodium, magnesium etc. Copper and silver are metals which occur in the free state as well as in the combined state.
II. Minerals and Ores
The natural substances in which metals or their compounds occur either in native state or combined state are called minerals.
The minerals are not pure and contain different types of other impurities. The impurities associated with minerals are collectively known as gangue or matrix.
The mineral from which the metal can be conveniently and profitably extracted, is called an ore.
For example, aluminum occurs in the earth’s crust in the form of two minerals, bauxite
(,. ) and clay (. . ). Out of these two, aluminum can be conveniently and profitably extracted from bauxite. So, bauxite is an ore of aluminium.
Oxygen is the most abundant element on earth’s crust.
(a) Types of Ores
The most common ores of metals are oxides, sulphides, carbonates, sulphates, halides, etc. In general, very uncreative metals (such as gold, silver, platinum etc.) occur in elemental form or free state.
(i) Metals which are only slightly reactive occur as sulphides (e.g., CuS, Pbs etc.).
(ii) Reactive metals occur as oxides (e.g., , etc.)
(iii) Most reactive metals occur as salts as carbonates, sulphates, halides etc.
SOME COMMON ORES ARE LISTED IN THE TABLE
|
Nature
|
Metal
|
Name of the ore
|
Composition
|
|
Oxide ores |
Aluminum |
Bauxite |
|
|
|
Copper |
Cuprite |
|
|
|
Iron |
Magnetite |
|
|
|
|
Heamatite |
|
|
Sulphide ores |
Copper |
Copper pyrites |
|
|
|
|
Copper glance |
|
|
|
Zinc |
Zinc blende |
Zns |
|
|
Lead |
Galena |
PbS |
|
|
Mercury |
Cinnabar |
HgS |
|
Carbonate ores |
Calcium |
Limestone |
|
|
|
Zinc |
Calamine |
|
|
Halide ores |
Sodium |
Rock salt |
NaCI |
|
|
Magnesium |
Carnallite |
|
|
|
Calcium |
Fluors par |
|
|
|
Silver |
Horn silver |
AgCl |
|
Sulphate ores |
Calcium |
Gypsum |
|
|
|
Magnesium |
Epsom salt |
|
|
|
Barium |
Barytes |
|
|
|
Lead |
Anglesite |
III. Metallurgy
The process of extracting pure metals from their ores and then refining them for use is called metallurgy. In other words, the process of metallurgy involves extraction of metals from their ores and then refining them for use. The ores generally contain unwanted impurities such as sand, stone, earthy particles, limestone, mica, etc., these are called gangue or matrix.
The process of metallurgy depends upon the nature of the ore, nature of the metals and they types of impurities present. Therefore, there is not a single method for the extraction of all metals. However, most of the metals can be extracted by a general procedure which involves the following steps.
IV. Steps involved in Metallurgy
Crushing and Grinding of Ore
Most of the ores occur as big rocks in nature. They are broken into small pieces with the help of crusher. These pieces are then reduced to fine powder with the help of a ball mill or a stamp mill.
Concentration of Ore or Enrichment of Ore
The process of removal of unwanted impurities (gangue) from the ore is called ore concentration or ore enrichment.
i) Hydraulic washing (washing with water)
Principle: This method is based upon the difference in the densities of the ore particles and the impurities (gangue). Ores of iron, tin and lead are very heavy and, therefore, they are concentrated by this method.
ii) Froth floatation process
Principle: this method is based on the principle of difference in the wetting properties of the ore and gangue particles with water and oil.
This method is commonly used for sulphide ores.
iii) Magnetic separation
Principle: This method depends upon the difference in the magnetic properties of the ores and gangue. This method is used for the concentration of hematite, an ore of iron.
The froth floatation process as commonly used for the sulphide ores of copper, zinc, lead etc.
3.7 Extraction of Metals
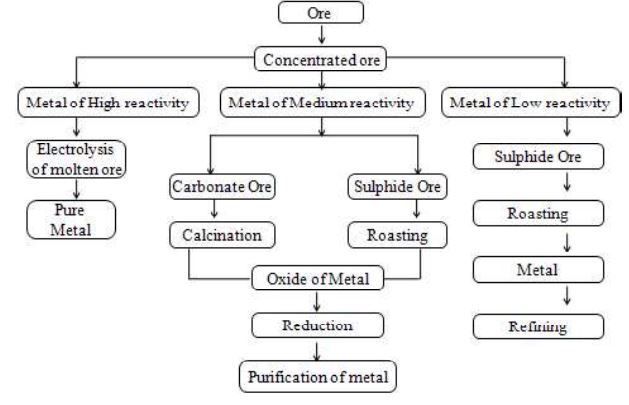
I. Three group of metals of reactivity series
Do you know that metals are classified into three groups on the basis of their reactivity series?
1. Metals of High Reactivity
2. Metals of Medium Reactivity
3. Metals of High Reactivity
Now, let’s see how each of this group of metals can be extracted…
II. Extraction of less reactive metals
These metals are unreactive. The oxides of these metals can be reduced easily on heating as follows:
Step I:
In the first step, cinnabar (an ore of mercury, HgS) is converted into mercury (II) oxide.
Sulphur dioxide is also released in the process.
Step II:
Mercury (II) oxide is reduced to mercury metal on further heating.
Similarly, copper which is found as in nature can be obtained from its ore by just heating in air.
Step I:
In the first step, copper glance (an ore of copper, ) is converted into Copper (I) oxide.
In the first step, copper glance (an ore of copper, ) is converted into Copper (I) oxide.
Sulphur dioxide is also released in the process.
Step II:
Copper (I) oxide is reduced to Copper metal on further heating.
Note:
Metals such as mercury and copper, which lie quite low in the reactivity series, exist in nature as sulphides. These ores when heated react with oxygen present in the air and get converted into oxides. When these oxides are further heated, pure metals are obtained. The process in which a sulphide ore is heated in the presence of air is known as roasting.
III. Extraction of moderately reactive metals
Metals of medium reactivity i.e., metals that are present in the middle of the reactivity series such as zinc, iron, lead, etc are quite reactive and exist in nature as oxides, sulphides, and carbonates. These metals are extracted from their ores by first converting ores to oxides and then by the reduction of these oxides, mostly using carbon. There are two methods by which ores are converted into their respective oxides: (a) Roasting (b) Calcination
(i) Roasting
It is the process of heating the concentrated ore strongly in the presence of excess air. This process is used for converting sulphide ores to metal oxide. In this process, the following changes take place
• the sulphide ores undergo oxidation to their oxides.
• moisture is removed
• volatile impurities are removed.
Examples
(ii) Calcination
It is the process of heating the concentrated ore in the absence of air.
The calcination process is used for the following changes:
• To convert carbonate ores into metal oxide.
• To remove water from the hydrated ores.
• To remove volatile impurities from the ore.
Examples
(iii) Reduction
After obtaining metal oxides from the ores, reduction of these metal oxides is done to obtain pure metals. Mostly, carbon in the form of coke is used for this. However, the oxides of metals, which are present relatively higher in the reactivity series such as manganese, cannot be reduced with coke. To reduce these oxides of metals, more reactive metals than manganese such as sodium, calcium, and aluminium are used.
Let’s see few examples
a) Reduction with carbon
Metals like zinc, copper, nickel, tin, lead etc. can be reduced by using carbon as reducing agent.
a) Reduction with carbon
Metals like zinc, copper, nickel, tin, lead etc. can be reduced by using carbon as reducing agent.
One disadvantage of using carbon as reducing agent is that small traces of carbon are added to metal as impurity. Therefore, it contaminates the metals.
Note:
Coke is very commonly used reducing agent because it is cheap
b) Reduction with Carbon Monoxide
Metals can be obtained from oxides by reduction with carbon monoxide in the furnace.
c) Reduction with Aluminium
Certain metal oxides are reduced by aluminum to metals.
IV. Extraction of highly reactive metals
Metals present at the top of the series such as sodium, potassium, calcium, manganese, and aluminium are very reactive. These metals CANNOT be reduced using coke as their affinity for oxygen is much more than that of carbon. Therefore, these metals are reduced by passing an electric current through their molten salts. This process is known as electrolytic reduction. For example, sodium metal is extracted from sodium chloride. To extract the metal, electrolytic reduction of molten sodium chloride is carried out. When an electric current is passed, sodium ions which have positive charge move towards the cathode and get deposited over it after accepting electrons. The chloride ions have negative charge and move towards the anode, lose their extra electrons, and escape out of the solution as chlorine gas.
V. Purification or refining of metals
The metal obtained any of the above methods is usually impure and is known as crude metal. The process of purifying the crude metal is called Refining.
(i) Liquation
This method is use for refining the metals having low melting points, such as tin, lead, bismuth etc. This is based on the principle that the metal to be refined is easily fusible (melt easily (but the impurities do not fuse easily.
(ii) Distillation
This method is used for the purification of volatile metals (which form vapours readily) such as mercury and zinc.
(iii) Electrolytic Refining
This is most general and widely used method for the refining of impure metals. Many metals such as copper, zinc, tin, nickel, silver, gold etc. are refined electrolytically. It is based upon the phenomenon of electrolysis. In this method, the crude metal is cast into thick rods and are made as anodes, while the thin sheets of pure metal are made as cathodes, An aqueous solution of some salt of the metal is used as an electrolyte. On passing current through the electrolyte, the pure metal from the anode dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the cathode. The soluble impurities go in the solution whereas the insoluble impurities settle down at the bottom of the node and are known as anode mud. In this way, the pure metal from anode goes into electrolyte and from electrolyte it goes to the cathode.
At anode :
At cathode :
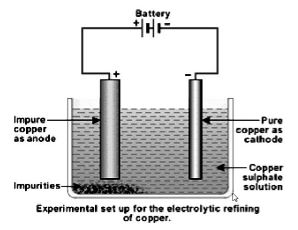
In electrolytic refining impure metal is made anode and pure metal is made cathode.
Zone refining and Van Arkel method are used for obtaining metals (Si, Ge etc.) of very high purity for certain specific applications.
EXTRACTION OF METALS – A SUMMARY
3.8 Corrosion of Metals
Surface of many metals is easily attacked when exposed to atmosphere. The react with air or water present in the environment and form undesirable compounds on their surface. These undesirable compounds are generally oxides.
Thus, corrosion is a process of deterioration of metal as a result of its reaction with air or water (present in environment) surrounding it.
I. Corrosion of Iron
iron corrodes readily when exposed to moisture and gets covered with a brown flaky substance called rust. This is also called Rusting of Iron. Chemically, the rust if hydrated iron (III) oxide, . Rusting is an oxidation process in which iron metal is slowly oxidized by the action of air (in presence of water). Therefore, rusting of iron takes place under the following conditions:
• Presence of air (or oxygen)
• Presence of water (moisture)
More the reactivity of the metal, the more will be the possibility of the metal getting corroded.
II. Experiment to show that rusting of iron requires both air and water
We take three test tubes and put one clean iron nail in each of the three test tubes:
(i) In the first test tube containing iron nail, we put some anhydrous calcium chloride to absorb water (or moisture) from the damp air present in the test tube and make it dry.
(ii) In the second test tube containing iron nail, we put boiled water because boiled water does not contain any dissolved air or oxygen in it. A layer of oil is put over boiled water in the test tube to prevent the outside air from mixing with boiled water.
(iii) In the third test tube containing an iron nail, we put unboiled water so that about two-third of the nail is immersed in water and the rest is above water exposed to damp air.
After one week, we observe the iron nails kept in all the three test tubes.
We will obtain the following observations from the experiment:
(i) No rust in seen on the surface of iron nail kept in dry air in the first test tube. This tells us that rusting of iron does not takes place in air alone.
(ii) No rust is seen on the surface of iron nail kept in air free boiled water in the second test tube, This tells us that rusting of iron does not take place in water alone.
(iii) Red brown rust is seen on the surface of iron nail kept in the presence of the air and water in the third test tube. This tells us that rusting of iron takes place in the presence of both air and water together.
III. Prevention of rusting
(i) Corrosion of metals can be prevented by coating the metal surface with a thin layer of pant, varnish or grease.
(ii) Iron is protected from rusting by coating it with a thin layer of another metal which is more reactive that iron. This prevents the loss of electrons from iron because the active metal loses electrons in process of covering iron with zinc is called galvanization. Iron is also coated with other metals such as tin known as tin coating.
(iii) By alloying: Some metals when alloyed with other metals become more resistant to corrosion. For example, when iron is alloyed with chromium and nickel, it form stainless steel. This is resistant to corrosion and does not rust at all.
(iv) To decrease rusting of iron, certain antirust solutions are used. For example, solutions of alkaline phosphates are used as antirust solutions.
3.9 Alloys
I. Introduction
An alloy is a homogenous mixture of two or more metals or a metal and a non-metal. For example, iron is the most widely used metal. But it is never used in the pure form. this is because iron is very soft and stretches easily when not. But when it is mixed with a small amount of carbon (about 0.05%), it becomes hard and strong. The new form of iron is called steel.
II. Objective of Alloy Making
Alloys are generally prepared to have certain specified properties which are not possessed by the constituent metals. The main objects of alloy-making are:
(a) To increase resistance to corrosion: For example, stainless steel is prepared which has more resistant to corrosion than iron.
(b) To modify chemical reactivity: The chemical reactivity of sodium is decreased by making an alloy with mercury which is known as sodium amalgam.
(c) To increase the hardness: Steel, an alloy of iron and carbon is harder than iron.
(d) To increase tensile strength: Magnesium is an alloy or magnesium and aluminum.
It has greater tensile strength as compared to magnesium and aluminum.
(e) To produce good casting: Type metal is an alloy of lead, tin and mercury.
(f) To lower the melting point: For example, solder is an alloy of lead and tin (50) Pb and 50% Sn). It has a low melting point and is used for welding electrical wires together.
III. Some Important Alloys
The approximate composition and used of some important alloys are given below:
A) Alloys of Iron
Steel: Steel is an alloy of iron and carbon containing 0.1 to 1.5% carbon. Steel is very hard, tough and strong. It is used for making rails, screws, girders, bridges, railway lines etc. Steel can also be used for the contraction for building, vehicles, ships etc.
Alloy Steel: Steel obtained by the addition of some other elements such as chromium, vanadium, titanium, molybdenum, manganese, cobalt or nickel to carbon steel are called Alloy Steel.
B) Alloys of Aluminum
This common alloys of aluminum are:
i) Duralumin: It is an alloy containing aluminum, copper and traces of magnesium and manganese. Its percentage composition is – Al – 95%, Cu = 4%, Mg = 0.5 % Mn = 0.5% It is stronger than pure aluminum, Since duralumin is light and yet strong, it is used for making bodies of aircrafts, helicopter, jets and kitchenware’s like pressure cookers etc.
ii) Magnalium: It is an alloy of aluminum and magnesium having the composition: Al – 95%, Mg = 5% It is very light and hard. It is more harder than pure aluminum. It is used for making light instruments, balance beams, pressure cookers etc.
iii) Alnico: It is an alloy containing aluminum, iron, nickel, and cobalt. It is highly magnetic in nature and can be used for making powerful magnets.
C) Alloys of Copper
The important alloys of copper are Brass and Bronze.
i) Brass – It is an alloy of copper and zinc having the composition: Cu = 80% Zn = 20%. Brass is more malleable and more strong than pure copper. It is used for making cooking utensils, condenser sheets, pipes, hardware, nuts, bolts, screws, springs etc.
ii) Bronze – It is an alloy of copper and tin having the composition: Cu = 90% Sn = 10% Bronze is very though and highly resistant to corrosion. It is used for making utensils, statues, cooling pipes, coins, hardware etc.
iii) German Silver – It is an alloy of copper, zinc and nickel having the composition: Cu = 60%, Zn = 20%, Ni = 20%. It is used for making silverware, utensils and for electroplating.
D) Alloying of Gold
Pure gold is very soft and cannot be used as such for jewellery. Therefore, it is generally alloyed with other metals commonly copper or silver to make it harder and modify its colour. The purity of gold is expressed as carats. Pure gold is of 24 carat. A 18 carat gold means that is contains 18 parts of gold is 24 parts by weight of alloy. Most of the jewellery is made of 22 carat gold.
Amalgams
Amalgams are homogenous mixtures of a metal and mercury. For example, sodium amalgam contains sodium and mercury. Different amalgams are prepared according to their used. For example,
i) Sodium amalgam is produced to decrease the chemical reactivity of sodium metal. It is also used as a good reducing agent.
ii) Tin amalgam is used for silvering cheap mirrors.
iii) The process of amalgamation is used for the extraction of metals like gold or silver from their native ores.








