2.1 Acids
An acid is a compound which on dissolving in water gives hydronium ion as the only positively charged ion.
Note: In actual practice, the acids dissolve in water to form H+ ion or a proton. As H+ ion or proton cannot exist independently in an aqueous solution, it binds itself with water molecule to form hydronium ion.
2.2 Classification of acids
I. On the basis of their sources.
Acids are mostly obtained from natural sources. One the basis of their source acids are of two types
a) Mineral acids
b) Organic acids
(a) Mineral Acids:
Acids which are obtained from rocks and minerals are called mineral acids.
(b) Organic Acids:
Acids which are present in animals and plants are known as organic acids. A list of commonly used acids along with their chemical formula and typical uses, is given below
|
Name |
Type |
Chemical Formula |
Where found or used |
|
Carbonic acid |
Mineral acid |
H2CO3 |
In soft drinks and lends fizz, In stomach as gastric juice, used in tanning industry |
|
Nitric acid
|
Mineral Acid
|
HNO3
|
Used in the manufacture of explosives. (TNT, Nitroglycerine) and fertilizers (Ammonium nitrate, Calcium nitrate, Purification of Au, Ag.) |
|
Hydrochloric acid |
Mineral Acid |
HCl |
In purification of common salt, in textile industry as bealching agent, to make aqua regia mixture of HCl and HNO3 in ratio of 3 : 1 |
|
Sulphuric acid
|
Mineral Acid
|
H2SO4
|
Commonly used in car batteries, in the manufacture of fertilizers (Ammonium sulphate, super phosphate) detergents etc, in paints, plastics, drugs, in manufacture of artificial silk, in petroleum refining. |
|
Phosphoric acid
|
Mineral Acid
|
H3PO4
|
Used in antirust paints and in fertilizers.
|
|
Formic acid
|
Organic Acid
|
HCOOH(CH2O2)
|
Found in the stings of ants and bees, used in tanning leather, in medicines for treating gout disease of joints. |
|
Acetic acid
|
Organic Acid |
CH3COOH(C2H4O2) |
Found in vinegar used a solvent in the manufacture of dyes and perfumes |
|
Lactic acid
|
Organic Acid |
CH3CH(OH)COOH(C3H6O3)
|
Responsible for souring of milk in curd. |
|
Benzoic acid
|
Organic Acid
|
C6H5COOH |
Used as a food preservation. |
|
Citric acid
|
Organic Acid
|
C6H8O |
Present in lemons, oranges and citrus fruits. |
II. On the basis of elements present in them
1. Hydracids (Binary acids): Hydracids contain hydrogen and a non-metallic element, other than oxygen.
Examples:
Hydrofluoric acid : HF
Hydrochloric acid : HCl
Hydrobromic acid : HBr
Hydroiodic acid : HI
Hydrogen sulphide :
(Hydrosulphuric acid)
2. Oxyacids (Ternary acids): Oxyacids contain oxygen, along with hydrogen and another non-metallic element.
Examples:
(Sulphuric acid), HNO3 (Nitric acid), H3PO4 (Phosphoric acid),
(Sulphurous acid).
HClO (Hypochlorous acid), HClO2 (Chlorous acid)
(Chloric acid), HClO4 (Perchloric acid)
(Carbonic acid), CH3COOH (Acetic acid)
III. On the basis of basicity of an acid
Basicity: The number of hydronium ions [(aq)] which can be liberated by one molecule of an acid on complete ionisation is called its basicity. On the basis of basicity, the acids can be classified as under:
(a) Monobasic acids:
Definition: When one molecule of an acid on complete ionisation produces one hydronium ion [H+(aq)], then the acid is said to be monobasic acid.
Characteristics of a Monobasic acid:
(1) A monobasic acid ionises in one step in aqueous solution.
(2) A monobasic acid forms only single salt or normal salt
Examples: HCl, HF, HBr, CH3COOH, HCOOH,
(b) Dibasic acids:
Definition: When one molecule of an acid on complete ionisation produces two hydronium ions [2(aq)], then the acid is said to be Dibasic acid.
Characteristics of a dibasic acid:
(1) A dibasic acid ionises in two steps in aqueous solution.
(2) A dibasic acid forms two series of salts.
(i) An acid salt with one replaceable ion.
(ii) A normal salt with no replaceable ion.
[Sodium sulphate – a normal salt
Examples: , , , , , (oxalic acid), etc,.
(c) Tribasic acids:
Definition: When one molecule of an acid on complete ionisation produces three hydronium ions [3(aq)], then the acid is said to be Tribasic acid.
Characteristics of a tribasic acid:
(1) A tribasic acid ionises in three steps in aqueous solution.
(2) A tribasic acid forms three series of salts.
(i) An acid salt with two replaceable ions.
(ii) An acid salt with one replaceable ions.
(iii) A normal salt with no replaceable hydrogen ion
Examples:
IV. On the basis of state
|
1. Solids |
Phosphoric acid H3PO4 |
|
Boric acid H3BO3 |
|
|
2. Liquids |
Hydrochloric acid HCl |
|
Sulphuric acid H2SO4 |
|
|
Nitric acid HNO3 |
V. On the basis of strength of acid
Acids undergo ionisation in aqueous solution. The degree of ionisation is denoted by the.
Mathematically,
Degree of ionisation of an acid () =
VI. On the basis of ionisation of acid
Strong acids: The acids that undergo almost complete ionisation in an aqueous solution, thereby producing high concentration of (aq) ions are called strong acids.
Note: Any acid having a degree of ionisation of 30% or above is a strong acid.
Examples:
(i) Nitric acid
(ii) Hydrochloric acid
(iii) Sulphuric acid.
Weak acids: The acids that undergo partial ionisation in an aqueous solution, such that their degree of ionisation does not exceed 30% are called weak acids.
Examples:
(i) Carbonic acid
(ii) Sulphurous acid
(iii) Hydrogen sulphide
(iv) Acetic acid
(v) Formic acid
(vi) Oxalic acid
(vii) Hydrocyanic acid
VII On the basis of concentration of acid
The measure of amount of water present in a given sample of acid is called concentration.
Based on the concentration, the acids are classified as follows:
Concentrated acid: A sample of an acid which contains very little or no amount of water is called concentrated acid.
Dilute acid: A sample of an acid which contains far more amount of water than its own weight is called dilute acid.
Note: It must be kept in mind that the concentration of an acid merely tells the measure of water in an acid. It should not be confused with strength of an acid which is determined by the measure of the concentration of (aq) ions in the aqueous solution.
Examples :
(1) A strong acid will remain strong acid, even if it is dilute, because it produces high concentration of (aq) ions.
(2) A weak acid will remain weak acid, even, when concentrated, because it does not produce high concentration of (aq) ions.
VIII On the basis of Volatility
|
1. Volatile |
Hydrochloric acid HCl |
|
Nitric acid HNO3 |
|
|
Carbonic acid H2CO3 |
|
|
Sulphurous acid H2SO3 |
|
|
2. Liquids |
Sulphuric acid H2SO4 |
|
Phosphoric acid H3PO4 |
2.3 Methods of preparation of acids
1. By direct synthesis of hydrogen with non-metals:
Note: The acids containing hydrogen and one non-metal are called Hydracids.
2. By dissolving non-metallic oxides in water
Non-metallic oxide + Water Acid
Note: The acids containing hydrogen, one non-metal and oxygen are called oxyacids.
3. By heating salts of more volatile acid with a less volatile acid:
Salt of more volatile acid + Less volatile acid Salt + More volatile acid
4. By oxidation of non-metals with oxyacids:
(i)
(ii)
(iii)
2.4 General properties of acids
(1) Taste: Acids have sharp sour taste.
(2) Effect of indicators: The substances which are used to identify acids or alkalis due to the change in their colour are called indicators.
(i) Acids turn blue litmus paper red.
(ii) Acids turn methyl orange solution pink.
(iii) Acids turn alkaline pink phenolphthalein in aqueous solution colourless.
(3) Effect on skin: Strong mineral acids have corrosive action on skin and cause painful burns.
(i) Conc. sulphuric acid stains the skin black.
(ii) Conc. nitric acid stains the skin yellow.
(iii) Conc. hydrochloric acid stains the skin to amber colour.
Note: All mineral acids are not corrosive. For example, carbonic acid is used in making soft drinks.
(4) Effect of electric current: All aqueous solutions of mineral acids are good conductors of electricity. They decompose with the liberation of hydrogen at cathode.
(5) Action with metallic oxides: All metal oxides react with dilute mineral acids to form their respective metallic salts and water only.
Metallic oxide + Mineral acid (dil) Metallic salt +
(6) Action with metallic hydroxides: All metal hydroxides react with dilute mineral acids to form their respective salts and water only.
Metal hydroxide + Mineral acid (dil) Metal salt + Water
(7) Action with metallic carbonates or metal hydrogen carbonates: All metallic carbonates or metal hydrogen carbonates react with dilute mineral acids to form their respective salts, water and carbon dioxide.
Metal carbonate / + Acid (dil) Metal salt + Water + (g)
Metal hydrogen carbonate
(8) Action with metallic sulphites / metal hydrogen sulphites: All metallic sulphites or metal hydrogen sulphites, react with dilute mineral acids to form their respective salts, water and sulphur dioxide gas.
Metal sulphite/ + Acid (dil) Metal salt + Water + SO2 (g)
Metal hydrogen sulphite
(9) Action with metallic sulphides / metal hydrogen sulphides: All metallic sulphides or metal hydrogen sulphides react with dilute mineral acids to form their respective salts and hydrogen sulphide.
Metal sulphide/+ Acid (dil) Metal salt + Hydrogen sulphide(g)
Metal hydrogen sulphide
10) Action with active metals: Mineral acids (dil) react with active metals to form their respective salts and hydrogen gas.
Metal + Acid (dil) Metal salt + Hydrogen (g)
2.5 Uses of Acids
|
Acids |
Use |
|
1. Acetic acid 2. Citric acid 3. Tartaric acid 4. Oxalic acid 5. Carbonic acid 6. Boric acid |
Cooking Food preservation; soft drinks Baking powder Inkstain remover Soft drinks Washing eyes |
2.6 Bases
Base: A compound which reacts with hydronium ions [ (aq)] of an acid to form salt and water as only products is called a base. (or)
A base is a compound which accepts protons [ (aq)] to form salt and water as only products.
Examples:
1. All metallic oxides (simple oxides) are bases.
2. All metallic hydroxides (also ammonium hydroxide) are bases.
Alkali: A base which is soluble in water is called alkali.
Modern concept of an Alkali: A compound which on dissolving in water furnishes OH- ions as only negative ions is called an alkali.
Point to Remember: All alkalis are bases, but all bases are not alkalis.
For example, potassium hydroxide [KOH] is a base. However, as it dissolves in water to furnish OH– ions, hence it is an alkali. On the other hand, zinc hydroxide is a base. However, it does not dissolve in water and hence, is not an alkali.
Examples of alkalis:
(i) Sodium oxide
(ii) Potassium oxide
(iii) Calcium oxide
(iv) Sodium hydroxide
(iv) Sodium hydroxide
(vi) Calcium hydroxide
2.7 Classification of acids/alkalies
I On the basis of strength:
Alkalis (soluble bases) undergo dissociation in an aqueous solution.
The degree dissociation of an alkali in an aqueous solution is called strength of an alkali.
Mathematically,
Degree of dissociation of a alkali () =
II On the basis of degree of dissociation
On the basis of degree of dissociation, the bases are classified as follows:
(i) Strong bases: The bases that undergo almost complete dissociation in an aqueous solution, to produce high concentration of hydroxyl ions () are called strong alkalis.
Examples:
(i) Potassium hydroxide
(ii) Sodium hydroxide
(ii) Weak bases: The alkalis that undergo partial dissociation in an aqueous solution, so as to produce low concentration of hydroxyl ions are called weak alkalis.
Examples:
(i) Ammonium hydroxide
(ii) Calcium hydroxide
(iii) Magnesium hydroxide
III. On the basis of concentration
The measure of amount of water present in a given sample of alkali is called concentration.
Based on the concentration, the alkalis are classified as follows:
(i) Concentrated alkali: A sample of a base which contains very little or no amount of water is called concentrated alkali.
(ii) Dilute alkali: A sample of an alkali which contains far more amount of water than its own weight is called dilute alkali.
For example, alkali solution having concentration less than 1mole / litre, is called a dilute alkali.
IV On the basis of acidity of base /alkali:
Acidity: The number of hydroxyl ions [ ion] furnished by one molecule of an acid on complete dissociation in water is called its acidity of an alkali.
On the basis of basicity the bases can be classified as under:
Monoacidic bases:
Definition: When one molecule of a base on complete dissociation in water produces one hydroxyl ion [aq.], then the base is said to be monoacidic base.
Note: A monoacidic base dissociates in one step in aqueous solution if it is a soluble base.
Examples:
Diacidic bases:
Definition: When one molecule of an alkali on complete dissociation in water produces two hydroxyl ions [2aq.], or a base whose one molecule reacts with two hydronium ions completely to form salt and water as only products is called a diacidic alkali/base.
Note: Dissociation of alkalis takes place in one step only.
Examples:
Diacidic alkalis: Calcium hydroxide
Magnesium hydroxide
Diacidic bases: Ferrous hydroxide –
Copper hydroxide –
Triacidic bases: The base whose one molecule reacts with three H+ (aq) ions completely to form salt and water as only products are called triacidic bases.
Examples: Aluminium hydroxide [], Ferric hydroxide
2.8 General methods for preparation of bases or alkalies
1. By the action of oxygen on metals
Metal + Oxygen Metallic oxide [Basic Oxide]
4K +
2Cu + 2CuO
2. By the action of water / steam on active metals
3. By dissolving oxides of highly active metals in water
4. By dissolving ammonia gas in water
Soluble salt sol. + Caustic alkali sol. Insoluble metallic + salt base
5. By strong heating of metallic carbonates [exception: ]
6. By heating nitrates of metals from calcium to copper in electrochemical series
2.9 General properties of soluble bases or alkalies
1. Taste: Alkalis have a bitter taste.
2. Touch: Alkalis have a soapy touch i.e., when their solution is felt between the finger tips.
3. Effect of indicators:
(i) Alkali solutions turn acidic red litmus solution blue.
(ii) Alkali solutions turn acidic methyl orange solution yellow.
(iii) Alkali solutions turn phenolphthalein solution pink.
(iv) Alkali solutions turn turmeric paper brown from its original yellow colour.
1. Action with metals
Metals like zinc, tin and aluminum react with strong alkalis like NaOH (caustic soda), KOH (caustic potash) to evolve hydrogen gas.
2. Action with non-metallic oxides
Acids react with metal oxides, but bases react with oxides of non-metals to form salt and water.
Example:
3. Action with acids:
Alkali solution + Acid Salt + Water
5. Action with ammonium salts
Ammonium salt + Alkali Metal salt + Water + Ammonia
2.10 Uses of some common alkalies
|
Name |
Commercial Name |
Chemical Formula |
Uses |
|
Sodium hydroxide |
Caustic Soda |
NaOH
|
In manufacture of soap, paper, pulp, rayon, refining of petroleum etc. |
|
Potassium hydroxide |
Caustic potash |
KOH |
In alkaline storage batteries, manufacture of soap, absorbing CO2 gas etc. |
|
Calcium hydroxide
|
Slaked lime
|
Ca(OH)2
|
In manufacture of bleaching powder softening of hard water etc. |
|
Magnesium hydroxide
|
Milk of Magnesia
|
Mg(OH)2
|
As an antacid to remove acidity from stomach |
|
Aluminium hydroxide
|
|
Al(OH)3
|
As foaming agent in fire extinguishers. |
|
Ammonium hydroxide |
|
NH4OH |
In removing greases stains from cloths and in cleaning window panes. |
Neutralisation: A chemical reaction in which hydronium ions [ (aq) ions] of an acid and hydroxyl () ions of a base combine to form unionized water molecules, is called neutralization.
2.11 Conducting nature of acid and base solutions
Acids are the substances which contain one or more hydrogen atoms in their molecules which they can release H+ ions in water . Similarly, bases are the substances which contain one or more hydroxyl groups in their molecules which they can release ions in water. Since the ions are the carries of charge therefore, the aqueous solutions of both acids and bases are conductors of electricity.
Experiment
In a glass beaker, take a dilute solution of hydrochloric acid (HCl). Fix two small nails of iron in a rubber cork in the beaker as shown in the figure. Connect the nails to the terminals of a 6 volt battery through a bulb. Switch on the current and bulb will start glowing. This shows that the electric current has passed through the acid solution. As the current is carried by the movement of ions, this shows that is solution HCl has ionised to give H+ and ions. Current will also be in a position to pass if the beaker contains in it dilute ( ions are released in aqueous solution). Similarly, aqueous solutions containing NaOH or KOH will also be conducting due to release of ions.
Bulb will not glow if glucose () or ethyl alcohol () solution is kept in the beaker. This means that both of them will not give any ions in solution.
Comparison between properties of Acids and Bases
|
Acids |
Bases |
|
1. Sour in taste. 2. Change colours of indicators etc. Litmus turns from blue to red, phenolphthalein remains colourless. 3. Shows electrolytic conductivity in aqueous solution. 4. Acidic properties disappear when reacts with bases (Neutralisation). 5. Acids decompose carbonate salts. |
1. Bitter in taste. 2. Change colours of indicators eg, Litmus turns from red to blue, phenolphthalein turns from colourless to pink. 3. Shows electrolytic conductivity in aqueous solutions. 4. Basic properties disappear when react with acids (Neutralisation). 5. No decomposition of carbonate salts by bases. |
2.12 Role of water in the ionisation of Acids and Bases
Substances can act as acids and bases only in the presence of water (or) in aqueous solution. In dry state which is also called anhydrous state, these characters cannot be shown. Actually, water helps in the ionization of acids or base by separating the ions. This is also known as dissociation and is explained on the basis of a theory called Arrhenius theory of acids and bases.
In the dry state, hydrochloric acid is known as hydrogen chloride gas i.e. HCl(g). It is not in the position to give any ions. Therefore, the acidic character is not shown. Now, let us pass the gas through water taken in a beaker with the help of glass pipe. molecules are of polar nature which means that they have partial
negative charge () on oxygen atom and partial positive charge () on hydrogen atoms. They will try to form a sort of envelope around the hydrogen atoms as well as chlorine atoms present in the acid and thus help in their separation as ions. These ions are said to be hydrated ions.
The electrical current is carried through these ions. The same applied to other acids as well as bases. Thus we conclude that –
(i) acids can release ions only in aqueous solution.
(ii) base can release ions only in aqueous solution.
(iii) hydration helps in the release of ions from acids and bases.
2.13 Neutralization
You must have observed that antacid tablets are used during indigestion. An antacid contains magnesium hydroxide, which is a mild base. Magnesium hydroxide neutralizes the effect of excess hydrochloric acid produced in the stomach during indigestion. Hence, it helps relieve the pain.
Do you know what actually happens when acids and bases react with each other?
When acids are mixed with bases, they neutralize or cancel each other’s effect. The products formed on mixing acids with bases are salt and water.
The process of treating an acid with a base to form salt and water is called Neutralization.
The general reaction for neutralization can be written as follows:
Acid + Base Salt + Water + Heat
Note:
A lot of heat is produced during the reaction. Hence, it is an exothermic process. Do you know that all acids generate hydrogen () and all bases generate hydroxyl ( ) ions in their aqueous solutions?
ions cannot exist independently; rather they combine with water molecules () to form hydronium ions ().
Similarly, bases also dissociate in aqueous solutions. However, not all bases dissolve in water. The bases that dissolve in water are known as alkalis. Thus, it can be said that ‘all bases are not alkalis, but all alkalis are bases’. Alkalis dissolve in water and produce ions.
These hydroxide ions ( ions) can exist freely in water or in an aqueous solution. These hydrogen (H+) and hydroxyl (OH-) ions react with each other in neutralization reactions to form water. Hence, neutralization reactions, in terms of hydrogen and hydroxide ions can be represented as:
HX + M OH MX + HOH (or) + ()
Applications of Neutralization
(i) People particularly of old age suffer from acidity problems in the stomach which is caused mainly due to release of excessive gastric juices containing HCl. The acidity is neutralized by antacid tablets which contain sodium hydrogen carbonate (baking soda), magnesium hydroxide etc.
(ii) The sting of bees and ants contain formic acid. Its corrosive and poisonous effect can be neutralized by rubbing soap which contains NaOH (an alkali).
(iii) The stings of wasps contain an alkali and its poisonous effect can be neutralized by an acid like acetic acid (present in vinegar).
(iv) Farmers generally neutralise the effect of acidity in the soil caused by acid rain by adding slaked lime (Calcium hydroxide) to the soil.
2.14 Indicators
Indicator indicated the nature of particular solution whether acidic, basic or neutral. Apart from this, indicator also represents the change in nature of the solution from acidic to basic and vice versa. Indicators are basically coloured organic substances extracted from different plants. A few common acid base indicators are
I. Litmus
Litmus is a purple dye which is extracted from ‘lichen’ a plant belonging to variety Thallophytic. It can also be applied on paper in the form of strips and is available as blue and red strips. A blue litmus strip, when dipped in an acid solution acquires red colour. Similarly a red strip when dipped in a base solution becomes blue.
II. Phenolphthalein
It is also an organic dye and acidic in nature. In neutral or acidic solution, it remains colourless while in the basic solution, the colour of indicator changes to pink.
III. Methyl Orange
Methyl orange is an orange coloured dye (yellow) and basis in nature. In the acidic medium the colour of indicator becomes red and in the basic or natural medium, it colour remains unchanged.
IV. Red Cabbage Juice
It is purple in colour in natural medium and turns red or pink in the acidic medium. In the basic or alkaline medium, its colour changes to green.
V. Turmeric Juice
It is yellow in colour and remains as such in the neutral and acidic medium. In the basic medium its colour becomes reddish or deep brown.
|
Sample |
Blue litmus solution |
Red litmus solution |
Phenolphthalein |
Methyl orange |
|
HCl |
Changes to red |
No colour change |
Remains colourless |
Changes to red |
|
Changes to red |
No colour change |
Remains colourless |
Changes to red |
|
|
NaOH |
No colour change |
Changes to blue |
Changes to light pink |
No changes in colour |
|
KOH |
No colour change |
Changes to blue |
Changes to light pink |
No changes in colour |
2.15 Concept of pH
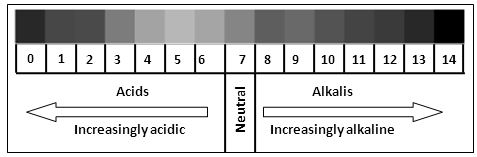
A scale for measuring hydrogen ion concentration in a solution called pH scale, has been developed by S.P.L. Sorrensen. The ‘p’ in pH stands for potenz’ in German meaning power. On the pH scale we can measure pH from O (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of solution. Higher the hydrogen ion concentration, Lower is the pH scale.
Characteristic of pH scale are –
(i) For acidic solution, pH < 7
(ii) For alkaline solution, pH > 7
(iii) For neutral solution, pH = 7
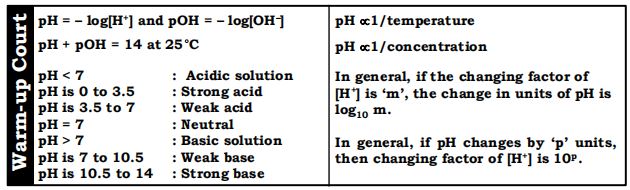
i) pH of a solution can be defined as the negative logarithm of its ion concentration.
pH = – log[]
(ii) The pH of a solution is also defined as the logarithm of the reciprocal of ion concentration.
If [] = M, then
(i) Similarly, pOH of a solution can be defined as the negative logarithm of its ion concentration.
(ii) The pOH of a solution is also defined as the logarithm of the reciprocal of ion concentration.
Note: pH + pOH = 14
pH range for common fluids
|
Substances |
pH range |
|
Substances |
pH range |
|
|
1) |
Gastric juice |
1.0 – 3.0 |
10) |
Milk |
6.3 – 6.6 |
|
2) |
Blood (human) |
7.3 – 7.5 |
11) |
Tomato |
4.0 |
|
3) |
Sea water |
8.5 |
12) |
Black coffee |
5.0 |
|
4) |
Saliva (human) |
6.5 – 7.5 |
13) |
Milk of magnesia |
10.5 |
|
5) |
Tears |
7.4 |
14) |
Apples |
2.9 – 3.3 |
|
6) |
Urine (human) |
4.8 – 8.4 |
15) |
Vinegar |
2.4 – 3.4 |
|
7) |
Rain water |
6.0 |
16) |
Soda water |
Less than 7.0 |
|
8) |
Soft drinks |
2.0 – 4.0 |
17) |
Small intestine (human) |
Approx 8.0 |
|
9) |
Lemons |
2.2 – 2.4 |
|
|
|
Nature of solution and pH range
The acidity and basicity of a solution can be expressed in terms of [] ion concentration, its [] ion concentration (or) its pH.
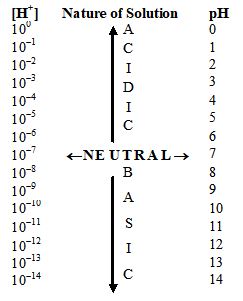
a) If pH < 7, the solution is acidic. Also, if the range of pH is
(i) 0 to 3.5, the solution is a strong acid
(ii) 3.5 to 7, the solution is a weak acid.
b) If pH = 7, the solution is neutral.
c) If pH > 7, the solution is basic. Also, if the range of pH is
(i) 7 to 10.5, the solution is a weak base.
(ii) 10.5 to 14, the solution is a strong base.
Effect of temperature on pH: Increasing temperature decreases the pH of the aqueous solution.
Effect of concentration: If [] concentration increases by 10 times pH decreases by 1 unit and if [] concentration decreases by 10 times, the pH increases by 1 unit.
In general, if the changing factor of [] is ‘m’, the change in units of pH is m.
In general, if pH changes by ‘p’ units, then changing factor of [] is .
Significance of pH in daily life
(i) pH in our digestive system
Dilute hydrochloric acid produced in our stomach helps in the digestion of food. However, excess of acid causes indigestion and leads to pain as well as irritation. The pH of the digestive system in the stomach will decrease. The excessive acid can be neutralized with the help of antacid which are recommended by the doctors. Actually, these are group of compounds (basic in nature) and have hardly and side effects. A very popular antacid is ‘Milk of Magnesia’ which is insoluble magnesium hydroxide. Aluminum hydroxide and sodium hydrogen carbonate can also be used for the same purpose. These antacids will bring the pH of the system back to its normal value. The pH of human blood varies between 7.36 to 7.42. it is maintained by the soluble bicarbonates and carbonic acid present in the blood. These are known as buffers.
(ii) pH change leads to tooth decay
The white enamel coating on our teeth is of insoluble calcium phosphate which is quite hard. It is not affected by water. However, when the pH in the mouth falls below 5.5 the enamel gets corroded. Water will have a direct access to the roots and decay of teeth will occur. The bacteria present in the mouth break down the sugar that we eat in one form or the other to acids, Lactic acid is one these. The formation of these acids causes decrease in pH. It is therefore advisable to avoid eating surgery foods and also to keep the mouth clean so that sugar and food particles may not be present. The tooth pastes contain in them some basic ingredients and they help in neutralising the effect of the acids and also increasing the pH in the mouth.
(iii) Role of pH in curing stings by insects
The stings of bees and ants contain methanoic acid (or formic acid). When stung, they cause lot of pain and irritation. The cure is in rubbing the affected area with soap. Sodium hydroxide present in the soap neutralises acid injected in the body and thus brings the pH back to its original level bringing relief to the person who has been stung. Similarly, the effect of stings by wasps containing alkali is neutralized by the application of vinegar which is ethanoic acid (or acetic acid)
(iv) Soil pH and plant growth
The growth of plants in a particular soil is also related to its pH. Actually, different plants prefer different pH range for their growth. it is therefore, quite important to provide the soil with proper pH for their healthy growth. Soils with high iron minerals or with vegetation tend to become acidic. This soil pH can reach as lows as 4. The acidic effect can be neutralized by ‘liming the soil’ which is carried by adding calcium hydroxide. These are all basic in nature and have neutralising effect. Similarly, the soil with excess of lime stone or chalk is usually alkaline. Sometimes, its pH reaches as high as 8.3 and is quite harmful for the plant growth. In order to reduce the alkaline effect, it is better to add some decaying organic matter (compost or manure). The soil pH is also affected by the acid rain and the use of fertilizers. Therefore soil treatment is quite essential.
2.16 Salts
A substance formed by neutralization of an acid with a base is called a salt.
Example
Classification of Salts
Salts have been classified on the basis of chemical formulae as well as pH values.
(a) Classification Based on Chemical Formulae
(i) Normal salts
A normal salt is the one which does not contain any ionisable hydrogen atom or hydroxyl group. This means that it has been formed by the complete neutralization of an acid by a base.
For e.g. etc.
(ii) Acidic salts
An acidic salt still contains some replaceable hydrogen atoms, This means that the neutralization of acid by the base is not complete. For example, sodium hydrogen sulphate (), sodium hydrogen carbonate () etc.
(iii) Basic salts
A basic salt still contains some replaceable hydroxyl groups. This means that the neutralization of base by the acid is not complete. For example, basic lead nitrate Pb (OH) . basic lead chloride, Pb(OH)Cl, etc.
Classification Based on pH Values
Salts are formed by the reaction between acids and bases. Depending upon the nature of the acids and bases or upon the pH values, the salt solutions are of three types.
(i) Neutral salt solutions
Salt solutions of strong acids and strong bases are neutral and have pH equal to 7. They do not change the colour of litmus solution.
For e.g.: NaCl, etc.
(ii) Acidic salt solutions
Salt solutions of strong acids and weak bases are of acidic nature and have pH less than 7. They change the colour of blue litmus solution to red.
For e.g. etc.
In both these salts, the base is weak while the acids and HCl are strong.
(iii) Basic salt solutions
Salt solutions of strong bases and weak acids are of basic nature and have pH more than7. They change the colour of red litmus solution to blue.
For e.g. etc.
In both the salts, bases NaOH and KOH are strong while the acids and are weak.
Uses of Salts:
(i) As a table salt,
(ii) In the manufacture of butter and cheese.
(iii) In leather Industry.
(iv) In the manufacturing of washing soda and baking soda.
(v) For the preparation of sodium hydroxide by electrolysis of brine.
(vi) Rock salt is spread on ice to melt it in cold countries.
2.17 Some Important Chemical Compounds
Sodium chloride – Common Salt (Table Salt)
Sodium chloride (NaCl) also called common salt or table salt is the most essential part of our diet. Chemically it is formed by the reaction between solutions of sodium hydroxide and hydrochloric acid. Sea water is the major source of sodium chloride where it is present in dissolved form along with other soluble salts such as chlorides and sulphates of calcium and magnesium. it is separated by some suitable methods. Deposits of the salts are found in different part of the world and is known as rock salt. When pure, it is a white crystalline solid, However, it is often brown due to the presence of impurities.
(a) Uses:
(i) Essential for life
Sodium chloride is quite essential for life. Biologically, it has a number of function to perform such as in muscle contraction, in conduction of nerve impulse in the nervous system and is also converted in hydrochloric acid which helps in the digestion of food in the stomach. When we sweat, there is loss of sodium chloride along with water. It leads to muscle cramps. Its loss has to be compensated suitably by giving certain salt preparations to the patient. Electrol powder is an important substitute of common salt.
(ii) Raw material for chemical compounds
Sodium chloride is also a very useful raw material for different chemical. A few out of these are hydrochloric acid (HCl), washing soda (), baking soda () etc. Upon electrolysis of a strong solution of the salt (brine), sodium hydroxide, chlorine and hydrogen are obtained. Apart from these, it is used in leather industry for the leather tanning. In severe cold, rock salt is spread on icy roads to melt ice. it is also used as fertilizer for sugar beet.
Washing Soda
Chemical name
Sodium carbonate decahydrate
Chemical formula:
(a) Preparation
Sodium carbonate is recrystallized by dissolving in water to get washing soda it is a basic salt.
(b) Uses
(i) It is used as cleansing agent for domestic purposes.
(ii) It is used in softening hard water and controlling the pH of water.
(iii) It is used in manufacture of glass.
(iv) Due to its detergent properties, it is used as a constituent of several dry soap powders.
(v) It also finds use in photography, textile and paper industries etc.
(vi) It is used in the manufacture of borax (Na2B4O7. 10H2O)
Baking Soda
Baking soda is sodium hydrogen carbonate or sodium bicarbonate (NaHCO3).
(a) Preparation
It is obtained as an intermediate product in the preparation of sodium carbonate by Solvay process. In this process, a saturated solution of sodium chloride in water is saturated with ammonia and then carbon dioxide gas is passed into the liquid. Sodium chloride is converted into sodium bicarbonate which, being less soluble, separates out from the solution.
(b) Properties
(i) It is a white, crystalline substance that forms an alkaline solution with water. The aqueous solution of sodium bicarbonate is neutral to methyl orange but gives pink colour with phenolphthalein orange. (Phenolphthalein and methyl orange are dyes used as acid-base indicators.)
(ii) When heated above 543 K, it is converted into sodium carbonate.
(c) Uses
(i) It is used in the manufacture of baking powder. Baking powder is a mixture of potassium hydrogen tartarate and sodium bicarbonate. During the preparation of bread the evolution of carbon dioxide causes bread the evolution of carbon dioxide causes bread to rise (swell).
(ii) It is largely used in the treatment of acid spillage and in medicine as soda bicarb, which acts as an antacid.
(iii) It is an important chemical in the textile, tanning, paper and ceramic industries.
(iv) It is also used in a particular type of fire extinguishers. The following diagram shows a fire extinguisher that uses and to produce gas. The extinguisher consists of a conical metallic container (A) with a nozzle (Z) at one end. A strong solution of is kept in the container. A glass ampoule (P) containing is attached to a knob (K) and placed inside the solution. The ampoule can be broken by hitting the knob. As soon as the acid comes in contact with the solution, gas is formed. When enough pressure in built up inside the container, gas rushes out through the nozzle (A). Since CO2 does not support combustion, a small fire can be put out by pointing the nozzle towards the fire. The gas is produced according to the following reaction.
Bleaching Powder
Bleaching powder is commercially called ‘chloride of lime or’ chlorinated lime’. It is principally calcium oxychloride having the following formula :
Bleaching powder is prepared by passing chlorine over slaked lime at 313 K.
Uses
(i) It is commonly used as a bleaching agent in paper and textile industries.
(ii) It is also used for disinfecting water to make water free from germs.
(iii) It is used to prepare chloroform.
(iv) It is also used to make wool shrink-proof.
Plaster of Paris
(a) Preparation
It is prepared by heating gypsum () at about 373 K in large steel pots with mechanical stirrer, or in a revolving furnace.
The temperature is carefully controlled, as at higher temperature gypsum is fully dehydrated. The properties of dehydrated gypsum are completely different from those of plaster of Paris.
(b) Properties
(i) Action with water: When it is dissolved in water, it gets crystallized and forms gypsum
(c) Uses
When finely powered Plaster of Paris is mixed with water and made into a paste, it quickly sets into a hard mass. In the process, its volume also increases slightly. These properties find a number of uses. Addition of water turns Plaster of Paris back into gypsum.
(i) It is used in the laboratories for sealing gaps where airtight arrangement is required.
(ii) It is also used for making toys, cosmetic and casts of statues.
(iii) It is used as a cast for setting broken bones.
(iv) It also find use in making moulds in pottery.
(iv) It is also used for making surfaces smooth and for making designs on walls and ceilings.
Hydrated Salts – Salts containing water of crystallization
Certain salts contain definite amount of some molecules loosely attached to their own molecules. These are known as hydrated salts and are of crystalline nature. The molecules of present are known as ‘water of crystallization’.
In coloured crystalline and hydrated salts, the molecules of water of crystallization also account for their characteristic colours. Thus, upon heating of hydrated salt, its colour changes since molecules of water of crystallization are removed and the salt becomes anhydrous, For example, take a few crystals of blue vitriol i.e. hydrated copper sulphate in a dry test tube or boiling tube. Heat the tube from below. The salt will change to a white anhydrous powder and water droplet will appear on the walls of the tube. Cool the tube and add a few drops of water again. The white anhydrous powder will again acquire blue colour.








