4.1. Introduction
The compounds like urea, sugars, fats, oils, dyes, proteins vitamins etc., which were isolated directly or indirectly from living or indirectly from living organism such as animals and plants were called organic compounds.
The branch of chemistry which deals with the study of these compounds is called ORGANIC CHEMISTRY.
I. Bonding in Carbon
• Most carbon compounds are poor conductors of electricity.
• The boiling and melting points of the carbon compounds are low.
• Forces of attraction between these molecules of organic compounds are not very strong.
• As these compounds are largely non-conductors of electricity hence the bonding in these compound does not give rise to any ions.
The reactivity of elements is explained at their tendency to attain a completely filled outer shell, that is, attain noble gas configuration. Element forming ionic compounds achieve this by either gaining or losing electrons from the outermost shell. In the case of carbon, it has four electrons in its outermost shell and needs to gain or lose four electrons to attain noble gas configuration. (i) It could gain four electrons forming anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
(ii) It could lose four electrons forming cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
II. Molecule formed by sharing of electrons
1. Formation of Hydrogen molecule
Each hydrogen atom has a single electron in its valence shell.
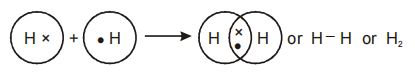
Each hydrogen atom additionally requires 1 electron to attain the stability and the nearest inert gas (He) configuration. For this, two hydrogen atoms combine and contribute 1 electron each for sharing. The bond is formed due to attraction between negatively charged electron pair and the positively charged nuclei.
2. Formation of Chlorine molecule
Electronic configuration of chlorine is 2, 8, 7.
Each chlorine atom has 7 electrons in its valence shell.
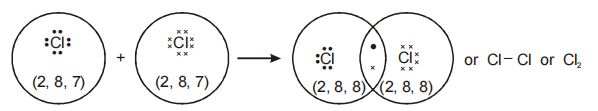
Each hydrogen atom additionally requires 1 electron to attain stability and the nearest inert gas (Ar) configuration. For this, two hydrogen atoms combine and contribute 1 electron each for sharing.The bond is formed due to attraction between a negatively-charged electron pair and the positively charged nuclei.
3. Formation of Ammonia molecule
Electronic configuration of nitrogen is 2, 5. Nitrogen atom has 5 electrons in its valence shell.
Nitrogen is short of 3 electrons to attain octet configuration. Hydrogen atom has 1 electron in its valence shell. Hydrogen is short of 1 electron to attain stability nearest inert gas [He] configuration.
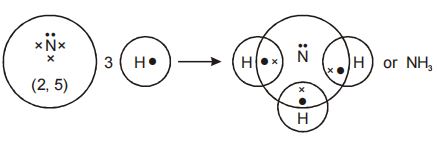
For this, nitrogen atom combines and contributes 3 electrons with 3 hydrogen atoms which contribute 1 electron each for sharing.
III. Types of Covalent Bond
Based on various criteria, covalent bonds are further classified.
1. Single Covalent bond
The covalent bond formed by Sharing of ONE pair of electrons is called a Single covalent bond.
Formation of fluorine molecule
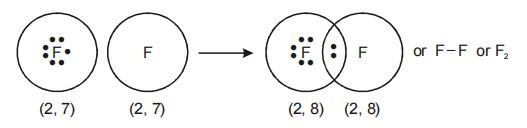
We observe that one pair of electrons is shared between two fluorine atoms, resulting in the formation of a single covalent bond.
Examples:
2. Double Covalent bond
The covalent bond formed by Sharing of TWO pairs of electrons is called a Double covalent bond.
Formation of oxygen molecule
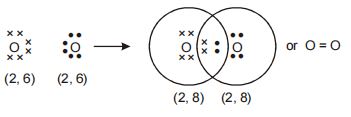
We observe that two pairs of electrons are shared between two oxygen atoms, resulting in the formation of a double covalent bond.
Examples :
3. Triple Covalent bond
The covalent bond formed by sharing of THREE pairs of electrons is called a Triple covalent bond.
Formation of nitrogen molecule
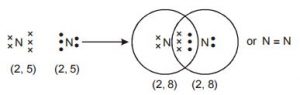
We observe that three electron pairs are shared between two nitrogen atoms, resulting in the formation of a triple covalent bond.
Examples:
4.2. Properties of Covalent Compounds
I. Physical state
Generally, covalent compounds are gases, liquids or soft solids at room temperature. Covalent compounds are composed of molecules. There exists a intermolecular force of attraction known as Vander Waal’s force of attraction between these molecules’. These Vander Waal’s forces are weak and hence covalent compounds exist as soft solids, liquids and gases.
Exceptions: Graphite, Diamond. Let’s see why.
Each carbon atom in a diamond is strongly bonded with the four adjacent carbon atoms by covalent bonds. So we have a network of strongly bonded carbon atoms.This results in a closely-packed three dimensional structure. This makes diamond the hardest of all the substances. As more energy is needed to set the carbon atom free from this network of strongly bonded carbon atoms, the melting and boiling points are very high in diamond.
II. Melting and boiling Points
Covalent compounds possess low melting and boiling points.
Melting and boiling involves the breaking of intermolecular forces of attraction between the molecules. As the intermolecular forces are weak between the molecules, less energy is required to break them. Hence, melting and boiling points of covalent compounds are low.
III. Solubility
Covalent compounds are soluble in non-polar solvents.
Like dissolves like. Covalent compounds have molecules without the opposite polarity and hence they are non-polar compounds. Therefore, covalent compounds are soluble in non-polar solvents like kerosene, benzene, ether, carbon disulphide, carbon tetrachloride, etc.
IV. Conductivity
Charge carriers are required for substances to conduct electricity. Ionic solutions have charge carriers in the form of mobile ions. Metals have charge carriers in the form of electrons. But covalent compounds do not have any charge carriers and hence they are bad conductors of electricity.
Exceptions: Polar covalent compounds, Graphite
Reason: The polar compounds dissociate into ions when dissolved in water. These free ions are responsible for conductivity. Let’s understand why graphite conducts electricity in graphite In graphite, each carbon is bonded with three other carbon atoms. There is one free electron in each carbon that does not participate in bonding. This free electron in graphite is responsible for the conductivity of graphite.
V. Speed of reaction
Reactions of covalent compounds are slow.
The reactions of covalent compounds involve, firstly, the breaking of the existing bonds in the molecules and then the formation of new bonds. Breaking of bonds requires extra energy, and until sufficient energy is available, the reaction is not initiated. Further, to initiate the reaction, the reacting molecules should collide. And among all the collisions, only a small fraction is fruitful. Since a covalent reaction involves a number of operations, it is slow.
VI. Directional property
Covalent bonds are directional. The direction is along the inter-nuclear axis (line joining the nuclei of two reacting atoms). This directional property leads to different arrangement of the atoms along the line of direction.
The result being that different molecules are formed with the same structure. This phenomenon is called Isomerism and the molecules are called Isomers.
4.3. Allotropy
The property by virtue of which an element exists in more than one form in the same physical state, having the same chemical properties but different physical properties, is called allotropy.
Reasons for allotropy
a) The different methods by which each form is or was prepared.
b) Different atomic arrangements in the molecules of each form.
c) Different amounts of energy associated with each form during preparation.
Allotropes of Carbon – A Chart
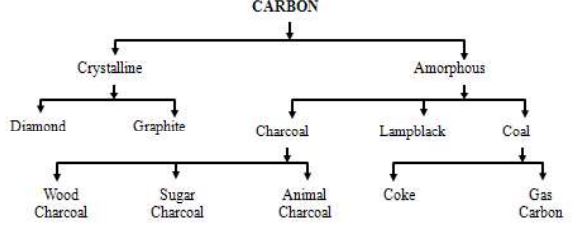
Carbon exhibits allotropy. Diamond, graphite, are some important allotropic forms of carbon. In diamond, carbon atoms are arranged in tetrahedral structure. Each carbon is attached to four other carbon atoms. In graphite, carbon atoms are arranged in hexagonal structure. Many hexagons form a graphite layer. , Buckminster fullerene, is a football like structure containing pentagonal and hexagonal rings.
Carbon forms two types of oxides, viz., CO and .
CO is a poisonous gas. is a fire extinguisher. Plants absorb for photosynthesis. Solid is called dry ice.
Carbon exhibits unique properties like catenation and isomerism. Petroleum, coal, wood and natural gas are the main sources of carbon compounds. Distillation of petroleum gives variety of carbon compounds. Dry distillation of coal gives many important carbon compounds.
4.4. Diamond
Physical Properties
1. Physical state
A brilliant solid transparent with extraordinary brilliance.
2. Structure
In diamond, each carbon atom is attached to four other carbon atoms. Each of the four valence electrons of the carbon atoms are bonded strongly with another carbon atom by covalent bonds. Thus, in a regular diamond structure, each carbon atom is held firmly in place by four bonds (Tetrahedral units). These carbon-carbon bonds are the strongest bonds to be found in any substance at ordinary temperatures.
3. Hardness
Diamond is the hardest known substance. Diamond has three dimensional network of tetrahedral units in which each carbon is strongly bonded to four adjacent carbon atoms. This produces a rigid 3 – dimensional structure which makes diamond the hardest substance. Though diamond is hard it is brittle and can be broken due to the property of cleavage. Cleavage is a property of splitting of crystals of some minerals, in certain directions, to produce a flat, even surface.
4. Density
The density of diamond is 3.5 g/cc. The distance between C – C bond is less, which makes the crystal compact. Therefore the density of diamond is very high.
5. Melting point
The element with the highest melting point is carbon in the form of diamond. It has a melting poing of 3,500 degree centigrade. In diamond, each carbon atom is bound to the four adjacent carbon atoms, by strong covalent bonds. Large amounts of energy is required to break these strong covalent bonds and hence the melting point is very high.
6. Solubility
Diamond has a sparkling brilliance. The reason is its high refractive index.
7. Electrical Conductivity
Diamond is an extremely bad conductor of electricity. In diamond, each carbon atom is covalently bonded with four other carbon atoms. So, the four outermost electrons of a carbon atom are engaged or trapped in the covalent bonds, leaving no free electrons, making it a bad conductor of electricity. Diamond is a bad conductor of heat due to the lack of free electrons.
8. Units of weight
The weight of diamon is measured in carat. 1 carat = 200 mg.
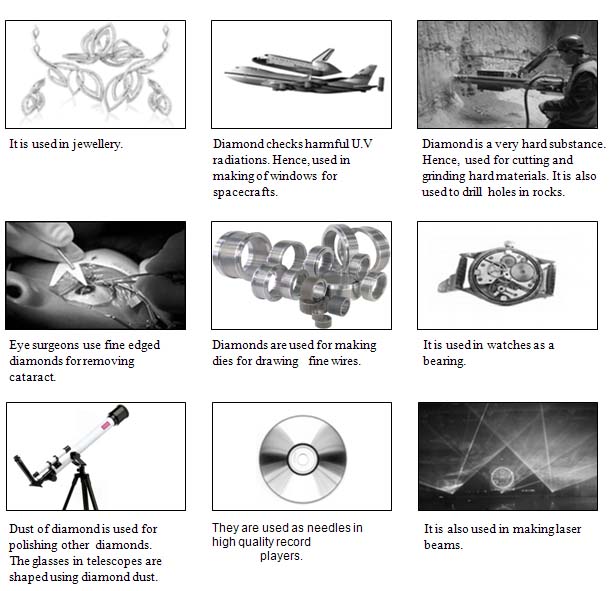
II. Uses
4.5. Graphite
Physical properties of graphite
1. Structure
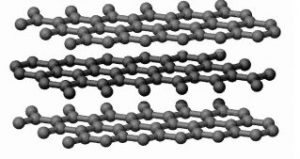
Graphite is a soft solid. In graphite, the carbon atoms are arranged in flat planes of hexagonal rings, stacked one on another.
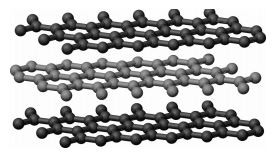
Each carbon atom is attached to just three others within the plane. As such, only three out of the four valence electrons are involved in carbon-carbon bonding. The remaining electron wanders freely between the planes. This free electron accounts for the electrical conductivity of graphite. The links between the adjacent layers are held by weak van der Waals forces. Therefore, the planes can slide over each other easily and make graphite soft, slippery and a good lubricant.
2. Electrical and thermal conductivity
Graphite is a good conductor of electricity. In graphite, each carbon atom forms a covalent bond with three adjacent carbon atoms. Hence three outermost electrons are trapped in thee covalent bonds, leaving the fourth electron free. This free electron is responsible for good electrical conductivity as well as thermal conductivity.
3. Softness and lubricating property
Graphite is a very soft and good lubricant. As the distance between two successive layers in graphite is more, covalent bonds are not formed between the layers. The layers in graphite are held together by weak forces and hence they can slide over each other on application of small forces. This makes graphite soft and a good lubricant. Due to the same property, graphite makes paper black, and hence its name black lead.
Uses


Comparison of physical properties of Diamond and Graphite
|
Property |
Diamond |
Graphite |
|
1. Nature |
Brittle solid |
Flaky solid |
|
2. Physical state |
Transparent with extraordinary brilliance. |
Black opaque solid with metallic lustre. |
|
3. Structure |
Compact, three dimensional structure in which the atoms are held firmly by strong covalent bonds. |
Layers of arranged sheets held by weak Vanderwaal’s forces. |
|
4. Hardness |
Hardest known substance |
Soft and slippery. |
|
5. Specific gravity |
3.52 |
2.3 |
|
6. Electrical conductivity |
Bad conductor of electricity. |
Good conductor of electricity. |
|
7. Thermal conductivity |
Bad conductor of heat. |
Good conductor of heat. |
|
8. Reaction with oxygen |
Catches fire at 8000C. |
Catches fire at 700 0C. |
4.6. Fullerenes
In 1990, scientists discovered another allotrope of carbon. Its molecular structure looks like a football developed by American engineer Buckminster fuller. This form of carbon was therefore named as Buckminster fullerene or Bucky balls. Fullerene is a new allotrope of carbon and it is a crystalline form having 30 to 960 atoms in its molecule. It is made by condensing vapourized graphite in helium.
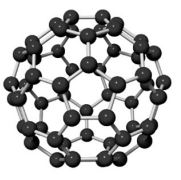
Occurrence
It occurs naturally in interstellar dust and in some carbon-rich rocks.
Structure
Each molecule of Buckminster fullerenes has 60 atoms arranged in hexagons and pentagons. Double covalent bonds join hexagons to hexagons. Fullerenes contain between 30 and 960 carbon atoms. Fullerenes are crystalline forms of carbon containing large, even numbers of carbon atoms in cage-like structures. Some examples are , , , , , , . Fullerenes are present in interstellar (between the stars) dust and so they were not expected to be found on earth.
Buckminster fullerene
C60 was artificially obtained in 1985. And a few years later (1992), it was found in a rare, carbon-rich rock of northeastern Russia, believed to have been formed at least 600 million years ago.
1. Preparation
C60 and some other fullerenes are prepared by striking an electric arc between graphite electrodes in a low-pressure atmosphere of the inert gas helium. Graphite evaporates to form a fluffy solid called ‘fullerene soot’. Nearly 10% of the soot is C60 and 1% is the higher fullerenes. They are separated by chromatography using benzene as the solvent.
2. Structure
Its structure is different from that of diamond and graphite. It is cage-like, with 60 carbon atoms arranged in a sphere. The spherical shape is formed by patching together 20 hexagons and 12 pentagons of carbon atoms. Each carbon atom is shared among two hexagons and one pentagon. This arrangement of hexagons and pentagons is just like that on some designs of a football that is why it is also commonly called a Buckyball.
Note
It has been named after the American architect, Richard Buckminster. It resembles Fuller, who was famous for geodesic (spherical) domes.
3. Physical Properties
1. is a mustard yellow solid. The solid formed of molecules is called fullerite.
2. It is insoluble in water but soluble in organic solvents, e.g., carbon disulphide (), benzene, chloroform, etc. Solutions are magenta coloured.
3. It has a density of 1.72 g/.
4. It has a refractive index of 2.2.
5. It is not a conductor of electricity (Insulator).
4. Chemical properties
Buckminster fullerene is chemically more active than either diamond or graphite. Many new compounds have been prepared keeping the cage intact.
1. On heating in the absence of air, fullerite sublimes at about 530°C. In an excess of air, it is stable only upto about 390°C. It forms CO2 slowly above 390°C and rapidly at 444°C.
2. It undergoes addition reactions with ,, and .
3. Metal atoms can associate with the molecule both inside and outside the cage.
5. Uses
Many applications of the material have been suggested but they are not very operational yet.
Other fullerenes
Many fullerenes have been discovered which have ellipsoidal (egg-like) or tube-like shape. Carbon ‘onions’ have also been discovered which consist of carbon cages one inside another. It is the number of carbon pentagons and hexagons that makes the difference in structure.
4.7. Versaltile Nature of Carbon
About 3 million organic compounds are known today. The main reasons for this huge number of organic compounds are:
I. Catenation
The property of self linking of carbon atoms through covalent bonds to form long straight or branched chains and rings of different sizes is called catenation. Carbon shows maximum catenation in the periodic table due to its small size, electronic configuration and unique strength of carbon – carbon bonds.
II. Tetravalency of carbon
Carbon belongs to group 14 of periodic table. Since the atomic number of carbon is 6. The electronic configuration of carbon atom is 2,4. It has four electrons in the outermost shell. Therefore, its valency is four.
Thus carbon forms four covalent bonds in its compounds. A methane molecule () is formed when four electrons of carbon are shared with four hydrogen atoms are shown below.
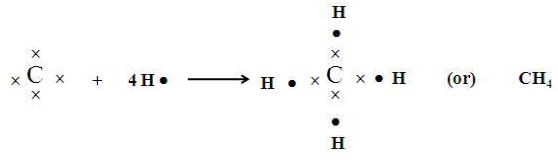
III. Tendency to form multiple bond
Due to small size of carbon it has a strong tendency to form multiple bond (double & triple bonds) by sharing more than one electron pair. As a result, it can form a variety of compound. For example –
4.8. Vital force theory of berzelius hypothesis
Organic compounds cannot by synthesized in the laboratory because they require the presence of a mysterious force (called vital force ) which exists only in living organisms.
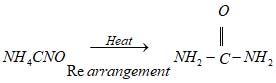
Wohler’s Synthesis
In 1828, Friedrich Wohler synthesized urea (a well-known organic compound) in the laboratory by heating ammonium cyanate. Urea is the first organic compound synthesized in the laboratory.
4.9. Hydrocarbons
The organic compounds containing only carbon and hydrogen are called hydrocarbons. These are the simplest organic compounds and are regarded as parent organic compounds. All other compounds are considered to be derived form them by the replacement of one or more hydrogen atoms by other atoms or groups of atoms. The major source of hydrocarbons is petroleum.
II. Types of Hydrocarbons
The hydrocarbons can be classified as:
1. Saturated hydrocarbons (Alkanes)
Alkanes are saturated hydrocarbons containing only carbon – carbon and carbon – hydrogen single covalent bonds.
For e.g.: (Methane) (Ethane)
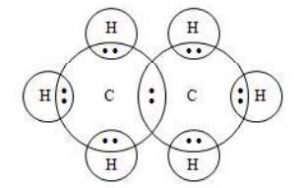
Electron dot structure of ethane
2. Unsaturated hydrocarbons
i) Alkenes
These are unsaturated hydrocarbons which contain carbon – carbon double bond. They contain two hydrogen less than the corresponding alkanes.
General formula:
For e.g.: (Ethane) (Propane)
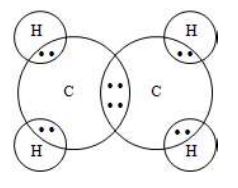
4.10. Classification of Organic Compounds
The organic compounds are very large in number on account of the self – linking property of carbon called catenation. These compounds have been further classified as open chain and cyclic compounds.
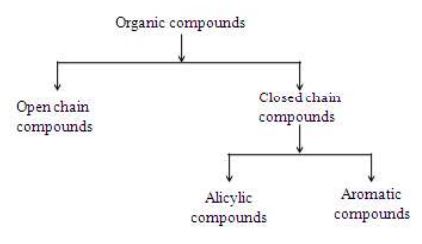
I. Open chain compounds
These compounds contain an open chain of carbon atoms which my be either straight chain or branched chain in nature. Apart from that, they may also be saturated or unsaturated based upon the nature of bonding in the carbon atoms. For example.
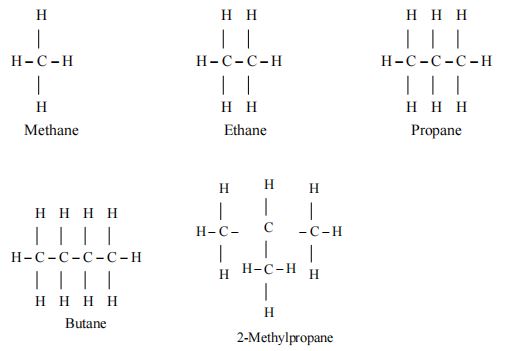
Butane is a straight chain alkane while 2- Methyl propane is branched chain in nature.
II. Closed chain or Cyclic compounds
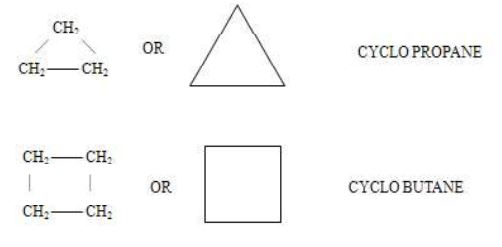
Apart from the open chains, the organic compounds can have cyclic or ring structures. A minimum of three atoms are needed to form a ring. These compounds have been further classified into following types.
1. Alicyclic compounds
Those carboxylic compounds which resemble aliphatic compounds in their properties are called alicyclic compounds.
2. Aromatic compounds
Organic compounds which contain one or more fused or isolated benzene rings are called aromatic compounds.
For eg.
4.11. Homologous Series
Homologous series may be defined as a series of similarly constituted compounds in which the members possess similar chemical characteristics and the two consecutive members differ in their molecular formula by – CH2.
I. Characteristics of Homologous Series
(a) All the members of series can be represented by the same generally formula.
For eg. General formula for alkane series is .
(b) Any two consecutive members differ in their formula by a common difference of – and differ in molecular mass by 14.
(c) Different member in a series have a common functional group.
For eg. All the members of alcohol family have – OH group.
(d) The members in any particular family have almost identical chemical properties. Their physical properties such as melting point, boiling point, density etc. show a regular gradation with the increase in the molecular mass.
(e) The members of a particular series can be prepared almost by the identical methods.
II. Homologues
The different members of a homologous series are known as homologues.
For example:
(i) Homologous series of alkanes
General formula:
Value of n Molecular formula IUPAC name
n = 1 Methane
n = 2 Ethane
n = 3 Propane
(ii) Homologous series of alkenes
Value of n Molecular formula IUPAC name Common name
n = 1 Ethane Ethylene
n = 3 Propane Propylene
n = 4 C4H8 But – 1 – ene – Butylenes
(iii) Homologous series of alkynes
General formula:
Value of n Molecular formula IUPAC name Common name
n = 2 Ethyne Acetylene
n = 3 Propyne Methyl acetylene
n = 4 But – 1 – yne Ethyl acetylene
4.12. Nomenclature of organic compounds
Nomenclature means the assignment of names to organic compounds. There are two main systems of nomenclature of organic compounds.
(i) Trivial system
(ii) IUPAC system (International Union of Pure and Applied Chemistry)
I. Basic Rules of Nomenclature or Compounds in IUPAC System
For naming simple aliphatic compounds, the normal saturated hydrocarbons have been considered as the parent compounds and the other compounds as their derivatives obtained by the replacement of one or more hydrogen atoms with various functional groups.
Each systematic name has first two or all three of the following parts:
(a) Word root
The basic unit is a series of word root which indicate linear or continuous number of carbon atoms.
(b) Secondary suffix
Suffixes added after the primary suffix to indicate the presence of a particular functional group in the carbon chain are known as secondary suffixes.
II. Name of Straight Chain Hydrocarbons
The name of straight chain hydrocarbon may be divided into two parts
(i) Word root (ii) Primary suffix
(i) Word roots for carbon chain lengths
|
Chain length
|
Word root
|
Chain length
|
Word root
|
|
C1 C2 C3 C4 C5 |
Meth – Eth – Prop – But – Pent – |
C8 C7 C8 C9 C10 |
Hex – Hept – Oct – Non – Dec – |
(ii) Primary suffix
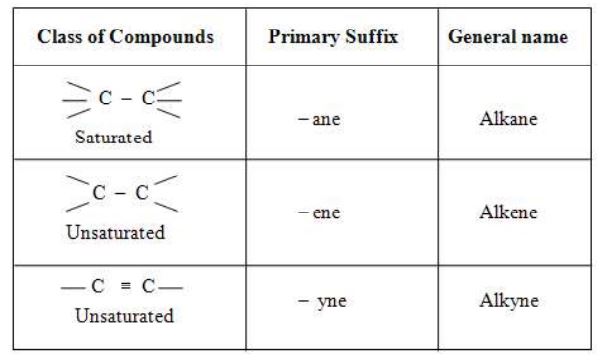
Examples :
|
Molecular Word root formular |
Primary suffix |
IUPAC Name |
|
CH4 Meth – CH3-CH3 Eth- CH3CH2CH3 Prop- CH3CH2CH2CH3 But- CH2 = CH2 Eth- CH3 –CH = CH2 Prop- CH3 -C º CH Prop- |
– ane – ane – ane – ane – ane – ane – yne |
Methane Ethane Propane Butane Ethene Propene Propyne |
III. Names of Branched Chain Hydrocarbon
The carbon atoms in branched chain hydrocarbons are present as side chain. These side chain carbon atoms constitute the alkyl group or alkyl radicals.
An alkyl group is obtained from an alkane by removal of a hydrogen.
General formula of an alkyl group is
An Alkyl group is represented by R.
Examples
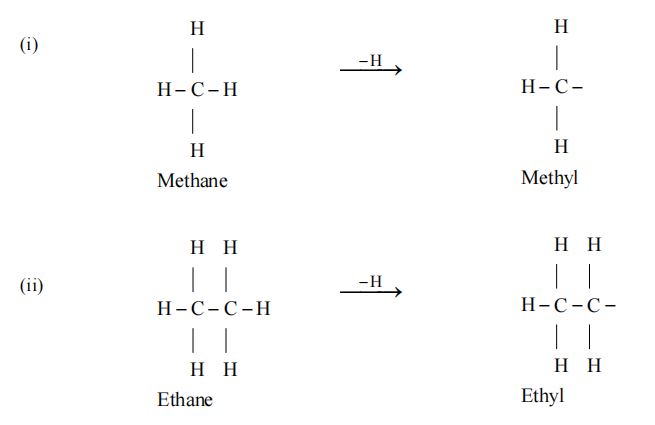
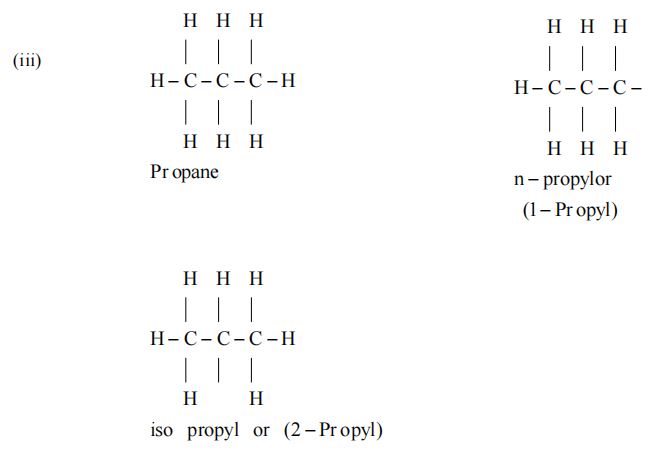
IV. Naming a Branched Chain
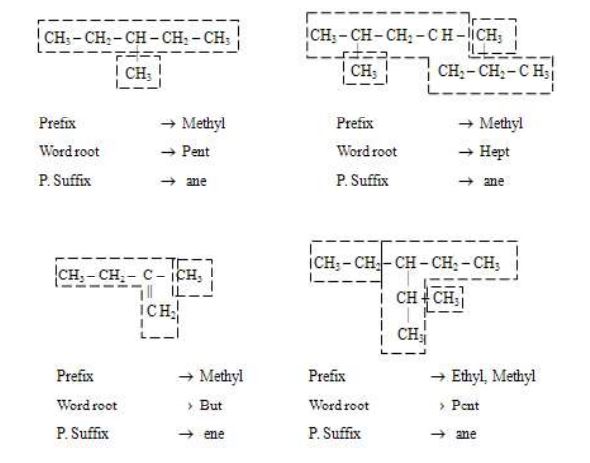
Rule 1 – Longest chain rule
Select the longest possible continuous chain of carbon atoms. If some multiple bond is present, the chain selected must contain the multiple bond.
(i) The number of carbon atoms in the selected chain determines the word root.
(ii) Saturation or unsaturation determines the primary suffix (P. suffix).
(iii) Alkyl substituents are indicated by prefixes.
Examples
Rule 2 – Lowest number Rule
The chain selected in numbered in terms of Arabic numerals and the position of the alkyl groups are indicated by the number of the carbon atom to which alkyl group is attached.
(i) The numbering is done in such a way that the substituents carbon atom has the lowest possible number.
(ii) If some multiple bonds is present in the chain, the carbon atoms involved in the multiple bond should get lowest possible numbers.
Examples :
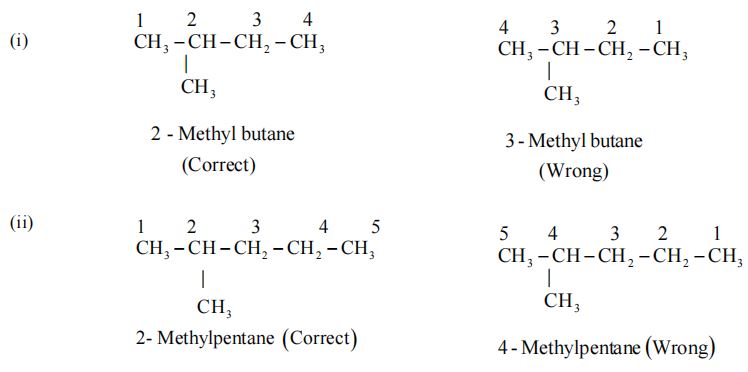
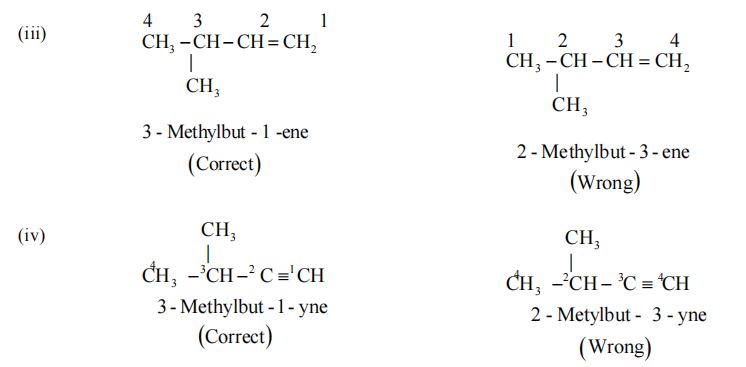
The name of the compound, in general, is written in the following sequence. (Position of substituents) – (prefixes) (word root) (p – suffix)
Rule 3 – Use of prefixed di, tri etc.
If the compound contains more than one similar alkyl groups, their positions are indicated separately and an appropriate numerical prefix, di, tri, etc., is attached to the name of the substituents. The positions of the substituents are separated by commas. Further it should follow the lowest sum
Rule 4 – Alphabetical arrangement of prefixes
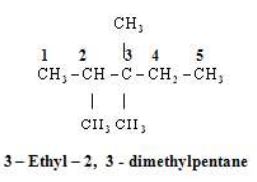
If there are different alkyl substituents present in the compound their names are written in the alphabetical order. However, the numerical prefixes such as di, tri etc. are not considered for the alphabetical order.
For Eg:
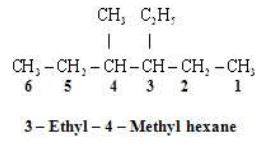
Rule 5 – Naming of different alkyl substituents at the equivalent positions
If two alkly substituents are present at the equivalent position then numbering of the chain is done is such a way that the alkyl group which comes first in alphabetical order gets the lower position.
Some More Examples
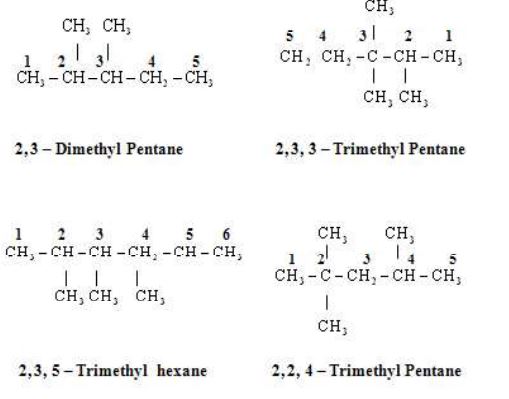
(i) 
Word root Hex
P.Suffix ane
Substituents Two methyl & one ethyl groups
IUPAC Name 4, Ethyl -2,4-dimethylhexane
(ii) 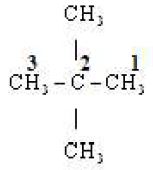
Word root Prop
P.Suffix ane
Substituents Two methyl groups
IUPAC Name 2, 2, di methyl propane
(iii) 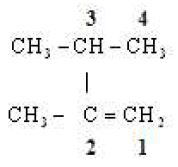
Word root But
P.Suffix ene
Substituents Two methyl groups
IUPAC Name 2, 3, di methyl But -1-ene
(iv) 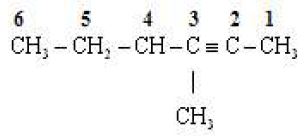
Word root Hex
P.Suffix yne
Substituents One methyl groups
IUPAC Name 3 methyl hex -2-yne
4.13. Functional Group
An atom or group of atoms in an organic compound or molecule that is responsible for the compound’s characteristic reactions and determines its properties is known as functional group. An organic compound generally consists of two parts –
(i) Hydrocarbon radical (ii) Functional group
Example
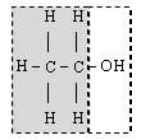
• Functional group is the most reactive part of the molecule.
• Functional group determines the chemical properties of an organic compound.
• Hydrocarbon radical determines the physical properties of the organic compound.
1. Main Functional Groups
A) Hydroxyl group (-OH)
All organic compounds containing – OH group are known as alcohols.
Example: Methanol (), Ethanol ( – – OH) etc.
B) Aldehyde group (-CHO)
All organic compounds containing CHO group are known as alde hydes.
Example: Methanol (HCHO), Ethanol () etc.
C) Ketone group (-CO-)
All organic compounds containing -CO- group are known as ketones.
Example: 2- Propanone (), 2-Butanone (), etc.
D) Carboxyl group ( -COOH)
All organic acids contain carboxyl group. Hence they are also called carboxylic acids.
Example: (Ethanoic acid)
(Propanoic acid)
E) Halogen group (X = F, Cl, Br, I)
All organic compounds containing – X(F, Cl, Br or I) group are known as halides.
Example: Chloromethane (CH3Cl), Bromomethane (CH3Br) etc.
II. Nomenclature of Compounds Containing Functional Group
In case come functional group (other than C = C and C C) is present, it is indicated by adding secondary suffix after the primary suffix. The terminal ‘e’ of the primary suffix is removed if it is followed by a suffix beginning with a, e, i, o, u. Some groups like – F, – Cl, – Br and – I are considered as substituents and are indicated by the prefixes.
Some groups like 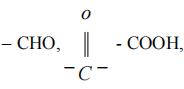 and – OH are considered as functional groups and are indicated by suffixes.
and – OH are considered as functional groups and are indicated by suffixes.
Lets understand the steps for naming compounds with functional group
Step 1 : Select the longest continuous chain of the carbon atoms as parent chain. The selected chain must include the carbon atom involved in the functional groups like – COOH, – CHO etc., or those which carry the functional groups like – OH, – Cl etc.
Step 2: The presence of carbon – carbon multiple bond decides the primary suffix.
Step 3: The secondary suffix is decided by the functional group.
Step 4: The carbon atoms of the parent chain are numbered in such a way so that the carbon atom of the functional group gets the lowest possible number. In case the functional group does not have the carbon atom, then the carbon atom of the parent chain attached to the functional group should get the lowest possible number.
Step 5: The name of the compound is written as:
Prefixes – word root – primary suffix – secondary suffix
The number of carbon atoms in the parent chain decides the word root.
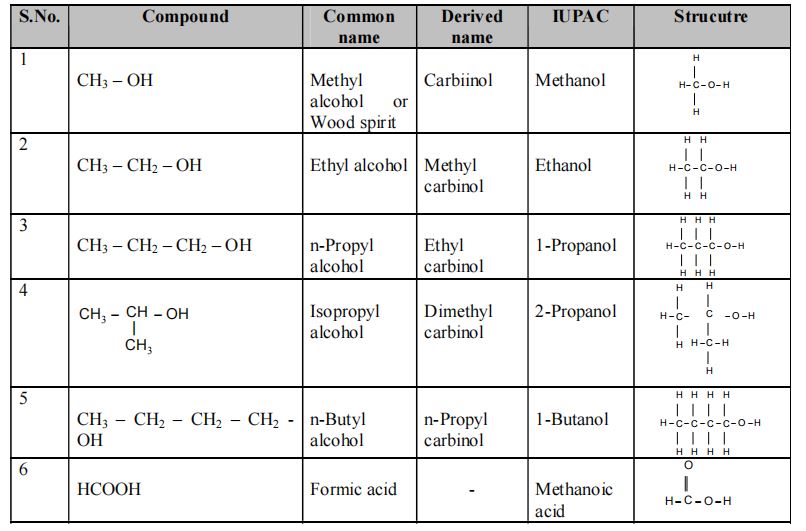
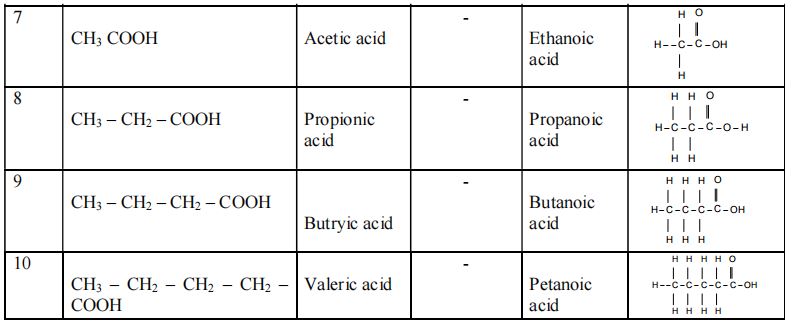
Table showing the IUPAC Names and Structures of different Compounds
(e) Some More Examples
(i) 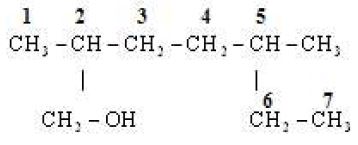
No.of C-atom in longest chain : 7
Word root : Hept
Primary suffix : ane
Functional group :
Secondary suffix :
IUPAC Name : 2,5 – Dimethylheptan -1 -ol
(ii) 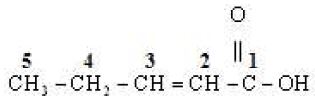
No.of C-atom in longest chain : 5
Word root : Pept
Primary suffix : ene
Functional group :
Secondary suffix :
Position of double bond : 2nd
IUPAC Name : Pent – 2 – en – 1 – oic acid
(iii) 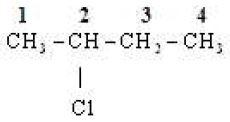
No.of C-atom in longest chain : 4
Word root : Bute
Primary suffix : ane
Functional group :
Prefix : chloro
IUPAC Name : 2 – Chlorobutane
4.14. Chemical Properties of Organic Compounds
The important chemical properties of organic compounds are discussed below:
I. Combustion
Carbon in all its allotropic forms burns in air or oxygen to give carbon dioxide and releases energy in the form of heat and light.
Most carbon compound also release a large amount of heat and light on burning.
Heat and light
Heat and light
Heat and light
Heat and light
Heat and light
Saturated hydrocarbons will generally give a clean flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke. This results in a sooty deposit on the metal plate. However, limiting the supply of air results in incomplete combustion of even saturated hydrocarbons giving a sooty flame.
II. Oxidation
Oxidation is a process in which oxygen is added to a substance. The substances which add oxygen to other substances are called oxidising agents. There are many oxidising agents such as alkaline potassium permanganate (alk. KMnO4), acidified potassium dichromate (K2Cr2O7), nitric acid (HNO3) etc. which are commonly used in organic chemistry. Some common reactions of oxidation are
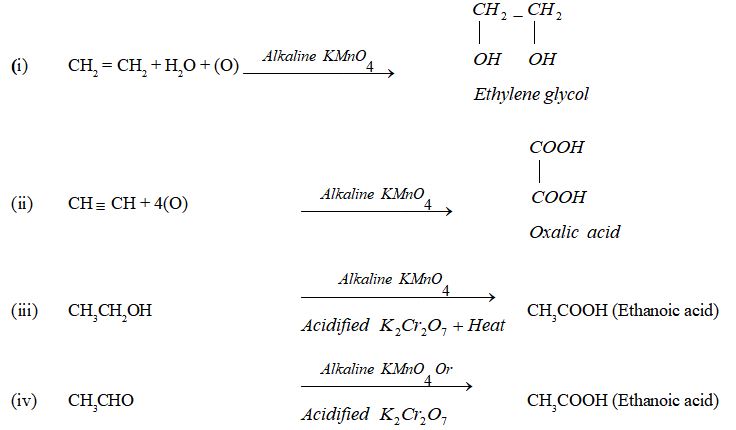
III. Substitution Reaction
The reaction in which an atom or group of atoms in a molecule is replaced or substituted by different atoms or group of atoms are called substitution reactions. Saturated hydrocarbons are fairly uncreative. For example, chlorine does not react with methane at room temperature. However, in the presence of sunlight the reaction of chlorine and hydrocarbons is fairly fast reaction. It gives a variety of products.
Methane Chlorine Chloromethane Hydrogen chloride
In this reaction H – atom of methane has been replaced by a – Cl atom converting to . However, if is used in excess, all the hydrogen atoms are replaced by chlorine atom one by one.
Chloromethane Chlorine Dichloromethane Hydrogen chloride
Dichloromethane Chlorine Trichloromethane Hydrogen chloride
Trichloromethane Chlorine Tetrachloromethane Hydrogen chloride
(Carbon tetrachloride)
IV. Addition Reaction
The reactions in which two molecules react to form a single product having all the atoms of the combining molecules are called addition reactions. Unsaturated compounds such as alkenes contain double bond between carbon atoms. Because of the presence of double bond, they undergo addition reaction.
(i) Addition of halogen to alkenes:
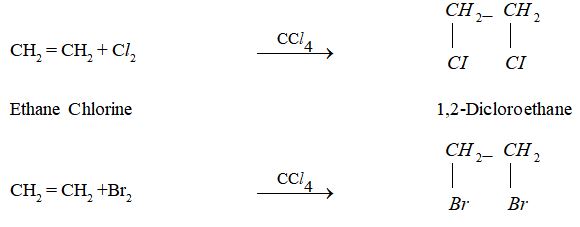
Ethene Bromine 1,2- Dibromoethane
(ii) Addition of hydrogen:
This reaction is called hydrogenation. Hydrogenation reaction is used in the manufacture of vanaspati ghee from vegetable oils. The vegetable oil such as ground nut oil, cotton speed oil and mustard oil contain bonds (C = C) in their molecules. When reacted with hydrogen in the presence of nickel as catalyst, they are converted into vanaspati ghee which is solid at room temperature like butter or ghee.
4.15. Ethanol or (Ethyl alcohol)
• Ethanol is the second member of the homologous alcoholic series.
• It is also known as methyl carbinol.
• Structural formula.
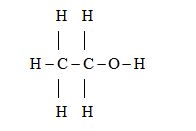
Physical properties
• Ethanol is colourless liquid having a pleasant smell.
• Ethanol boils at 351 K.
• It is miscible with water in all proportions.
• It is a nonconductor of electricity (it does not contain ions)
• It is neutral to litmus.
Chemical properties
A) Combustion
Ethanol burns in air with a blue flame to form
B) Oxidation
(a) By mild oxidizing agent (Chromic anhydride).
(b) By strong oxidizing agent ( + or alkaline KMnO4).
C) Reaction with sodium
Ethanol reacts with sodium to produce hydrogen gas and sodium ethoxide.
D) Reaction with carboxylic acids:
[ESTERIFICATION] The process of formation of an ester by the combination of an alcohol with carboxylic acid is known as Esterification.
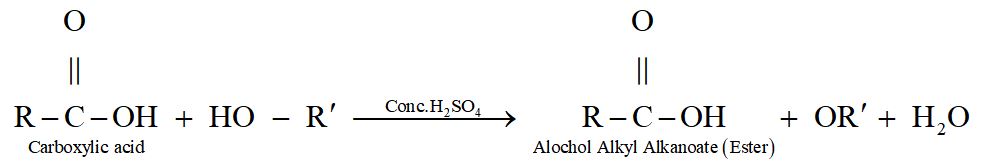
When ethanol reacts with ethanoic acid in presence of concentrated sulphuric acid ethyl ethanoate and water are formed.
Esters are sweet smelling substances and thus are used in making perfumes.
E) Action with concentrated sulphuric acid
Ethanol reacts with concentrated sulphuric acid at 443 K to produce ethylene. This reaction is known as acidic dehydration of ethanol because in this reaction, water molecule is removed from ethanol.
The concentrated sulphuric acid may be regarded as a dehydrating agent because it removes water from ethanol.
The concentrated sulphuric acid may be regarded as a dehydrating agent because it removes water from ethanol.
Some Important Terms:
(a) Denatured alcohol: To prevent the misuse for drinking purpose, the alcohol supplied for industrial purpose is rendered unfit by mixing it with some poisonous substances like methanol, pyridine, copper sulphate etc. It is known as denatured alcohol.
(b) Rectified spirit: Ethanol cottoning 5 percent water is known as rectified spirit.
(c) Absolute alcohol: Rectified spirit is heated under reflux over quicklime for about 5 to 6 hours and then allowed to stand for 12 hours. One distillation, pure alcohol ( = 100%) is obtained. This is called absolute alcohol.
(d) Power alcohol: Alcohol, which is used for generating power is called power alcohol. It consists of a mixture of absolute alcohol petrol roughly in the ratio 20: 80. Since alcohol itself, does not mix with petrol, therefore, a third solvent such as benzene, ether etc. is added as a co-solvent.
Uses of Ethanol
(i) Ethanol is a constituent of beverages like beer, wine, whisky and other liquors.
Beer = 3 – 6% Ethanol
Whisky = 50% Ethanol
Wine = 10 – 20% Ethanol
(ii) Ethanol is used to sterilize wounds and syringes.
(iii) Antifreeze: It is a mixture of ethanol and water which has a much lower freezing point than that of water. It is used in radiators of vehicles in cold countries.
(iv) It is used in manufacture of paints, des, medicines, soaps and synthetic rubber. Solution of ethanol prepared in pharmaceutical industry are known as tinctures.
Harmful effects of drinking alcohol
(i) If ethanol is mixed with and consumed, it may cause serious poisoning and loss of eyesight.
(ii) It causes addiction (habit forming) and mixes with blood. It damages liver if taken regularly.
(iii) Higher amount of consumption of ethanol leads to loss of body control & consciousness. It may even cause death.
4.16. Ethanoic acid (or acetic acid)
(i) Molecular formula: CH3COOH
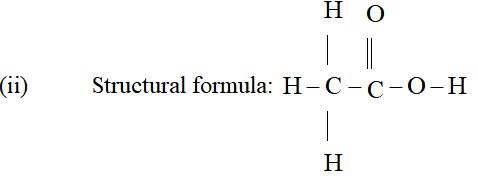
(iii) It dissolves in water, alcohol and ether. Its dissolution in water takes place with the evolution of heat and decrease in volume of the solution.
(iv) The melting point of ethanoic acid in 290 K and hence it often freezes during winter in cold climates. Therefore, it is names as glacial acetic acid.
Chemical properties
(A) Acidic character
Ethanoic acid is a monobasic acid. It has a replaceable hydrogen atom in its – COOH group. Therefore, it neutralizes alkalies.
(i) It reacts with a solution of sodium hydroxide to form sodium ethanoate and water.
Sodium ethanoate is an ionic compound which dissolves in polar solvents such as water, but does not dissolves in non-polar solvents such as alcohol, propanone etc.
The aqueous solution of sodium ethanoate is alkaline due to hydrolysis.
(ii) It reacts with sodium carbonate and sodium bicarbonate with the evolution of gas.
(iii) It reacts with metals like sodium, zinc and magnesium to liberate hydrogen gas.
(B) Ester formation
When ethanoic acid in heated with ethanol in presence of small quantity of conc. ethyl ethanoate, a sweet smelling ester, is formed.
(C) Decarboxylation
When sodium ethanoate is heated with soda lime, methane is formed.
The term’ decarboxylation ‘ is used when the elements of carbon dioxide are removed from a molecule.
Uses
1. Dilute aqueous solution (5-8%) of ethanoic acid is called vinegar, which is used to preserve food (sausage, pickles, etc.)
2. Pure ethanoic acid is used as a solvent and chemical reagent.
3. As cellulose ethanoate, it is used in making photographic films and rayon.
4. Ethanoic acid finds application in the preparation of propanone, choroethanoic acid, ethanoates of metals etc.
5. It is widely used in the manufacture of textiles.
6. It is used in the preparation of white lead.
Tests
1. Litmus test: Add small amount of blue litmus solution to the given compound. If the blue litmus solution turns red, it indicates that the organic compound is ethanoic acid.
2. Sodium bicarbonate test: Take a small portion of the organic compound in a test tube and add a pinch of solid sodium bicarbonate. Evolution of carbon dioxide with brisk effervescence shows the presence of carboxylic acid.
3. Ester formation: When a mixture of ethanoic acid and ethanol is heated in the presence of concentrated sulphuric acid, a fruity smelling ester, ethyl ethanoate, is produced.
4.17. Soaps and Detergents
The word ‘detergent’ means’ cleansing agent and so the detergents are substances which remove dirt and have cleansing action in water. According to this definition of detergents, soap is also a detergent and has been used for more than two thousand years. There are two types of detergents:
(a) Soapy detergents or soaps
(b) Non – soapy detergents or soapless soaps.
I. Soap
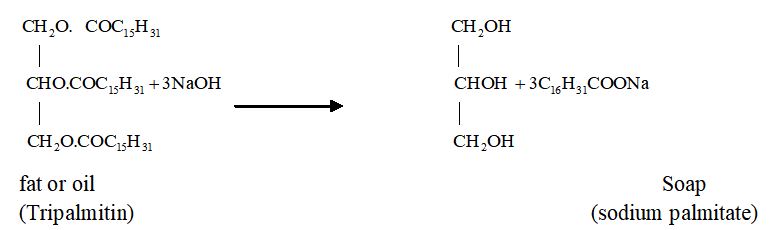
A soap is a sodium or potassium salt of some long chain carboxylic acids (fatty acid). Sodium salts of fatty acids are known as hard soaps and potassium salts of fatty acid are known as soft soaps. A soap has a large non-ionic hydrocarbon group and an ionic COO-Na+ group. The structure of soap can be represented as:

where ![]() represents the hydrocarbon group and represents negatively charged carboxyl group. Some example so soaps are sodium stearate, – , sodium palmitate, – Na+ and sodium oleate, – .
represents the hydrocarbon group and represents negatively charged carboxyl group. Some example so soaps are sodium stearate, – , sodium palmitate, – Na+ and sodium oleate, – .
Hard water, which contains salts of magnesium and calcium, reacts with soap to form magnesium and calcium salts of fatty acid.
Preparation
Soap is prepared by heating oil or fat of vegetable or animal origin with concentrated sodium hydroxide solution (caustic soda solution). Hydrolysis of fat takes place and a mixture of sodium salt of fatty acids and glycerol is formed. Since the salt of fatty acids thus formed are used as soap so alkaline hydrolysis of oils and fats is commonly known as saponification.
Limitation
Soap is not suitable for washing clothes with hard water because of the following reasons.
1. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions of hard water react with soap forming insoluble calcium and magnesium salts of fatty acids.
Therefore, a lot of soap is wasted if water is hard.
2. When hard water is used, soap forms insoluble precipitates of calcium and magnesium salts, which stick to the cloth being washed. Therefore, it interferes with the cleaning ability of the soap and makes the cleaning process difficult.
These calcium and magnesium salts of fatty acid are insoluble in water and separate as cruddy white precipitate
Detergents
These are also called synthetic detergents or soapless soaps. A synthetic detergent is the sodium salt of a long chain benzene sulphonic acid or the sodium salt of a long chain alkyl hydrogen sulphate.
Preparation
Synthetic detergents are prepared by reacting hydrocarbons from petroleum with conc. sulphuric acid and converting the product into its sodium salt.
Example
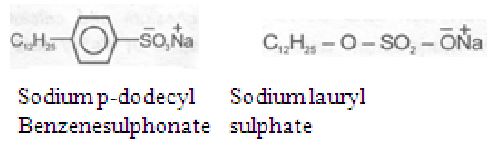
Washing powders available in the market contain about 15 to 30 percent detergents by weight.
Alkaline hydrolysis of oils and fats is commonly known as saponification
Comparisons Between Soap and Detergent
|
S.No |
Soaps |
Synthetic detergents |
|
1 |
Soaps are sodium salts of higher fatty acids |
Synthetic detergents are sodium alkyl sulphates or sodium alkyl benzene sulphonates with alkyl group having more than ten carbon atoms. |
|
2 |
Soaps are prepared form natural oils and fats. |
Synthetic detergents are prepared form the hydrocarbons of petroleum. |
|
3 |
Soaps form insulable salts (curdy white ppt.) with calcium and magnesium which are present in hard water and hence, cannot be used in hard water/ |
Calcium and magnesium salts of detergents are soluble in water and, therefore, no curdy white precipitates are obtained in hard water and hece, can be used even in hard water. |
|
4 |
Soad cannot be used in acidic medium and they are decomposed into carboxylic acids in acidic medium. |
They cay be used in acidic medium as they are the salt of strong acids and are not decomposed in acidic medium. |
|
5 |
Soaps are biodegradable. |
Some of the synthetic detergents are not biodegradable. |
Advantages of Synthetic Detergents Over Soap
Synthetic detergents are widely used as cleaning agents these days. Some of their advantages over soaps are:
(i) Synthetic detergents can be used for washing even in hard water. On the other, soaps are not suitable for use with hard water.
(ii) Synthetic detergents can be used even in acidic solutions because they are not readily decomposed in acidic medium. On the other hard, soaps cannot be used in acidic medium because they are decomposed into carboxylic acids in acidic medium.
(iii) Synthetic detergents are more soluble in water than soaps.
(iv) Synthetic detergents have a stronger cleaning action than soaps.
Cleaning Action of Soaps and Detergents
A molecule of soap is made up of two parts: a non-polar part consisting of a long chain 12 – 18 carbon atoms and a polar part, . The polar end in water soluble and is thus hydrophilic whereas hydrocarbon part is insoluble in water and is thus hydrophobic. In a soap solution, the hydrocarbon portions of several soap molecules huddle together to form aggregates of molecules (or ions) called micelles. The soap micelles are negatively charged due to the presence of carboxylate ions at the surface. Repulsion between similarly charged micelles keeps them dispersed in the solution.
The hydrocarbon part is however soluble in non-polar solvents and is sometimes called lipophilic.
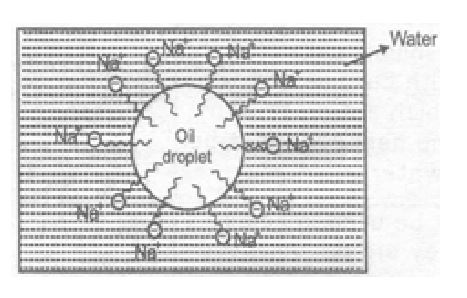
(i) Cleansing action of soap
Mostly the dirt is held to any surface such as cloth by the oil or grease which is present there. Now since the oil and grease are not soluble in water, the dirt particles cannot be removed by simply washing the cloth with water. However, when soap is applied, the non-polar hydrocarbon part of the soap molecules dissolves in oil droplets while the polar groups remain attached to water molecules. In this way, each oil droplet gets surrounded by negative charge.
These negatively charged oil droplets cannot coalesce and continue breaking into small droplets. These oil droplets (containing dirt particles) can be washed away with water along with dirt particles. So, the action of soap or detergents in to emulsify oil or grease, this loosens the soil particles of dirt and they are removed
In a soap molecule hydrophilic polar end in water soluble and hydrophobic hydrocarbon part is insoluble in water.
Soap or detergent helps in cleansing in another way. Not only it emulsifies oil or grease but it also lowers the surface tension of water. As a result of this water wets things more effectively.
When water is added on to the surface of the cloth then water molecules tend to stay as close to each other as possible because of the strong forces of attraction (hydrogen bonding) for each other and do not wet the cloth properly. If some soap solution is added to this away then polar end of soap dissolves in water and non-polar hydrocarbon end remains away from the water.
Thus, soap molecules arrange themselves between the water molecules on the surface of water and decrease the forces of attraction between the water molecules. Water can now spread on the surface of cloth and can make it wet effectively.
Synthetic Detergent: A Serious Problem
It may be noted that in the past, the widespread use of detergents caused pollution of rivers and other water bodies. Earlier the synthetic detergents were made from long chain of hydrocarbons having a lot of branched chains in them. These branched chain detergent molecules were degraded very slowly by the microorganisms present in water bodies like lakes or rivers.
Therefore, they tend to remain in water bodies for a long time and make water unfit for aquatic life. For example, detergents containing phosphates can cause rapid growth of algae and therefore, deplete the dissolved oxygen present in the water of lakes and rivers. As a result of lack of oxygen, fish and other aquatic animals may die. To solve these problems, now-a-days, the detergents are prepared from hydrocarbons which have minimum branching. These are degraded more easily than branched chain detergents. Therefore, these are biodegradable and create fewer problems.








