5.1 Introduction :
You must have visited a library. There are thousands of books in a large library. In spite of this if you ask for a particular book, the library staff can locate it easily.
How is it possible?
In a library, the books are classified into various categories and sub-categories. They are arranged on shelves accordingly. Therefore location of books becomes easy. Let us come back to chemistry. Most of the matter that we see, touch and feel is made up of compounds. There are millions of such compounds existing presently. You will be surprised to know that compounds are formed as a result of various permutations and combinations of only about 110 odd elements. To study properties of these elements and their compounds individually is a tremendous task.
How then was this task simplified?
This task was simplified by simple classification of elements into a few groups. Instead of studying each and every element or compounds, we just learn the properties of groups. The attempts were made by different scientists to classify elements based on their properties. Necessity for classification of elements
Following are the reasons for the classification of elements.
1. The classification may help to study them better.
2. The classification may lead to correlate the properties of the elements with some fundamental property that is characteristic of all the elements.
3. The classification may further reveal relationship between the different elements.
Origins of the periodic table
Chemists would be overwhelmed with isolated pieces of information if they did not have some way of relating the facts they know about the more than 100 known elements. The Periodic Table provides a means of organizing information so that relationships among elements can be clearly seen and understood.
Even before so many elements were known, scientists were searching for relationships among elements. Three important attempts to determine such relationships were made during the nineteenth century by the English physician William Prout, the German chemist Johann Döbereiner, and the English chemist John Newland. All three of these scientists based their work on the model of the atom proposed in 1803 by the English scientist John Dalton.
5.2. Earlier attempts in classifying elements
1. Greeks classification
The ancient Greeks erroneously suggested that all matter consisted of four elements only – Earth, air fire and water. But their idea could not be supported by the experiments.
2. Dalton’s contribution
The following are some important points of Dalton’s contribution.
(i) All matter consists of simple bodies (elements) and compound bodies (compounds). The smallest part of a simple body is the atom. The smallest part of a compound body is the compound atom (later called the molecule).
(ii) All atoms of the same element have the same properties, such as shape, size, and mass. Atoms of different elements have different properties.
(iii)When matter undergoes chemical change, atoms of different elements either combine or separate from one another.
(iv)Atoms cannot be destroyed, even during chemical change.
In 1808, John Dalton published the first list of atomic weights in his ‘Table of the relative atomic weights of the ultimate particles of gaseous and other bodies’.
He changed chemists’ ideas from a qualitative to a quantitative basis, and started the chemical revolution during the 19th century.
3. Prout’s Hypothesis
In 1814, Prout suggested that all of the other elements are developed from hydrogen. In other words, Prout proposed that hydrogen is the fundamental element. Prout reached this conclusion because he observed that the masses of atoms are whole number multiples of the atomic mass of hydrogen. The notion of atomic mass as the relative mass of an atom on a scale where hydrogen has a mass of one unit grew out of this idea. Later, oxygen became the standard. Later still, the concept of atomic mass unit (amu), with carbon-12 as a standard, was developed.
At the time, Prout’s idea seemed revolutionary. Now, it appears that Prout may have been close to the truth. Evidence from studies of radioactive changes led modern scientists to believe that all elements may be derived from hydrogen.
4. Classification on the Basis of Valency
Realising the importance of valency in chemistry, an attempt was made to classify elements on this basis.The monovalent elements were classed together and so were the divalent ones, the trivalent ones and so on.
However, such classification suffers from the following drawbacks.
(i) Several elements have variable valency, e.g., iron has a valency of 2 and 3, copper 1 and 2, tin 2 and 4, lead 2 and 4, etc. This makes the position of such elements uncertain.
(ii) Such classification does not explain the diverse nature of elements having the same valency. For example, both sodium and chlorine are monovalent, but they are quite different from each other in chemical behaviour. Sodium is a strongly electropositive metal whereas chlorine is a strongly electronegative nonmetal.
5. Lavoisier’s classification
By the late 1860’s, more than 60 chemical elements had been identified. Based on similar physical and chemical properties, Lavoisier and early chemists classified the elements into metals and non-metals.
(i) The elements which were malleable and ductile, good conductors of heat and electricity and possessed characteristic metallic lustre were named as metals.
(ii) The elements which were brittle, bad conductors of heat and electricity and did not possess metallic lustre were named as non-metals.
Certain elements such as antimony, arsenic, boron, silicon and tellurium resembled metals in some respects and non-metals in certain respects and were therefore metalloids.
Reasons for rejection
i) Some of the elements behave both as metals and non-metals.
ii) The elements were divided only into two broad categories which does not help much in the study of elements.
5.3. Major Contributions
I. Dobereiner’s Triads
In 1817, Dobereiner noticed that certain groups of three elements have related properties. Dobereiner called these groups triads.
|
If the elements of a triad arranged in order of increasing atomic mass, the atomic mass of the middle element is the average of the atomic masses of the other two elements. |
The properties of the middle element of a triad are also approximately midway between the properties of the other two elements. Thus bromine is less reactive than chlorine but more reactive than iodine.
Examples of Dobereiner’s Triads
|
Set-1 |
Set-2 |
Set-3 |
|
|
Element |
Li Na K |
Cl Br I |
Ca Sr Ba |
|
Atomic weight |
7 23 39 |
35.5 80 127 |
40 87.5 137 |
|
Average of the atomic weights of the two extremes |
Significance of Dobereiner Triads
This classification of elements in triads had greater significance in predicting the atomic mass and properties of the middle element. However, only a few elements could be arranged in such triads.
Defects of Triad Classification
i) Quite a large number of similar elements could not be grouped into triads.
Example: Iron, manganese, nickel, cobalt, zinc and copper are similar elements but cannot be placed in the triads.
ii) It was possible that quite dissimilar elements could be grouped into triads. As Dobereiner failed to arrange all the known elements in the form of triads, his classification was not very successful.
Example: For example, carbon (12), nitrogen (14) and oxygen (16) can form a triad but their properties are entirely different from each other.
II. Newland’s classification
John Alexander Reina Newland was a chemist as well as a lover of music.He arranged many of the known elements in the increasing order of their atomic masses. It was noticed that the eighth element was similar in properties to the first element, just like the eighth note in music – Western as well as Indian.
|
Western |
Indian |
Elements |
|
|
Do |
Sa |
Lithium |
Sodium |
|
Re |
Re |
Beryllium |
Magnesium |
|
Me |
Ga |
Boron |
Aluminum |
|
Fa |
Ma |
Carbon |
Silicon |
|
So |
Pa |
Nitrogen |
Phosphorus |
|
La |
Da |
Oxygen |
Sulphur |
|
Ti |
Ne |
Fluorine |
Chlorine |
|
Do |
Sa |
– |
– |
The eighth element after lithium is sodium. It is similar to lithium in many of its chemical properties. Similarly, the eighth element after sodium is potassium, whose properties are similar to sodium. The eighth element from fluorine is chlorine both of which are similar in their properties. The eighth element from nitrogen is phosphorus and both these elements are similar in properties.
Based on this observation, Newland stated his law of octaves.
|
When elements are arranged in increasing order of their atomic mass the eighth element resembles the first in physical and chemical properties just like eight note on a musical scaler esembles the first note |
However, a very important conclusion was made that there is some systematic relationship between the order of atomic masses and the repetition of properties of elements. This gave rise to a new term called Periodicity. It is the recurrence of characteristic properties of elements arranged in a table, at regular intervals of a period.
Achievements of the Law of Octaves
i) The law of octaves was the first logical attempt to classify elements on the basis of atomic weights.
ii) The periodicity of elements was recognised for the first time.
Defects of Law of Octaves
i) This law could be best applied, only up to the element calcium.
ii) The newly discovered elements could not fit into the octave structure.
iii) It failed to exhibit this feature with heavier elements.
5.4. Mendeleev’s Periodic Classification
With the failures of many attempts, there was a chaotic mess in the arrangement of elements. An end to this chaotic mess of elements was put by Mendeleev.Dmitri Ivanovich Mendeleev, a Russian chemist, was the first to put forward the successful arrangement of elements. In 1869, he published a periodic table of elements.
|
Meaning of Periodic Table A periodic table is a chart in which the elements are arranged in such away that : i) The elements having similar properties are placed in the same vertical column, called group. ii) In the term “periodic table”, the word “periodic” means that the elements having similar properties are repeated after certain definite intervals or periods. The word “table” means that the elements have been arranged in tabular form. |
Mendeleev’s periodic law:
Mendeleev studied the chemical properties of all 63 elements known at that time. On the basis of their properties, he proposed that when elements are arranged in the increasing order of their atomic masses, the elements with similar properties appear at regular intervals or periods, i.e., physical and chemical properties of the elements are a periodic function of their atomic masses (atomic weights).
Later on, when Mendeleev came to know about the work of Lother Meyer, he integrated the two statements (Lother Meyer’s and his own statement) in the form of a law called Mendeleev Lother Meyer periodic law or simply Mendeleev’s periodic law.
This law states that :
|
The physical and chemical properties of the elements are a periodic function of their atomic weights i.e, when the elements are arranged in the increasing order of their atomic weights, the elements with similar propeties are repeated after cert an regular intervals. |
What is Mendeleev’s periodic table?
Mendeleev (1871) arranged all the then known 63 elements in the increasing order of their atomic masses. The arrangement of elements was made in horizontal rows (called periods) and vertical columns (called groups). This arrangement showed that the elements having similar chemical properties came directly under one another in the same group. This arrangement of elements was called Mendeleev’s periodic table, which can thus be defined as an arrangement of elements in the increasing order of their atomic masses in different groups and periods (see the table in next page).
Main features of Mendeleev’s periodic table
1. In Mendeleev’s table, the elements were arranged in vertical columns called groups.
2. There were in all eight groups: Groups I to VIII. The group numbers were indicated by Roman numerals. i.e., I, II, III, IV, V, VI, VII & VIII.
3. Except VIII group, every group is further divided into subgroups i.e., A and B. Groups VIII occupy three triads of three elements each, i.e., in all nine elements
4. The properties of the elements in same group or subgroup are similar.
5. There is no resemblance in the elements of subgroups A and B of same group except valency.
6. The horizontal rows of the periodic table are known as periods.
7. There were seven periods, represented by Arabic numerals 1 to 12. To accommodate more elements, the periods 4, 5, 6 and 7 were divided into two halves. The first half of the elements are placed in the upper left corner and second half in the lower right corner.
For example, the elements occupying the box corresponding to group I and period 4 are potassium (K) and copper (Cu). K is written in the top left corner, while Cu is written in the lower right corner.
8. A period comprises the entire range of elements after which the properties repeat themselves.
9. In a period, the properties of the elements gradually change from metallic to nonmetallic while moving from left to right.
10. There were gaps left in the periodic table. Mendeleev left these gaps knowingly, as these elements were not discovered at that time.
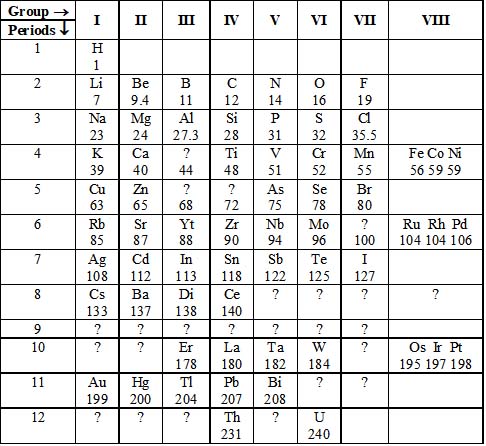
Simplified version of Mendeleev’s periodic table (1871)
The number indicates the atomic masses of elements.
How Useful was Mendeleev’s Periodic Table?
Mendeleev’s periodic classification brought about a revolution in chemistry. His periodic table classifies elements in a more systematic manner than the earlier methods of classification.
Contributions of Mendeleev’s Periodic Table
i) Systematic study of elements
Mendeleev’s Periodic Table simplified the study of elements. It became useful in studying and understanding the properties of a large number of elements, in a simpler manner. This is because the elements showing similar properties belonged to the same group.
ii) Prediction of new elements
While arranging the elements in increasing order of atomic mass, Mendeleev left three blanks for elements that were not discovered at that time. He was able to predict the properties of these unknown elements more or less accurately. He named them eka-boron, eka-aluminium and eka- silicon. He named them so, as they were just below boron, aluminium and silicon in the respective sub-groups. Later, eka-boron was named as scandium, eka-aluminium as gallium and eka-silicon as germanium.
A comparative study of the properties of the elements predicted and later discovered
|
Property |
Eka boron |
Scandium |
|
Atomic weight |
44 |
44.9 |
|
Oxide |
Eb2O3 |
Sc2O3 |
|
Specific gravity |
3.5 |
3.864 |
|
Sulphate |
Eb2(SO4)3 |
Sc2(SO4)3 |
|
Property |
Eka aluminium |
Gallium |
|
Atomic weight |
68 |
44.9 |
|
Oxide |
Eb2O3 |
Ga2O3 |
|
Specific gravity |
5.9 |
5.94 |
|
Melting point |
Low |
303.15k |
|
Solubility in acid and alkali |
Dissolves slowly in both acid and alkali |
Dissolves slowly in both acid and alkali |
|
Property |
Eka silicon |
Gallium |
|
Atomic weight |
72 |
72.32 |
|
Oxide |
5.5 |
5.47 |
|
Specific gravity |
Hight |
9.580C |
|
Melting point |
4 |
4 |
|
Reaction with acid and alkali |
Slightly attacked by acids, resists attack by alkali |
Dissolves neither in HCl nor in NaOH |
iii) Correction of atomic masses
Mendeleev’s periodic table helped in correcting the atomic masses of some of the elements, based on their positions in the periodic table. For example, atomic mass of beryllium was corrected from 13.5 to 9. Atomic masses of indium, gold, platinum were also corrected.
Defects of Mendeleev’s Periodic Table
i) Position of hydrogen:
The position of hydrogen is not correctly defined. It is still not certain whether to place hydrogen in group I A or group VII A.
ii) Anomalous pairs of elements:
In certain pairs of elements like Ar (40) and K (39); Co (58.9) and Ni (58.6); Te (127.6) and I (126.9) the arrangement was not justified. For example, argon was placed before potassium, whereas its atomic mass is more than potassium. In this case, the periodic law is violated.
iii) Position of isotopes
Isotopes are atoms of the same element having different atomic mass but same atomic number. For example, there are three isotopes of hydrogen with atomic mass 1, 2, and 3. According to Mendeleev’s periodic table, these should be placed at three separate places. However, isotopes have not been given separate places in the periodic table.
iv) Grouping of elements
Certain chemically dissimilar elements have been grouped together. Elements of group IA, such as lithium, sodium and potassium, were grouped with dissimilar elements, such as copper, silver and gold.
v) Cause of periodicity
Mendeleev’s table was unable to explain the cause of periodicity among elements. That is by the elements with similar properties fall one below the other, if they are arranged in the increasing order of their atomic weights.
vi) Position of lanthanides and actinides
Fourteen elements that follow lanthanum called lanthanides and fourteen elements following actinium called actinides were not given proper places in Mendeleev’s periodic table.
5.5. Moseley’s Modifications
Basis for Moseley’s classification: Discovery of radioactivity, isotopes, isobars and atomic nuclei led Moseley (in 1913) to change the periodic law as given by Mendeleev. He observed regularities in the characteristic X-ray spectra of the elements and found that plot (atomic number) is a straight line while (atomic weight) is not, and where a and b are constants that are same for all elements. Thus he concluded that atomic number is a more fundamental property than atomic weight.
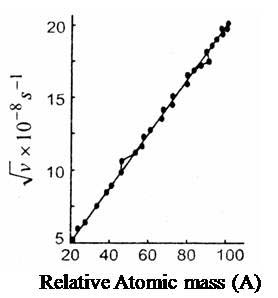
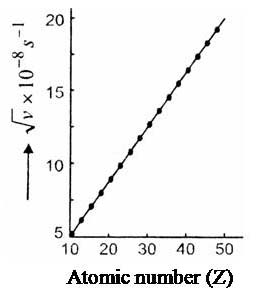
Moseley’s experiment
Modern justification for Moseley’s modification
Later, Henry Gywn-Jeffreys Moseley showed that the atomic number of an element is numerically equal to the number of electrons round the nucleus. The number of electrons in turn is equal to the number of protons in the nucleus. He suggested that atomic number is a more fundamental property of an element than its atomic mass. When the elements are arranged in the increasing order of their atomic number, most of the defects of Mendeleev’s classification get rectified.
Relation between Mass Number and Atomic Number:
Atomic Number (Z) is the number of protons in the nucleus of an atom. It is also equal to the number of electrons since the atom is electrically neutral.
Mass Number ((A) is the total number of neutrons and protons present in the nucleus of an atom.
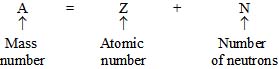
The periodic law given earlier is now modified and followed today.
“The properties of the elements are periodic functions of their atomic numbers”
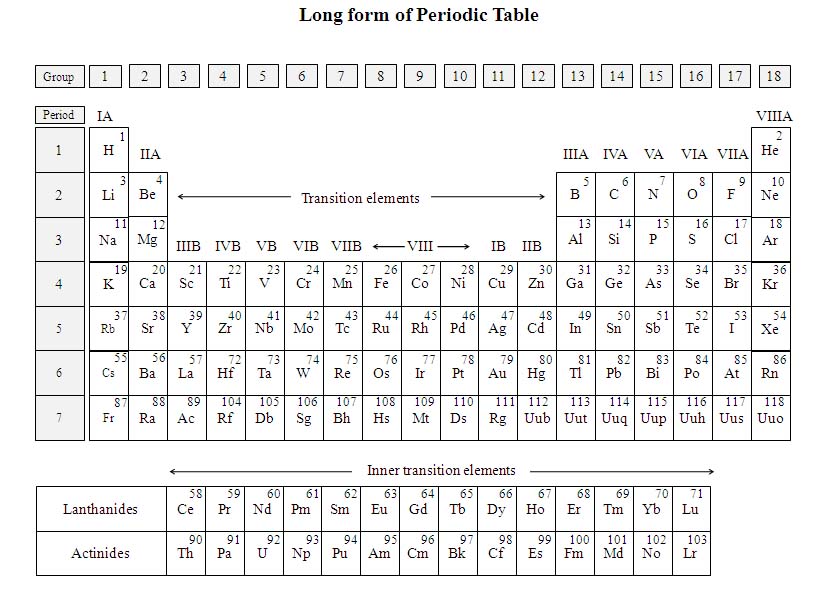
The modern periodic table is also known as the long form of the periodic table or the extended form of the periodic table.
Automatic Removal of Some Discrepancies in Mendeleev’s Periodic Table
When the basis of arrangement is changed from atomic mass to atomic number, the following discrepancies of Mendeleev’s periodic table automatically vanish.
1. Position of isotopes: All the isotopes of an element have the same atomic number, no matter what the mass number is. Hence the different isotopes of an element do not require separate positions in the periodic table.
2. Anomalous pairs of elements: When arranged in increasing order of atomic number, (i) Ar(18) should precede K(19), (ii) Co(27) should precede Ni(28) and, (iii) Te(52) should precede I(53). Thus the positions of these elements stand justified and they no longer remain anomalous pairs of elements.
5.6. Main features of Modern periodic table
In order to make the periodic table more useful, another form has been evolved by separating the subgroups. The modern periodic table thus developed is also known as the long form or extended form of the periodic table (see the table in next page) as against the short form, i.e., Mendeleev’s periodic table.
1. Basis for classification: The elements are arranged in the increasing order of their atomic numbers.
2. Periods: The seven horizontal rows of the periodic table are known as periods. Each period begins with the outermost electron entering into a new principal quantum number and completes after the outermost shell’s p-subshell is complete. The period number denotes the outermost orbit’s number of that element. The first element of each period (except 1st period) is an alkali metal and the last element is an inert gas.
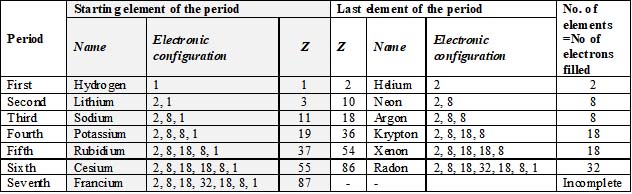
The description of periods is given below:
|
Period |
Length |
No. of elements |
|
1st period |
Very short period |
2 |
|
2nd and 3rd periods |
Short periods |
8 |
|
4th and 5th periods |
Long periods |
18 |
|
6th period |
Very long period |
32 |
|
7th period |
Incomplete period |
– |
To avoid inconvenience, 14 elements, which do not include lanthanum and actinium belonging to 6th and 7th period are placed in two separate rows at the bottom of the periodic table (now called lanthanides and actinides respectively).
Criterion for placement of an element in a period: The number of elements in each period of the periodic table is equal to the number of electrons filled in the corresponding electronic shell and a new period begins with an element that has one electron in a new main energy level (i.e., new shell)
3. Groups:
The elements present in vertical columns are called groups. The groups are again classified into two subgroups – A and B. There are sixteen groups under the headings: IA to VII A, 0 and IB – VII B and VIII.
4. Normal or Typical elements: The elements placed in ‘A’ subgroups, IA and IIA on the left and IIIA to VIIIA (0) on the right are called typical elements. They are also called representative, normal or main group elements.
5. Transitional elements: The ‘B’ subgroup elements present between IA and IIIA are called transition elements as they show transition properties from metals to non-metals.
6. Active metals: The strong metallic elements are placed in IA and IIA groups on the left hand side of the periodic table.
7. Active nonmetals: The strong nonmetallic elements are placed in VII A and VIA groups on the right hand side of the periodic table.
8. Non-reactive elements: The rare gases (noble gases) that are inert are placed in zero group at the end, extreme right hand side of the periodic table (He, Ne, Ar, Kr, Xe and Rn).
9. Lanthanides and Actinides : Lanthanides and Actinides are placed separately at the bottom of the periodic table due to their unique properties. Their details shall be learnt in pages ahead.
Common Names of Different Groups
|
Group |
General / Special Name |
Reason |
|
IA |
Alkali metals |
Due to the formation of strong oxides and hydroxides with a strong alkaline character (Basic in nature), these are called alkali metals. |
|
IIA |
Alkaline earth metals |
These oxides are alkaline in nature and exist in the earth. Hence, these elements are called Alkaline Earth metals. |
|
IIIA |
Boron family |
As all the elelements in this group represent similar properties and boron being the first of these elements, this group is call ed the Boron group. |
|
IVA |
Carbon family |
All the elements in this group have similar properties. Carbon being the first element of this group, this group is called the Carbon group. |
|
VA |
Pnicogens |
Pnicogen is a Greek word meaning ‘suffocation’. As the Hydrides of this group NH3, PH3, ASH3 have a pungentodour, and when inhaled causes suffocat ion, this group is called Pnicogen. |
|
VIA |
Chalcogens |
Chalcogens in Greek means ore-forming. Oxygen and sulphur are two important elements of this group, and these elements are associated with ores of many metals in the form of their oxides and sulphides. |
|
VII |
Halogens |
In Greek ‘halogen’ mean salt producer. A salt consists of anion and cation. For example common salt (NaCl) consist s Na+ (cation) and Cl– (anion). The elements of this group form the anion of salt easily, hence they are called Halogens. |
|
Zero group |
Rare gases Inert gases Noble gases |
Because of their presence in small quantities they are called rare gases. Due to their stable electronic configuration they are called Noble gases. As they have little tendency to react, they are also called Inert gases. |
|
VIII or VIIIB |
Iron triad/ ferrous metals / Platinum triads / Platinum metals |
VIII group consists of 3 triad series. The first triad series Fe, Co, Ni are called ferrous metals and second and third triad series Ru, Rh, Pd and Os, Ir, Pt are called Platinum metals. |
|
IB |
Coinage metals |
As these elements (copper group metals) were used for the manufacture of currencies in the olden days, they are called Coinage metals. |
5.7. Merits and demerits of Long form of periodic table
We have seen that the periodic table has developed in various stages. The classification evolving at every stage proved to be more useful than the previous one. We will now discuss the uses of the periodic table.
Advantages of the Long Form of Periodic Table
(i) This classification is based on the atomic number which is a more fundamental property of the elements.
(ii) The position of elements in the periodic table is governed by the electronic configurations which determine their properties.
(iii) The completion of each period is more logical. In a period as the atomic number increases, the energy shells are gradually filled up until an inert gas configuration is reached.
(iv) It eliminates the even and odd series of IV, V and VI periods of Mendeleev’s periodic table.
(v) The position of VIII group is also justified in this table. All the transition elements have been brought in the middle as the properties of transition elements are intermediate between s- and p-block elements.
(vi) Due to separation of two sub-groups, dissimilar elements do not fall together. One vertical column accommodates elements with same electronic configuration thereby showing same properties.
(vii) The table completely separates metals and non-metals. Non-metals are present in upper right corner of the periodic table.
(viii) There is a gradual change in properties of the elements with increase in their atomic numbers i.e., periodicity of properties can be easily visualised. The same properties occur after the intervals of 2, 8, 8, 18, 18 and 32 elements which indicates the capacity of various periods of the table.
(ix) Since this classification is based on the atomic number and not on the atomic mass, the position of placing isotopes at one place is fully justified.
(x) It is easy to remember and reproduce.
(xi) The position of some elements which were misfit on the basis of atomic mass is now justified on the basis of atomic number. For example, argon precedes potassium because argon has atomic number 18 and potassium has 19.
(xii) The lanthanides and actinides which have properties different from other groups are placed separately at the bottom of the periodic table.
(xiii) This arrangement of elements is easy to remember and reproduce.
Periodicity and periodic properties
1. When elements are arranged in increasing order of atomic numbers, elements with similar properties re-occur (due to similar outer electronic configuration) at regular intervals in the periodic table. This is known as periodicity.
2. Elements coming at intervals of 2, 8, 8, 18, 18, 32 will have similar properties and thus grouped in one particular group.
For example, elements with atomic number 1, 3, 11, 19, 37, 55 and 87. Elements with atomic number 4, 12, 20, 38, 56 and 88 will have similar properties.
Note: Two adjacent elements in a group generally differ by atomic number 2, 8, 8, 18, 18, 32.
5.8. Atomic Radius
In atoms, the electron cloud around the nucleus extends to infinity. The distance between the centre of the nucleus and the electron cloud of outermost energy level is called atomic radius.
Atomic radius cannot be determined directly, but can be measured from the internuclear distance of combined atoms, using X-ray diffraction techniques.
Units: Atomic radii is expressed in angstrom, nanometers, picometre units.
pico.metres
Factors affecting atomic radius
(a) Effective Nuclear Charge: The effect of increase in the number of protons increases the effective nuclear charge. This results in decrease in the value of atomic radius because protons attract the electronic orbits with greater force.
(b) Number of orbits: The effect of increase in the number of orbits in an atom increases the atomic size.
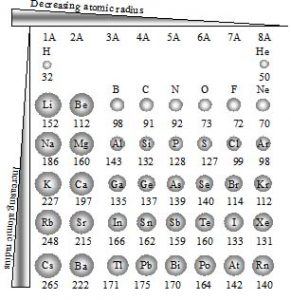
Variation of atomic radius in a period and group
In a period, the number of orbits remains same. On moving from left to right in a period while there is a unit increase in the atomic number. Thus the electron experiences more force of attraction towards nucleus. Hence atomic radius decreases from left to right in a period.
In a period from left to right, atomic radius decreases as the nuclear charge increases.
On moving from left to right across a particular period, the atomic radius decreases upto Halogens and increases to Inert gases.
In a given period, alkali metal is the largest and halogen is the smallest in size.
For atoms of Inert gases, only van der Waal radius is applicable.
In a group, from top to bottom, the atomic radius increases gradually due to the increase in the number of orbits and it overweighs the effect of increased nuclear charge.
Ionic radius
When a neutral atom loses one (or) more electrons, a positive ion called cation is formed.
The ionic radius of cation is less than that of neutral atom. It is because the cation has higher effective nuclear charge. For example, the size of
Among the cations as the positive charge increases, the ionic radius decreases. For example, the size of
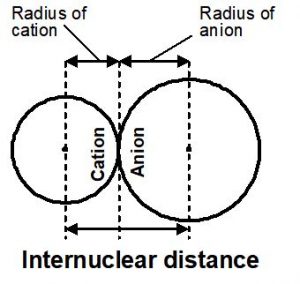
When a neutral atom gains one (or) more electrons, a negative ion called anion is formed. The radius of anion is more than that of its atom due to decrease in effective nuclear charge. For example, the size of
Among the anions, as the negative charge increases, the ionic radius increases.
For example, the size of
The decreasing order of the radii is :
Anion > Atom > Cation;
The species (atoms or ions) having the same number of electrons are known as iso – electronic species.
In isoelectronic species, the size decreases with increase of negative charge and decrease of positive charge.
Decreasing order of size
5.9. Metallic and Non-metallic Characters
I. Metallic Character
a) Metals are characterized by their big size and their ability to loose electrons.
b) IA and IIA groups contain big sized elements and hence are metallic in nature.
c) IA and IIA are called alkali metals and alkaline earth metals respectively.
Variation of metallic character in periodic table
• As we move down the group, the atomic size, the ability to lose electrons increases. Therefore, the metallic character increases as move from top to bottom.
• As we move across the period, the atomic size, the ability to lose electrons decreases. Therefore, the metallic character decreases as we move from left to right.
II. Non-metallic Character
a) Nonmetals are characterized by their small size and their ability to gain electrons.
b) VIA and VIIA groups contain small sized elements and hence are nonmetallic in nature.
c) VIA and VIIA elements are called chalcogens and halogens respectively.
Variation of metallic character and nonmetallic character
In a group, as we move from top to bottom, the size of atoms increases, resulting in an increase in electropositive character. Thus, the metallic character increases down the group.
As we move from top to bottom, the size of atoms increases resulting in the decrease in ionisation energy. Thus, the nonmetallic character decreases down the group. So, as we move down the group, the metallic character increases and the nonmetallic character decreases.
In a period, as we move from left to right, the size of atom and ability to lose electrons decreases. Thus, metallic character decreases as we move from left to right in a period. At the same time, the nuclear pull increases, increasing the ability to gain electrons. Thus, nonmetallic character increases, as we move from left to right in a period.
5.10. some noteworthy points
1. Triad rule – Dobereiner
2. Octet rule – Newland
3. Study of atomic volume – Lothar Meyer
4. Inventor of atomic number – Moseley
5. God father of periodic table – Mendeleev
6. Founder of modern periodic table – Bohr
7. Mg is bridge element, which joins metals of IIA and II B groups.
8. Elements after atomic number 92 are transuranic elements.
9. Artificial element is .
10. Liquid non-metal – Br
11. Liquid metal – Hg, Ga, Cs, Fr
12. Solid volatile non-metal – Iodine
13. Lightest metal – Li
14. Heaviest metal – Ir
15. Hardest metal – W
16. Noble metals – Pc, Pt, Au, Ag
17. Element most found on earth – Al
18. Gaseous elements – 11 (He, Ne, Ar, Kr, Xe, Rn, H2, N2, O2, Cl2, F2)
19. Liquid elements – 5(Br, Hg, Ga, Cs, Fr)
20. Submetals – 5(B, Si, As, Te, At)
21. Inert gases – 6
22. Most reactive solid element: Li
23. Most reactive liquid element: Cs
24. Largest atomic size: Cs
25. Total number of gaseous elements: 11 (, He, Ne, , Ar, Kr, Xe, Rn)
26. Total number of liquid elements : 4 (Ga, Br, Cs, Hg) (Fr and Eka are also liquid)
27. Most abundant element on earth: Oxygen followed with Si
28. Strongest alkali: Cs(OH)
29. Element stored in water: P
30. Elements kept in kerosene oil:Na, K, I, Cs
31. Liquid non-metal:
32. Bridge metals: Na, Mg
33. Lightest element: H
34. Poorest conductor of current: Pb (metal), S (non-metal)
35. Most abundant gas:








