1.1 Introduction
From the dawn of creation the world has been subjected to the inescapable phenomenon called change. In fact, the only thing which is permanent perhaps is change. Change is inexorable law of nature. It has manifested itself in every branch of human activity. Chemistry is that branch of science which deals with the reactions of various substances and the resulting changes. They range from very simple ones like change in state, colour, volume etc., to the more complex interconnecting reaction that keeps our bodies alive.
This chapter will take you on a journey through the different types of changes encountered in chemistry and are explained through instances drawn from our daily life.
1.2. Physical Changes
Take a dry hard glass test tube and put in it about 2g of candle wax. Heat the test tube gently on Bunsen flame. What do you observe?
The wax melts to form a colourless liquid. Now cool the tube by holding it in cold water. What do you observe? The molten wax solidifies. Thus, we can say that on heating wax melts and on cooling, the liquid wax solidifies, but no new products are formed.
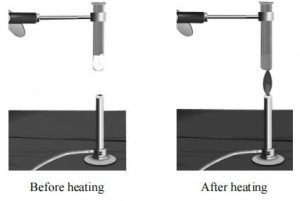
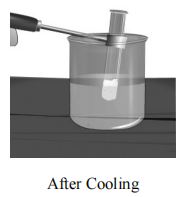
We observe that melting of wax is temporary and can be reversed. Such type of changes are called Physical Changes.
Examples of Physical changes
a) Heating of Zinc oxide and led oxide
Zinc oxide is a white powder. Place about 2g of zinc oxide in a dry test tube. Heat the test tube strongly. What do you notice after 2 minutes of heating?
The zinc oxide changes to yellow colour. Cool the test tube. In a few minutes, the colour of zinc oxide changes to white colour.
b) Formation of NaC solution
Take about 50 ml of water in a beaker. Add a spoonful of common salt in it and stir. You will observe that salt dissolves in water. Now evaporate the salt solution on heated sand bath. It is observed that water disappears leaving behind common salt. Furthermore, the mass of common salt remains same.
Thus, we can say that the above changes are
(i) temporary in nature, No new products are formed, and (iii) there is no change in weight when change takes place.
c) Melting of ice
Water in solid state (ice), on heating melts into liquid water.
On further heating above 100°C, liquid water changes to gaseous water (vapour), which on cooling forms liquid water. Further cooling of water makes it into ice. (original substance). In this case, a state of water can be retained on change of temperature. Thus, it is a physical change.
d) Burning of Platinum or Nichrome
Hold a short length of platinum wire or nichrome wire with the help of tongs. Hold the end of wire in a non-luminous Bunsen flame. What do you observe?
The end of wire becomes red hot. Take out the end of wire from the Bunsen flame and allow it to cool. What do you observe? The wire regains its original colour.
Thus, we can say that the change brought about in the appearance of the wire is temporary in nature. Similarly, when electric current flows through a bulb, its filament gets white hot and emits light. When current is switched off, the filament does not give off any light. Thus, the change in the appearance of the bulb is temporary in nature.
e) Magnetisation of iron
When a piece of iron is rubbed with a magnet, it becomes magnetic. The molecules in it are rearranged uniformly and the iron bar in turn behaves as a magnet. On heating, the piece of magnet loses its magnetic property and becomes just a piece of iron again, without change in its mass.
f) Some interesting physical changes
In general, a physical change is characterised by change in state, the logical sequence would be
But there are some physical changes where the intermediate transition state of liquid is missing, the change occurs directly from solid to vapour state. This type of physical change is called sublimation.
Examples
i) Sublimation of camphor,
ii) Sublimation of ammonium chloride.
iii) Sublimation of iodine.
Some more examples of physical changes
i) Condensation of water vapours, such as formation of clouds, mist, fog, etc.,
ii) Expansion of contraction of metals on heating.
iii) Formation of solutions of soluble substances in water.
iv) Crystallisation of salts from their solutions.
v) Beating of metals into sheets or drawing metals into wires.
vi) Shaping of glass by heat
Characteristics of Physical changes
i) No new substances are formed during physical change : Ice, on heating melts to form water. The water on further heating changes into steam. On cooling, the steam changes into water. On further cooling, the water solidifies to form ice.
However, the molecules of ice, water or steam always contain two atoms of hydrogen and one atom of oxygen. Thus, we can say that no new substances are formed.
Similarly, when we add common salt to water, salt solution is formed but no change takes place in the molecules. On evaporation, the water evaporates leaving behind salt.
ii) Physical changes can be generally reversed : The zinc oxide, on heating changes to yellow colour. However, on cooling its colour changes to white. Similarly, when a piece of iron is stroked with permanent magnet, it gets magnetised. However, if magnetised iron is hammered, it loses its magnetism. The wax on heating changes into liquid state. However, liquid wax changes into solid on cooling.
iii) There is no change in weight during physical change : 10g of solid wax on melting will form 10g of molten wax. If a salt solution is prepared by dissolving 20g salt in water, then on the evaporation of water, 20g of salt is left behind.
iv) Only a little heat (if any) is absorbed or given off during physical change : If water changes into steam by absorbing a certain amount of heat energy, then steam will change into water by giving off the same amount of heat energy. The heat energy supplied during physical change is in no way, utilised to change the composition of the molecules of a substance.
1.3 Chemical Changes
A change which alters the specific properties of a substance by bringing about a change in its molecular composition, followed by a change in its state, is called chemical change.
Examples of chemical changes
a) Burning of coal: Coal, on burning, gives a bright red appearance. What exactly is happening here?
Coal consists of carbon. The carbon combines with oxygen present in the atmosphere, producing carbon dioxide gas. This can be represented as :
As the chemical composition of coal changes and a new product is formed, the burning of coal is a chemical reaction.
This chemical reaction is a accompanied by the release of heat. The released heat, in turn, heats up the carbon atoms. These atoms at high temperature emit an orange red light, giving the burning coal its unique red hot appearance. The reaction occurring during burning is called combustion.
Combustion: The process of burning a substance in the presence of oxygen is called combustion.
b) Burning of crackers: When a small piece of magnesium wire is burnt, the wire burns with a flash of light. This happens because magnesium combines with atmospheric oxygen forming magnesium oxide.
In this reaction, as a result of chemical change, the apparent weight of the substance formed differs from the weight of the original substance.
c) Action of heat on mercuric oxide
Take a hard glass test tube and put in it 2g of red coloured mercuric oxide. Clamp the test tube on an iron stand. Heat the test tube strongly. You will notice that first of all the colour of mercuric oxide changes to black. On further heating, the test tube is filled with fumes. At this moment hold a glowing wooden splint in the test tube. The wooden splint bursts into flame. Also, tiny silvery droplets are seen sticking to the cooler parts of test tube. On cooling the test tube, the change is not reversed.
Actually, on heating mercuric oxide decomposes to form mercury and oxygen, i.e., two new products are formed. The mercury vapourises and liquefies on the cooler parts of the test tube. The oxygen is responsible for the glowing splint to burst into flame, because it supports combustion.
Thus, strong heating of mercuric oxide is a permanents change, which cannot be reversed. Furthermore, new products are formed.
d) Action of heat on sugar
Take a hard glass test tube and put 2g of sugar in it. Heat the test tube strongly. What do you observe?
The sugar melts and then turns brown. On further heating it gives off steam which condenses on the cooler parts of the test tube. The residue left in the test tube is black in colour. On cooling, the change does not reverse itself. Thus, heating of sugar is a permanent change.
Actually, sugar decomposes to form charcoal which is a form of carbon and is black in colour. It also gives off water in the form of steam.
Some interesting chemical change – Lighting of a matchstick :
The two important components involved in the process of lightning a matchstick are the head the matchstick and striking surface of the matchbox. The head of a matchstick comprises two substances – Antinomy trisulphide and potassium chlorate. The former is used as a combustible substance, while the latter is an oxidising agent, which on decomposition supplies oxygen. The striking surface of a matchbox comprises of red phosphorous and sand or glass powder. Red phosphorous has very low ignition temperature and its plays an important role in the initial ignition process. Sand or glass powder provides a suitable surface for friction.
Process of lighting: When the head of the matchstick is struck against the striking surface, friction results. Due to the friction, a trace of phosphorous gets detached from the surface. The heat produced during friction is sufficient to ignite red phosphorus. This ignited tiny spark of red phosphorus in turn ignites the antimony trisulphide present on the head of the matchstick. The matchstick continues to glow with the help of oxygen obtained from air and the decomposition of potassium chlorate.
From the above explanation it is clear that lighting of a matchstick is an example of chemical reaction.
Characteristics of chemical changes
i) When a chemical change occurs, new substances with entirely new properties are formed.
Candle wax on burning forms entirely new substances, i.e., carbon dioxide gas and steam. Mercuric oxide (red in colour) on strong heating forms new substances (mercury and oxygen gas). Sugar on strong heating forms carbon and steam. Magnesium on burning forms magnesium oxide.
ii) Chemical change cannot be easily reversed.
The carbon dioxide and steam formed during the burning of the candle cannot be converted into wax by altering the conditions of experiment. Carbon and water vapour, formed during the heating of sugar, cannot be recombined to form sugar. Magnesium oxide formed during the burning of magnesium cannot be easily changed to original metal and oxygen.
iii) There is usually a change in weight during chemical reaction.
When magnesium is burnt in air, then the weight of white ash (magnesium oxide) is more than magnesium metal. For every 3g of magnesium metal, 5g of magnesium oxide is formed. When sugar is burnt, for every 7g of sugar approximately 3g of sugar charcoal is left. Similarly, when iron rusts, the weight of rusted iron is more than that of original metal, because the oxygen combines with iron.
iv) Lot of heat is usually given off or absorbed during a chemical change.
When magnesium burns in air, it produces a large amount of heat and light energy. When mercuric oxide decomposes to form mercury and oxygen, it absorbs a large amount of heat energy. When sugar decomposes to sugar charcoal and steam, it absorbs large amount of heat energy.
1.4 Combination Of Both Physical Change And Chemical Change
a) Burning of candle ( a combination of a physical and a chemical change)
A candle is made of wax with a wick in it. The wick of the candle is the thread which stands out from the body of the candle and consists of cellulose a carbon compound. Wax is a higher hydrocarbon consisting of carbon and hydrogen.
Through the capillary action of the wick, the molten wax is pushed up. It combines with the oxygen in the atmosphere. The resultant products are CO2 and H2O, which have independent identity from that of the wax. Hence, burning of wax can be considered a chemical change.
The heat generated during the process of burning melts the wax which on cooling can be solidified. the change in the process of melting the wax, is temporary because on reversing the conditions, the original substance is regenerated, therefore it can be termed as a physical change.
b) Watering of plants : When you water a plant, the water can be evaporated out of the soil. However, once the plants uses the water, the water is broken down into oxygen and hydrogen by the plant in photosynthesis process for the glucose formation. The process cannot be reversed i.e., it is not possible to remove the water from the plant and make it shrink to the state it was in, before the growth. Thus, before the plant grows, the change is physical but after it grows the change becomes chemical.
c) Boiling water using a gas cylinder : Many a times, you must have observed the sight of boiling water in a container. Two interesting things are happens – one, inside the container and the other beneath it.
1. Inside the container, water is boiling. During boiling, heat is absorbed and steam is evolved.
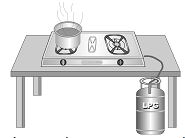
2. Below the container, LPG gas consisting mainly of butane is burning. During burning butane () combines with oxygen in air, resulting in the formation of new compounds like carbon dioxide and water. A large amount of heat is also released. This heat is used in boiling the water. The whole action is summarized in the following equation.
Here, boiling of water is a physical change as can be reversed and burning of butane is a chemical change as it cannot be reversed.
Differences between physical and chemical changes
|
Physical change |
Chemical change |
|
Change |
|
|
The change that takes place in the physical properties of substances like state, colour, volume, density etc. |
The change that takes place in the chemical composition of a substance. |
|
Composition |
|
|
The identity of the molecules or the substance remains constant, as no new substance is formed. |
The identity of the molecule or the substance changes, and a new substance is formed, whose fundamental properties differ from those of the original substance. |
|
Temporary or permanent change |
|
|
During a physical change, the original substance can be recovered merely reversing the conditions. Hence, physical change is temporary change. |
New molecules or substances, which cannot be converted to the original substance, are formed. Hence, the chemical change is a permanent change. |
|
Recovery of original substances |
|
|
On reversing the conditions, the original substance is recovered. |
On reversing the conditions, the original substance cannot be recovered. |
|
The weight of a substance |
|
|
The weight of a substance, before and after a physical change, remains constant. |
As a result of a chemical change, the apparent weight of the substance formed differs from the weight of the original substances. |
1.5 Chemical Reaction and its characteristics
A chemical reaction is a process that always results in the interconversion of chemical substances. The substance or substances initially involved in a chemical reaction are called reactants. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. For example, when hydrogen and oxygen react together they form water. Similarly, when hydrogen reacts with chlorine they form hydrogen chloride. These changes are known as ‘Chemical reactions’, Millions of examples of chemical reaction can be given. Chemical reaction are of various types. Some reactions may start in old in the other require some sort of energy (say heat, electricity, etc.)
Classically, chemical reactions encompass changes that strictly involve the motion of electrons in the forming and breaking of chemical bonds, although the general concept of a chemical reaction, in particular the notion of a chemical equation, is applicable to transformations of elementary particles, as well as nuclear reactions. Different chemical reactions are used in combination in chemical synthesis in order to get a desired product.
Characteristics of a chemical reaction
Chemical reactions are characterised by changes that are quite easily observed.
Some of these typical changes are,
i) Evolution of gas: In many chemical reactions one of the products is a gas.
Examples
a) When zinc reacts with dilute sulphuric acid, hydrogen gas is evolved. This can be seen in the effervescence that results.
Effervescence
The formation of gas bubbles in a liquid during a reaction is called effervescence.
In cases, where one of the reactants is a liquid, the gas produced forms bubbles in the liquid.
b) When potassium chlorate is heated strongly, it breaks up to produce oxygen gas, along with potassium chloride.
c) When sodium sulphite is treated with dilute hydrochloric acid, a foul smelling gas (like burning sulphur), sulphur dioxide, is liberated.
ii) Change of colour: Certain chemical reactions are characterised by a change in the colour of the reactants.
Examples
a) When a few pieces of iron are dropped into a blue coloured copper sulphate solution, the blue colour of the solution fades and eventually turns into light green.
b) When blue coloured copper sulphate reacts with hydrogen sulphide gas, a black coloured substance copper sulphide is formed.
c) Lead nitrate is a white crystalline solid. When heated strongly, it decomposes to produce the light yellow lead monoxide, the reddish brown nitrogen dioxide gas and oxygen.
iii) Formation of precipitates : Certain chemical reactions are characterised by the formation of insoluble solid substances called precipitates, as is the case when the aqueous solutions of two soluble solid substances are mixed with one another.
Examples
a) When a solution of silver nitrate is added to a solution of sodium chloride, a white insoluble substance (precipitate), silver chloride, is formed.
b) When ferrous sulphate solution is added to sodium hydroxide solution, a green precipitate, ferrous hydroxide, is formed.
c) When calcium bicarbonate solution is heated, a white precipitate, calcium carbonate, is produced.
iv) Change in energy : A chemical reaction involves breaking of chemical bonds (i.e., force of attraction) between atoms resulting in the absorption of energy in the form of heat, simultaneously formation of bonds take place with the release of energy. These two types of energy are different from each other i.e., there is either a surplus or deficit of energy during the reaction. Therefore, in a chemical reaction, energy is either absorbed or released.
1. Reactions in which heat is given out :
a) When carbon burns in oxygen to form carbon dioxide, a lot of heat is produced.
b) When water is added to quick lime a lot of heat energy is produced [along with the alkaline calcium hydroxide (slaked lime)].
During respiration, rusting, burning of coal, petrol, kerosene, etc., heat is given out.
2. Reactions in which heat is absorbed :
a) When nitrogen and oxygen together are heated to a temperature of about 3000°C, nitric oxide gas is formed.
b) Similarly calcium carbonate decomposes into carbon dioxide and calcium oxide when it is heated to a temperature of about 1000°C.
v) Change of state
In many chemical reactions, a change of state is and vice versa.
For example, the reaction between hydrogen sulphur (solid) and hydrogen chloride (gas).
The physical states of the reactants and the products are represented by the symbols s, l, g and (aq) attached to their symbols and formulae, to show whether they are a solid, or a liquid, or a gas, or in the aqueous form respectively.
1.6 Conditions favourable for a Chemical Reaction
1. Contact
No reaction takes place if the reactants are kept apart. For them to react, they should be in contact with each other.
i) Powdering or grinding of reactants :
This increases the effective surface area of the reactants, and hence the extent of contact increases. Consider the reaction of hydrochloric acid with large marble chips and smaller marble chips to understand it.
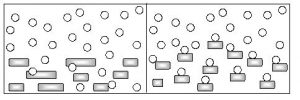
From the above figure, it is clear that number of collisions are less when the surface area of CaCO3 is less. When the marble chips are powered or broken into smaller particles, the surface is increased, there by increasing the number of collisions between the marble chips and HCl molecules. This increases the rate of reaction. Thus, more is the surface area, more is the rate of reaction.
ii) Reactants in solution state:
When reactants are taken in solution state, a closer contact is achieved more quickly by the reactant particles.
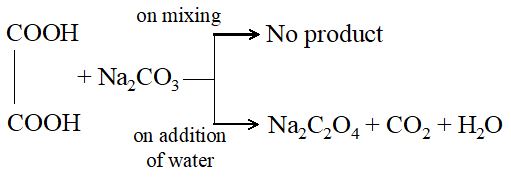
Eg: Oxalic acid and sodium carbonate do not react on mere mixing. When water is added, both form a solution and increases the contact favouring more reaction, which proceeds vigorously.
2. Supply of energy
|
Type of Energy
|
Effect |
Examples |
|
Heat
|
Most of the reactions are brought about by heating the reactants together. Heating initiates the reacting particles to move faster and collide more frequentl. |
|
|
Electrical
|
A few reactions are brought about by passing electric current through the reactants. The reactants split up into ions and then move to the opposite electrodes, forming new compounds.
|
|
|
Sound
|
Certain chemical reactions proceed by absorption of sound energy. This energy speeds up the reacting molecules, atoms or ions, causing a reaction to occur.
|
|
|
Light
|
Some reactions are influenced by the presence of light. The molecules of the reactants absorb light and become active to react rapidly.
|
3. Application of pressure
When reactants are subjected to pressure, the reacting molecules aggregate. Increase of pressure also results in more number of collisions among particles, resulting in more product formation.
Example :
4. Catalyst
The rate of reaction can be altered by a catalyst. The catalyst itself does not undergo any change, chemically or quantitatively.
Example :
The reactant particles of every reaction require certain amount of energy called activation energy, to initiate a reaction. The role of a catalyst is to affect this activation energy. If it lowers the activation energy, the reaction proceeds faster.
Role of catalysts in a chemical reaction
A substance which changes the rate of reaction without itself taking part in the (chemical) reaction is called a catalyst.
If a catalyst speeds up the rate of chemical reaction, it is called a positive catalyst. The following example illustrates the point.
Example: Potassium chlorate on strong heating decomposes to form potassium chloride and oxygen gas.
However, if a mixture of 4 parts of potassium chlorate and one part of manganese dioxide is heated,, it liberates oxygen at a much lower temperature. Moreover, the rate of evolution of oxygen is very fast. Thus, manganese dioxide is a positive catalyst.
Similarly, nickel (finely divided) is used as a ctalyst for converting vegetable oils into vanaspati ghee and platnized asbestos is used as catalyst in the manufacture of sulphuric acid from sulphur dioxide, etc.
If a catalyst slows down the rate of chemical reaction, it is called a negative catalyst. For example, addition of a little alcohol in hydrogen peroxide slows down its decomposition into water and oxygen.
1.7 Chemical equation
There are a large variety of substances in nature, and we come across innumerable transformations of substances in our day-to-day life.
For example, rusting of iron, curdling of milk, transformation of raw vegetables/spices into delicious dishes etc. All these transformations are due to the change in the chemical composition of the original substances.
Such a chemical change, which a substance undergoes, is called a chemical reaction.
To understand a chemical reaction, it is important to know which compound has transformed into what compound.
Note: The compounds which are involved in the chemical reaction are called reactants and the compounds obtained after the reaction are called products.
We know that elements and compounds are represented by symbols and formulae, respectively. In the same way, a chemical reaction can also be represented symbolically.
Such a symbolic representation of a chemical reaction in terms of a chemical formula is called a chemical equation.
Example: Magnesium reacts with Oxygen to form Magnesium Oxide. This can be symbolically conveyed with a chemical equation as follows :
Mg +
How to write a chemical equation
Following are the steps for writing a chemical equation.
Step – 1: Write down the symbols and the formulae of the reactants, on the left hand side.
Note: The different reactants are separated by plus (+) sign.
Step – 2: Write down the symbols and the formulae of the products, on the right hand side.
Note: The different reactants are separated by plus (+) sign.
Step – 3: The products and reactants are separated, by an arrow ().
The equation obtained by the above steps is called a skeleton equation.
The skeleton equation has to be supported with some additional information to make it a complete equation.
What does a chemical equation convey?
A chemical equation conveys the following information:
1. The reactants that enter into a reaction.
2. The products which are formed by the reaction.
3. The number of atoms and molecules of the reactants and the products involved.
4. The state of matter, in which the substance is present, or formed.
|
State of substance |
symbol |
|
Solid |
s |
|
Liquid |
|
|
Gas |
g |
|
Aqueous solution |
aq |
6. The sign means “yields”, and shows the direction of the action.
7. A ‘’ above the arrow shows that the reaction takes place in the presence of heat.
8. i) Light or sun light above the arrows shows that the reaction is photochemical reaction.
ii) Catalyst mentioned above the arrow shows that the reaction is catalytic reaction.
|
–1 Charge Name of Ion |
Formula |
–2 Charge Name of ion
|
Formula
|
–3 Charge Name of ion
|
Formula |
|
Bromide ion |
Oxide ion |
Nitride ion |
|||
|
Chloride ion |
Sulp hide ion |
Phosphide ion |
|||
|
Fluoride ion |
|
|
Boride ion |
||
|
Iodide ion |
|
|
|
|
|
|
Hydrogen carbonate or (bisulp hate ion) |
Carbonate ion |
Phosphate ion |
|||
|
Hydrogen sulphate or (bisulp hate ion) |
Manganate ion |
Arsenate ion |
|||
|
Hydroxide ion |
Thiosulphate ion |
Arsenite ion |
|||
|
Nitrate ion |
Silicate ion |
|
|
||
|
Chlorate ion |
Sulp hate ion |
Phosphite ion |
|||
|
Nitrite ion |
Sulp hite ion |
Borate ion |
|||
|
Permanganate ion |
Chromate ion |
Ferricyanide ion |
|||
|
Acetate ion |
Dichromate ion |
|
|
||
|
Cyanide ion |
Hydrogen Phosphate ion |
|
|
||
|
Hyp ophosphite ion |
Oxalate ion |
|
-4 Charge |
||
|
Meta aluminate ion |
|
|
Carbide ion |
||
|
|
+1 Charge |
|
|
Ferrocyanide ion |
|
|
Ammonium ion |
|
|
|
Charge Table
Balancing a chemical equation
Let us consider the formation of hydrogen chloride.
Hydrogen + Chlorine Hydrogen Chloride
(or)
Is this equation correct in all aspects?
We observe that the number of atoms of each element on reactants side is not equal to the number of atoms of the same element on the products side. Hence, the above equation is not balanced and should be balanced.
What is a balanced equation?
A balanced equation is one, in which the number of atoms of each element, are the same on the side of the reactants (i.e., on the left hand side of the arrow) and also on the side of the products (i.e., on the right hand side of the arrow).
Why should an equation be balanced?
All equations must be balanced in order to comply with the Law of conservation of matter, which states that ‘matter is neither created nor destroyed.’ In the course of a chemical reaction, an unbalanced equation would imply that atoms have been created or destroyed, which is not possible, and hence, an equation should be balanced.
Limitations of a balanced chemical equation
1. It does not give information about the physical state of reactants and products. For example, the equation given below does not tell whether the substances involved in chemical reaction are in solid, liquid or gaseous states.
How is the above limitation overcome in a balanced equation?
This difficulty is overcome by putting symbols like (s) for solids, () for liquids and (g) for gases. For chemicals, which react in solution form, a symbol (aq) is used. Following examples will illustrate the point :
2. A balanced chemical equation does not tell whether a chemical reaction will come to completion or not.
3. A balanced chemical equation does not tell anything about the speed of a chemical reaction.
For example, the reaction between silver nitrate solution and sodium chloride solution completes in a few seconds. However, decomposition of lead nitrate crystals takes place in a few minutes.
4. A balanced chemical equation does not tell about the physical conditions which bring about the chemical reaction, e.g., it does not tell whether heat energy, light energy, pressure, catalyst, etc., are required for a chemical reaction or not.
The problem is partly solved by writing the conditions of reaction on the arrow head as illustrated by a balanced equation.
From the above equation we can say that 1 volume of nitrogen gas reacts with 3 volumes of hydrogen gas, under a pressure of 900 atmospheres, at a temperature of 450°C in the presence of catalyst iron containing molybdenum, when a reversible reaction takes place with the formation of 2 volumes of ammonia gas.
5. A balanced chemical equation does not tell about changes such as precipitation, change in colour, evolution of heat, light and sound energy during the chemical change.
1.8 Methods to balance a chemical equation
Things to remember, before balancing a chemical equation
1. Before beginning to balance an equation, check each formula, to see if it is correct, and NEVER change a formula while balancing an equation.
2. Balancing is done by placing coefficients in front of the formula to ensure the same number of atoms of each element on both sides of the arrow.
Hit and Trial Method
Balancing chemical equations
The simple equations are balanced by “hit and trial method”. Which is done in following steps.
- Step (i) – Count the no. of atoms of various elements on both sides of the equation
Example –
|
Element |
No. Of atom s in Reactants (LHS) |
No. Of atoms in products (RHS) |
|
Fe |
1 |
3 |
|
H |
2 |
2 |
|
O |
1 |
4 |
- Step (ii) – Start balancing with the compound which contains maximum number of atoms. It may be a reactant or product. In that compound select the element which has maximum number of atoms.
According to this rule has maximum number of atoms & oxygen has 4 atoms so it is selected.
|
S.No. |
Atoms of oxygen |
In reactants |
In products |
|
1 |
Initial |
1 |
4 |
|
2 |
To balance |
1 4 |
4 |
So the partly balanced equation is
- Step (iii) – Fe and H are not balanced in the above reaction so the above reaction repeat the above process for both i.e.
|
S.No. |
Atoms of oxygen |
In reactants |
In products |
|
1 |
Initial |
1 |
4 |
|
2 |
To balance |
1 3 |
3 |
- Now the equation becomes as
3
Step (v) – Finally check the correctness of the balanced equation by counting the number of atoms on both sides of the equation.
| Element |
No. Of atom s in Reactants (LHS) |
No. Of atoms in products (RHS) |
|
Fe |
3 |
3 |
|
H |
8 |
8 |
|
O |
4 |
4 |
Step (vi) – To make chemical equation more informative physical states of the reactants and products are mentioned as for solid (s), liquid (l), gas (g) and for aqueous solution of reactant or product (aq ) is written.
Now the equation becomes as
Symbol (g) with water is written to show that water is used in the form of steam in this equation.
If a gas is evolved in a reaction it can be shown by the symbol after the formula i.e. arrow pointing upwards e.g.
The symbol or ppt is be written for precipitate.
or ppt.
Reversible reaction is represented by () symbol and irreversible reaction by () symbol.
The heat evolved in chemical reaction is written on the right side by putting positive (+) sign and heat absorbed in the chemical reaction is written on the right hand side by putting negative (–) sign.
calorie (Exothermic reaction)
calorie (Endothermic reaction) Sometimes the reaction conditions such as temperature, pressure, catalyst etc. are written above or below the arrow in the equaton e.g.
calorie heat
450°C (Fe-Mo)
This method is generally employed to balance simple equations. It only involves hit and trial process till the number of atoms of each kind is the same on both sides of the equation. Following steps may be helpful in
balancing a chemical equation by this method:
a) Select the biggest formula and balance the same kinds of atoms in it on both sides of the arrow.
b) If the above step fails, then select the element which occurs at the minimum number of places and this element is balanced first. The element which occurs at the maximum number of places is balanced last of all.
c) In case the elementary gases like hydrogen, oxygen, etc., appear, the equation is balanced by keeping these gases in the atomic state.
d) Atoms of the elementary gases are balanced last of all.
e) The balanced equation in the atomic state is changed in the molecular form.
(If the number of atoms of the elementary gas is odd, multiply the whole equation by 2 to make it molecular)
Drawbacks of Hit and Trial method
Following are the drawbacks of the hit and trial method.
i) It is tedious and takes a long time.
ii) It is quite difficult to balance a chemical equation in which different atoms of the same element occur at a number of places on both sides of the arrow head. Try to balance the following equation.
iii) It does not given any information regarding the mechanism of reaction.
f-number Method
Frequency is the number of places at which an element occurs in a chemical equation.
If an atom of an element is present at one place towards the reactants side it must be present at one place towards the products side.
For example in the equation :
Potassium atom is present at one place towards the side of reactants and at one place towards the side of products. Thus, frequency of occurrence in the whole equation is 2. Similarly, nitrogen atom occurs at one place towards the side of reactants and at one place towards the side of products. Thus, frequency of occurrence of nitrogen in the whole equation is 2.
However, oxygen atom occurs at one place in the reactants side and two places in the products side; thus the frequency of occurrence of oxygen is 3.
The frequency of occurrence of various elements in an equation, in short is called f-number.
Thus, in the above equation f-number for potassium is 2, nitrogen is 2 and oxygen is 3.
Note: While calculating f-numbers of various elements just count the number of places where the given elements occur. Do not count the actual number atom as they do not represent places. In the above equation f-number of oxygen is 3, because it is at three places in the equation. Do not say f-number of oxygen is 7, as it has 7 atoms in the equation.
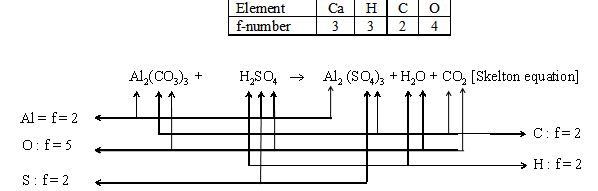
1. Examples for finding f-number :
2. 
|
Element |
Al |
C |
O |
H |
S |
|
F-number |
2 |
2 |
5 |
2 |
2 |
3.
|
Element |
K |
Cr |
O |
H |
c |
|
F-number |
2 |
2 |
2 |
2 |
4 |
As stated earlier, the balancing of chemical equation is related to the atomic number of element.
|
List of common metals in the increasing order of their atomic number |
|
|
Na |
11 |
|
Mg |
12 |
|
Al |
13 |
|
K |
19 |
|
Ca |
20 |
|
Cr |
24 |
|
Mn |
25 |
|
Fe |
26 |
|
Ni |
28 |
|
Cu |
29 |
|
Zn |
30 |
|
Ag |
47 |
|
Ba |
56 |
|
Au |
79 |
|
Hg |
80 |
|
Pb |
82 |
|
List of common non-metals in the increasing order of their atomic number |
|
|
H |
1 |
|
C |
6 |
|
N |
7 |
|
O |
8 |
|
F |
9 |
|
Si |
14 |
|
P |
15 |
|
S |
16 |
|
Cl |
17 |
|
Br |
35 |
|
I |
53 |
A list of common metallic elements and non-metallic elements in the increasing order of their atomic numbers is given above.
Rules for Balancing a Chemical Equation
1. Write the frequency numbers of all the elements in a given chemical equation.
2. Start balancing equation from that element which has least frequency number.
3. Other elements should be balanced in the order of increasing frequency numbers.
4. If two or more elements have same frequency number, then balance the metallic element first.
5. If there are two or more metallic elements with same frequency number, first balance the metal with highest atomic number. Then balance the next metallic elements with lower atomic number and so on.
6. If there are two or more non-metallic elements, with same frequency number, first balance the non-metallic element with highest atomic number. Other non-metallic elements should be balanced in the decreasing order of atomic numbers.
Note : Key to balance an equation is its f-number.
However, if f-numbers are same, then first metallic elements should be balanced in the order of decreasing atomic number, followed by non-metals in the order of decreasing atomic numbers.
Solved examples for writing chemical equations
Illustration.1 : Translate the following statements into chemical equations and then balance them.
(A) Hydrogen gas combines with nitrogen to form ammonia
(B) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(C) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(D) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Ans.
(A)
(B)
from air
(C)
White ppt.
(D)
Illustration.2 Balance the following chemical equations.
(A)
(B)
Ans.
(A)
(B)
Illustration.3 Write the balanced chemical equations for the following reactions.
(A) Calcium hydroxide + Carbon dioxide Calcium carbonate + water
(B) Zinc + Silver nitrate Zinc nitrate + Silver
(C) Aluminium + copper chloride Aluminium chloride + Copper
(D) Barium chloride + Potassium sulphate Barium sulphate + Potassium chloride.
Ans.
(A)
calcium carbon calcium
hydroxide dioxide carbonate
(B)
zinc silver nitrate
zinc nitrate silver
(C)
aluminium copper aluminium copper
chloride chloride
(B)
barium potassium barium potassium
chloride sulphate sulphate chloride
Illustration.4 Write the balanced chemical equation for the following and identify the type of reaction in each case –
(A) Potassium bromide(aq) + Barium iodide(aq) Potassium iodide(aq) + Barium bromide(s)
(B) Zinc carbonate (s) Zinc oxide (s) + Carbon dioxide(g)
(C) Hydrogen(g) + Chlorine(g) Hydrogen chloride(g)
(D) Magnesium(s) + Hydrochloridc acid(aq) Magnesium chloride(aq) + Hydrogen(g)
Ans.
(A) 2KBr(aq) + BaI2(aq) 2KI(aq) + BaBr2(aq)
This reaction is a double-displacement reaction.
(B) ZnCO3(s) ZnO(s) + CO2(g)
This reaction is a decomposition reaction
Illustration.5 Why should a magnesium ribbon be cleaned before burning in air?
Ans. Magnesium reacts with the constituent gases of the atmosphere to form various compounds which get deposited over its surface. The ribbon is cleaned before burning to remove the layer of these compounds so that pure magnesium can burn in air.
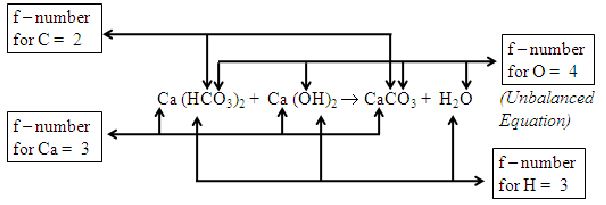
Example1: Calcium hydrogen carbonate solution reacts with calcium hydroxide solution to form calcium carbonate and water. This reaction can be written in equation form as under:
Writing f-numbers and order of balancing each element.
|
Element |
Ca |
H |
C |
O |
|
F-number |
3 |
3 |
2 |
4 |
|
Order of balancing each element |
2nd |
3rd |
1st |
4th |
Explanation
i) As the frequency number of carbon is 2, which is least f-number, we must start balancing with carbon.
The carbon atoms on reactants side are two, whereas towards products side is 1.
Therefore, we will multiply CaCO3 by numeral 2 as under:
ii) The elements calcium and hydrogen have f-number 3. However, calcium, being a metal should be balanced first and then the hydrogen.
In equation (a), counting calcium atoms towards reactants side, there are 2 calcium atoms. On the products side there are 2 calcium atoms. Thus, calcium atoms are balanced. In equation (a) counting hydrogen atoms towards reactants side, there are 2 atoms in and 2 atoms in . Thus, there are four hydrogen atoms. On the products side there are only 2 hydrogen atoms in . Thus, if we multiply by 2, there will be 4 hydrogen atoms as shown in equation (b).
iii) The element oxygen has f-number 4. Thus, it is balanced last of all. In equation (b) there are 6 atoms of oxygen in and 2 atoms of oxygen in . Thus, total number of atoms of oxygen towards the reactants side is 6 + 2 = 8 atoms. Towards the products side there are 6 atoms of oxygen in 2 molecules of calcium carbonate and 2 atoms of oxygen in 2 molecules of water. Thus, total number of oxygen atoms towards the products side is 6 + 2 = 8 atoms.
As number of atoms of all elements towards the reactants and the products side is same, the above equation (b) is fully balanced.
1.9 Types of Chemical reactions
Chemical reactions are the very heart of chemistry. They are nothing but the conversion of reactants into products.
Certain reactions like the explosion of dynamite or forest fire are more intense, while others like burning of matchsticks. LPG gas etc., are less intense. But all these reactions produce a detectable change, as the properties of products differ from those of the original reacting substances.
1.10 Combination reaction
The reactions in which two or more substances combine to form a single substance, are called combination reactions. In general, combination reactions are represented as

Examples :
1. Fe + S FeS
2.
3.
Classification of combination reaction
Combination reactions of elements and compounds. On this basis, these are classified as shown in below.
1. Element – Element Combination
This is a combination reaction in which two elements combine
Examples
1. Combination of hydrogen and oxygen to form water.
2. Synthesis of Ammonia
3. Burning of Carbon
2. Compound – Compound Combination
This is a combination reaction in which two compounds combine
Examples
1. Combination of ammonia gas and hydrogen chloride to form dense white solid ammonium chloride.
2. Reaction of quick lime in water
3. Compound – Element
This is a combination reaction in which an element and a compound combines
Examples
1. Combination of carbon monoxide and oxygen to form carbon dioxide
2. Combination of sulphur dioxide with oxygen to form sulphur trioxide.
1.11 Decomposition reactions
The reactions in which a compound splits up into two or more simpler substances are known as decomposition reactions. In general, a decomposition reaction is represented as:
Examples
Classification of decomposition reactions
Classification – I: Based on the type of substances formed on decomposition, they are classified as shown below:
A) Element – Element Decomposition
The decomposition reaction in which the products formed are only elements or elementary molecules, is called Element – Element decomposition reaction
1.
2.
B) Compound – Compound Decomposition
The decomposition reaction in which the products formed are different compound molecules, is called a compound – compound decomposition reaction
1.
2.
C) Compound – Element Decomposition
The decomposition reaction in which the products formed are in element and a compound molecules is called Compound – Element decomposition reaction
1.
2.
Classification – II: Based on the type of energy used for decomposition, they are classified as shown
A) Thermal decomposition
‘Thermo’ means heat. Hence, the decomposition which is brought about by heat is called Thermal decomposition
Examples :
1. Decomposition of lime: Lime () when heated strongly, decomposes to form quick lime (CaO) and carbon dioxide ()
2. Decomposition of lead nitrate: Colourless lead nitrate [] on heating decomposes to form black lead monoxide [PbO], reddish brown nitrogen dioxide [] and oxygen []
B) Photo decomposition (or) Photolysis :
‘Photo’ means light, lysis’ means breaking down or decomposition. Hence the decomposition reaction brought about by light is called photo decomposition or photolysis.
Examples :
1. Decomposition of hypochlorous acid: Hypochlorous acid [HClO), decomposes to give hydrochloric acid [HCl] and oxygen [].
2HCl (aq) 2HCl (aq) + (g)
2. Photosynthesis reaction :
Photosynthesis is the process by which plants prepare their food with the help of carbon dioxide of air and water from soil, in the presence of sun light.
Photosynthesis proceeds as in the following reaction :
C) Electro decomposition or Electrolysis
‘Electro’ means current. Hence the decomposition reaction brought about by electricity is called electrolysis.
Examples :
1. Electrolytic decomposition of water :
On passing electricity through acidified water, it decomposes into hydrogen and oxygen.
1.12 Displacement reactions
The reactions in which an element displaces another element from a substance are called a Displacement reactions. In general, they are represented as :
![]()
Examples:
1.
2.
3.
Metal activity series
The following metals are arranged according to their chemical reactivity.
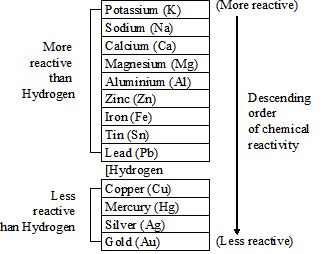
Utility of reactivity series
In the reactivity series, less reactive metal is placed below the series and more reactive metal is placed above it. Thus, as we move down, the metal in the reactivity series becomes less reactive. Therefore, in a metal displacement reaction, it is seen that a more reactive metal displaces a less reactive metal from its solution.
For example, Iron being more active than copper, it displaces copper from copper sulphate solution. The blue colour copper sulphate solution turns light green due to the formation of ferrous sulphate even as reddish brown copper is deposited on iron.
Placement of hydrogen
Though hydrogen is a non-metal, it is placed in metal activity series for the following reasons.
i) Just like metal, it readily loses its electron to form a positive ion.
ii) Metals readily combine with nonmetals like halogens, oxygen, sulphur etc.
Note: The metals above the hydrogen are more reactive than hydrogen and can displace hydrogen. The metals below hydrogen are less reactive than hydrogen and cannot displace hydrogen.
Examples:
a) Zinc being an active metal, it displaces hydrogen from dilute hydrochloric (or dilute sulphuric) acid
b) Similarly zinc displaces hydrogen from alkalis like
sodium hydroxide.
c) Zinc reacts with steam too to give hydrogen gas and zinc oxide.
Highly reactive metals like sodium and potassium react with water to displace hydrogen from it and form sodium hydroxide and potassium hydroxide respectively. [The reactions are violent due to the very reactive nature of sodium and potassium].
iii) A more reactive non-metal displaces a less reactive non-metal from the latter’s solution.
Examples:
Chlorine being more active than bromine, it displaces bromine from sodium bromide solution.
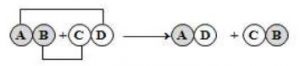
1.13 Double decomposition
reactions
The reactions where the constituent atoms of the reactants interchange to produce new compounds, are called ‘double decomposition reactions’. In general, it is represented as :
Examples :
1.
2.
3. Types of double decomposition reactions
|
Precipitation reactions |
Neutralisation reactions |
|
Definition |
|
|
When aqueous solutions of two compounds react, by exchanging their radicals, such that one of the products formed is an insoluble salt and appears in the form of a precipitate, the reaction is said to be precipitation reaction. |
When an acid solution reacts with a base by exchanging the radicals, such that salt and water are the only products, then the reaction is called neutralization reaction. Acid + Base Salt + Water
|
|
Examples |
|
|
Formation of magnesium hydroxide When an aqueous solution of magnesium chloride and potassium hydroxide are allowed to mix, a white precipitate of magnesium hydroxide is obtained. The process can be represented as:
|
Formation of zinc chloride: When a solution of hydrochloric acid reacts with sodium hydroxide, sodium chloride and water are formed. The process can be represented as: |
|
Ionic representation |
|
|
Some more examples |
|
|
1. 2. |
|
1.14. Redox reactions
Before we understand the redox reactions, let us get familiar with the terms related to redox reactions
|
Oxidation |
Reduction |
|
Definition |
|
|
The process in which a substance gains oxygen or loses hydrogen or electrons is known as oxidation. |
The process in which a substance loses oxygen or gains hydrogen or electrons is known as reduction. |
|
Examples |
|
|
Gain of oxygen: Magnesium undergoes oxidation (gains oxygen) to form magnesium oxide.
Loss of hydrogen: Hydrogen sulphide undergoes oxidation (loses hydrogen) to form sulphur.
Loss of electrons: Sodium undergoes oxidation (loses an electron) to form sodium ion. |
Loss of oxygen: Copper oxide undergoes reduction (loses oxygen) to form metallic
Gain of hydrogen: Bromine undergoes reduction (gains hydrogen) to form hydrogen bromide.
Gain of electrons: Chlorine undergoes reduction (gains an electron) to form chloride ion. |
|
Some more examples |
|
|
i) |
i) |
|
ii) |
ii) |
|
iii) |
iii) |
|
iv) |
iv) |
|
v) |
v) |
|
vi) |
vi) |
|
Remember |
|
|
The substance that undergoes oxidation is said to be oxidized. For example, in the reaction, carbon is said to be oxidized as it gains oxygen. |
The substance that undergoes reduction is said to be reduced. For example, in the reaction silver is said to be reduced as it loses oxygen. |
|
Oxidizing and reducing agents |
|
|
Substances that help in oxidation of other substances are known as oxidizing agents. For example, in the reaction, undergoes oxidation by giving oxygen. Hence, ZnO is the oxidizing agent.
Similarly, oxidising agents also gain electrons or hydrogen and |
Substances that help in reduction of other substances are known as reducing agents. For example, in the reaction, ‘Cl’ undergoes reduction by gaining ‘H’ from . That is, helps in reduction of ‘Cl’ by giving hydrogen. Hence, is the reducing agent. Similarly, reducing agents also lose electrons or oxygen and help the other substances to get reduced. |
|
What do oxidising and reducing agents undergo ? |
|
|
We have seen that an oxidising agent loses oxygen or gains electrons or hydrogen. Therefore, an oxidising agent undergoes reduction. |
We have seen that a reducing agent gains oxygen or loses electrons or hydrogen. Therefore, a reducing agent undergoes oxidation. |
Redox Reactions
The chemical reactions in which both oxidation and reduction takes place simultaneously are called redox reaction.
For example, consider the reaction that takes place when zinc pieces are added to blue copper sulphate solutions, the solution becomes colorless, due to the formation of zinc sulphate. The reaction is as follows :
Let us see the reaction with the change in the charges of the respective species :
Observe the changes that took place in the reaction:
a) The cupric ion () present in copper sulphate, gained two electrons and changed to neutral copper. That is copper has undergone reduction by gaining of electrons.
The reduction reaction can be shown as : .
b) The neutral zinc lost two electrons and changed to zinc ion (), to form zinc sulphate (ZnSO4). That is, zinc has undergone oxidation by losing of electrons.
The oxidation reaction can be shown as :
The zinc ion combines with sulphate radical to form zinc sulphate (). It can be shown as below:
We have seen that, in the above reaction, both oxidation and reduction have taken place simultaneously. Hence, it is a redox reaction.
Examples :
Electronic Interpretation of Oxidation
The electronic theory attempts to interpret oxidation on the basis of electron transfer. According to octet rule, atom will try to complete its octet by losing gaining or sharing electrons. Sodium chloride is an electrovalent compound and consists of an ion pair even in the solid state. In its formation, the neutral sodium loses and electron and becomes positively charged sodium ion. Sodium is said to be oxidised and loss of electrons is termed as oxidation.
Electronic Interpretation of Reduction
Reduction which is also referred to as electronation is a process involving the gain of electrons and is the reverse of oxidation.
For example
Mg combines with oxygen and is oxidized to MgO. According to electronic theory magnesium atom loses two electrons from its outermost shell (M) and is oxidised to mG which oxygen atom gains these two electrons and gets reduced to oxide anion, hence oxidation involves loss of electrons and it is also referred as de- electronation. Reduction involves gain of electrons so it is referred to as electronation.
EFFECT OF OXIDATION REACTIONS IN EVERYDAY LIFE
We are all aware of the fact that oxygen is most essential for sustaining life. One can live without food or even water for a number of days but not without oxygen. It is involved in a variety of actions which have wide range of effects on our daily life. Most of them are quite useful while a few may be harmful in nature. Some of these effects are briefly discussed. Some examples are-
(a) Combustion Reactions:
A chemical reaction in which a substance burns or gets oxidised in the presence of air or oxygen in called combustion reaction. For example, kerosene, coal, charcoal, wood etc. burn in air and thus, undergo combustion. Methane () a major constituent of natural gas undergoes combustion in excess of oxygen upon heating.
Similarly, butane () the main constituent of L.P.G. also undergoes combustion.
All combustion reactions are of exothermic nature and are accompanied by release of heat energy. The human body may be regarded as a furnace or machine in which various food stuffs that we eat undergo combustion or oxidation. The heat energy evolved keeps our body working. Carbohydrates such as glucose, fructose, starch etc. Are the major source of energy to the human body. They undergo combustion with the help of oxygen that we inhale to form carbon dioxide and water. For example.
All combustion reactions are not accompanied by flame. Combustion is basically oxidation accompanied by release of energy.
Respiration
Respiration is the most important biochemical reaction which releases energy in the cells. When we breathe in air, oxygen enters our lungs and passes into thousands of small air sacs (alveoli). These air sacs occupy a large area of membranes and oxygen diffuses from the membranes into blood. It binds itself to haemoglobin present in red blood cells and is carried to millions of cells in the body. Respiration occurs in these cells and is accompanied by the combustion of glucose producing carbon dioxide and water. Since the reaction is of exothermic nature, the energy released during respiration carry out many cell reactions and also keeps our heart and muscles working. It also provides the desired warmth to the body. Both carbon dioxide and water pass back into the blood and we ultimately breathe them out. Respiration takes place in the cells of all living beings.
Fish takes up oxygen dissolved in water through their gills while plants take up air through small pores (stomata) present in their leaves.
Harmful Effects of Combustion
We have discussed the utility of combustion in releasing energy which our body needs to keep warm and working; however, combustion has harmful effects also. The environmental pollution is basically due to combustion. Poisonous gases like carbon monoxide (CO), sulphur dioxide () sulphur trioxide () and oxide of nitrogen (NOX) etc. are being released into the atmosphere as a result of variety of combustion reactions which are taking place. They pollute the atmosphere and make our lives miserable. In addition to these, other harmful effects of combustion are corrosion and rancidity. These are briefly discussed.
(i) Corrosion: Corrosion may be defined as the process of slow eating up of the surfaces of certain metals when kept in open for a long time.
Quite often, when we open the bonnet of a car after a long time, we find a deposit around the terminals of the battery. This is an example of corrosion. Black coating on the surface of silver and green layer on the surface of copper are the examples of corrosion. In case of iron, corrosion is called rusting. Rust is a chemical substance brown in colour and is formed by the chemical action of moist air (containing and ) on iron. It is basically an oxidation reaction and the formula of rust is , . It is very slow in nature and once started keeps on.
Both corrosion and rusting are very harmful and case damage to the building, Railway tracks, cars and other objects / materials where metals are used. We quite often hear that an old building has collapsed on its own causing loss of both lives and property. This is on account of the rusting of iron which is used in making the structure particularly the roof.
(ii) Rancidity: Oxidation has damaging effects on food and eatables. When the fats and oils present in butter and margarine are oxidised, they become rancid. As a result, their smell and taste change. They become quite unpleasant. This is known as rancidity. It can be checked in a number of ways.
(A) Manufacturer sometimes add certain food additives to the food materials. These are known as antioxidant and check their oxidation.
(B) Keeping food in air tight containers prevents its oxidation.
(C) Refrigeration of food also slows down rancidity because the temperature inside refrigerator is very low and direct contact with air or oxygen is avoided.
(D) Chips manufacturers generally flush their bags with nitrogen before packing so that they may not be oxidised.
1.15 Exothermic and Endothermic reactions
|
Exothermic |
Endothermic |
|
Understanding |
|
|
When magnesium is added to sulphuric acid, the mixture fizzles as hydrogen is given out. The reactions like the one between magnesium and sulphuric acid release energy. Such type of chemical reactions where the energy is released, are called Exothermic reactions. |
Consider the decomposition of calcium carbonate.
Such type of chemical reactions where the energy is absorbed, are called endothermic reactions. |
|
Examples |
|
|
1. Burning of coal : 2. Respiratory Reactions : |
1. Decomposition of nitric oxide : 2. Formation of ethane from acetylene: |
Illustrations
Illustration1. What is the basis of a balanced chemical equation?
or
State the law on which a balanced chemical equation is based.
or
State the law of conservation of mass.
or
On what basis is a chemical equation balanced?
Ans. The basis of balanced chemical equation is the law of conservation of mass. Mass can neither be created nor destroyed in a chemical reaction.
Illustration2. Would you call digestion of food in our body a chemical change?
Ans. Yes. It is a chemical change.
Illustration3. Balance the following chemical equation
Ans. Balanced chemical equation is
Illustration4. Balance the following chemical equation :
Ans. Balanced chemical equation is
Illustration5. Write a combination reaction in which two gases combine.
Ans. Hydrogen and chlorine gases combine to form hydrogen chloride.
Illustration6. What change in colour is observed when white silver chloride is left exposed to sunlight? What type of chemical reaction is this?
Ans. When silver chloride is exposed to sunlight, the white colour of silver chloride changes to grey colour. This is a photochemical decomposition reaction.
Illustration7. Why do we apply paint on iron articles?
Ans. We apply paint on iron articles to protect them from corrosion.
Illustration8. How can you help your mother in keeping the fried items so that they do not develop a bad smell and their shelf life is increased?
Ans. Since we cannot pack the fried objects in the atmosphere of nitrogen at home but we can increase their shelf life by keeping them in airtight containers. In this way they do not come in contact with oxygen. Their shelf life can further be increased by keeping them in fridge at low temperature.
Illustration9. A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Ans.
(i) Calcium oxide or quick lime, its formula is CaO.
(ii)
Quick lime water Slaked lime
Illustration10. Write any two limitations of a chemical equation.
Ans. A chemical equation does not provide the following informations:-
(i) Whether the reaction is fast, slow or instantaneous i.e. the rate of the reaction is not shown by a chemical equation.
(ii) Whether the reaction goes to completion or is stopped in between i.e., the extent to which a reaction takes place is not known from a chemical equation.
Illustration11. Balance the following equations:
(i)
(ii)
Illustration12. Distinguish between an exothermic and an endothermic reaction. Amongst the following reactions, identify the exothermic reaction and the endothermic reaction:
(i) Heating coal in air to form carbon dioxide.
(ii) Heating limestone in alime kiln to form quick lime.
Ans. Exothermic reactions are those reactions in which heat is evolved.
Endothermic reactions are those which involve absorption of heat.
(i) Heating coal in air to form carbon dioxide is an exothermic reaction.
(ii) Heating limestone in a lime kiln to form quick lime is an endothermic reaction.
Illustration13. What is an oxidation reaction? Give an example of oxidation reaction. Is oxidation an exothermic or an endothermic reaction?
Ans. Reactions involving addition of oxygen are classified as oxidation reactions. For example magnesium reacts with oxygen to form magnesium oxide.
Oxidation reactions are generally exothermic in nature.
Illustration14. What is a redox reaction? When a magnesium ribbon burnsin air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
Ans. Those reactions in which oxidation and reduction reactions occur simultaneously, are called redox reactions. In these reactions one substance is oxidised and another substance gets reduced.
When a magnesium ribbon burns to form a white ash, magnesium metal is oxidised in this reaction because it combined with oxygen to form magnesium oxide.
Illustration15. In the reaction:
Identify the oxidising and reducing agents.
Ans. Mn loses oxygen, therefore it is reduced, thus it acts as an oxidizing agent.
HCl loses hydrogen. It itself is oxidized, thus it acts as a reducing agent.
In the above reaction
Mn is oxidizing agent.
HCl is reducing agent.
Illustration16. Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
Ans. Calcium carbonate decomposes on heating and forms calcium oxide and carbon dioxide.
Illustration17. Define a combination reaction. Give one example of a combination reaction which is also exothermic.
Ans. Reactions in which two or more substances combine to form a new compound are called combination reactions. An example of this type of reactions is the combustion of magnesium in air, where magnesium combines with oxygen to form magnesium oxide, it is an exothermic reaction also.
Illustration18. Dilute solution of ammonium hydroxide is added to aqueous solution of ferrous sulphate. Ferrous hydroxide is formed. What is the type of this reaction? Write chemical equation.
Ans. It is a double decomposition reaction.
Ferrous sulphate Ammonium hydroxide
Ferrous hydroxide Ammonium sulphate
Illustration19. (i) What is observed when a solution of potassium iodide is added to a sulution of lead nitrate taken in a test tube?
(ii) What type of reaction is this?
(iii) Write a balanced chemical equation to represent the above reaction.
Ans.
(i) A yellow precipitate of lead iodide will be formed.
(ii) This is a double displacement reaction.
(iii)
Illustration20. Why does the blue colour of copper sulphate solution changes to green colour when an iron nail is dipped? Write chemical equation.
Ans. The blue colour of the copper sulphate solution changes to green colour because copper of copper sulphate is replaced by iron and forms ferrous sulphate which is green coloured.
Iron Copper sulphate Ferrous sulphate Copper.








