NCERT TEXT BOOK EXERCISES
Q1. Fill in, the blanks:
(a) Most liquids that conduct electricity are solutions of ________, _______, and ________.
(b) The passage of an electric current through a solution causes ________ effect.
(c) If you pass current through copper sulphate solution, copper gets deposited on the __________ plate connected to the terminal of the battery.
(d) The process of depositing a layer of any desired metal on another metallic object, by means of electricity, is called ____________.
Ans. (a) acids, bases, salts
(b) chemical
(c) negative
(d) electroplating.
Q2. When the free ends of a tester are dipped into a solution the magnetic needle shows deflection. Can you explain the reason?
Ans. Yes, we can explain the reason. The magnetic needle will show deflection when circuit is complete. The deflection of magnetic needle shows that the solution is a good conductor.
Q3. Name three liquids, which when tested in the manner shown in Fig. may cause the magnetic needle to deflect?
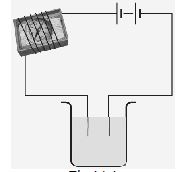
Ans. Tap water, hydrochloric acid, sodium hydroxide.
Q4. The bulb does not glow in the set up shown in Fig. List the possible reasons. Explain your answer.
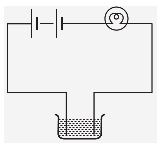
Ans. The possible reasons may be:
(i) The connections of the circuit may be loose.
(ii) Bulb may be fused.
(iii) Cells may be used up.
(iv) The liquid may be poor conductor.
Q5. A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows r dimly for liquid B. You would conclude that:
(i) liquid A is a better conductor than liquid B.
(ii) liquid B is a better conductor than liquid A.
(iii) both liquids are equally conducting.
(iv) conducting properties of liquid cannot be compared in this manner.
Ans. We conclude that option (i) ‘liquid A is a better conductor than liquid B’ is correct.
Q6. Does pure water conduct electricity? If not what can we do to make it conducting?
Ans. No, the pure water does not conduct electricity. Pure water can be made conducting by dissolving salt in it.
Q7. In case of a fire before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Ans. The water used in water hoses is good conductor of electricity. Firemen shut off the main electrical supply, because if the supply of electricity continues this may be high risk of electrocution due to water.
Q8. A child staying in the coastal region tests the drinking water and also the sea water with his tester. He finds that the compass needle deflects more in the case of sea water. Can you explain the reason?
Ans. Sea water contains more amount of salt than drinking water. So the sea water is a better conductor of electricity. This is the reason that the compass needle deflects more in case of sea water.
Q9. Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Ans. No, it is not safe for the electrician to carry out electrical repairs during heavy downpour. It is because during heavy downpour there is a higher risk of electrocution.
Q10. Paheli had heard that rain water is as good as distilled water. So she collected some rain water in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Ans. Rain water is like distilled water but some impurities are mixed in from the atmosphere. These impurities make the rain water conducting. This could be the reason for the deflection of compass needle.
Q11. Prepare a list of objects around you that are electroplated.
Ans. Pots of metals, bath taps, ornaments, rims of vehicles, handle bar of cycles and motor cycles, kitchen gas burner, bottom of cooking utensils, handles of doors, tin cons are the some objects around us that are electroplated.
Q12. The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
Ans. Copper ion is positively charged. It is attracted towards the plate which is connected to the negative terminal of the battery. As copper ions are transferred to the thin copper plate, this thin pure copper plate must be connected to the negative terminal of the battery. Consequently, impure copper rod is connected to the positive terminal of the battery.








