4.1 INTRODUCTION
In our earlier classes of chemical classification of matter, we have learnt that matter is made up of atoms and molecules. Atoms are the basis of chemistry. They are the basis for everything in the Universe. Atoms and the study of atoms are a world unto themselves. In this chapter, we are going to explore atom and its constituents. Now, let us learn a little more about this idea that has brought about a revolution in science.
4.2 ANCIENT IDEAS OF ATOM
The views of Kanad
Way back as the sixth century BC, the Indian philosopher Maharshi Kanad came forward with the following idea. Matter is not continuous, and made up of tiny particles, named paramanus. (In Sanskrit, param means final or ultimate and anu means particle.) Kanad further said that two or more paramanus combine to form bigger particles.
The Views of Democritus and Leukiposs
In the fifth century BC, the Greek philosophers Democritus and Leukiposs came up with a similar idea. They thought that on dividing a piece of a substance, one would ultimately get a particle that could not be divided any further. They gave the name atomos (in Greek, atomos means indivisible) to these ultimate particles.
4.3 DALTON’S THEORY
In 1803, an English Chemist, John Dalton, put forward his Atomic Theory. The main postulates of this theory are as follows:
1. Composition of matter: Matter is made of very small particles called atoms.
2. Indivisibility of atoms: Atoms are indivisible. They cannot be further broken down.
3. Invincibility of atoms: Atoms can be neither created nor destroyed in a chemical reaction.
4. Atoms of similar elements and dissimilar elements: Atoms of a given element are identical in all respects – same mass, same size and same properties. For example, atoms of hydrogen element are identical in all respects. Atoms of different elements are different. For example, atoms of hydrogen and oxygen elements are different in all respects.
5. Combination of atoms: Atoms of different elements combine in a whole number ratio to form compounds. For example, the ratio of combination of hydrogen and oxygen to form water molecule () is 2:1 which is a whole number ratio.
6. Role of atom in a chemical reaction: Atom is the smallest particle of matter that takes part in a chemical reaction.
Contradiction of Dalton’s atomic theory by modern atomic theory
Further experiments conducted by scientists have provided the evidence that atoms are further divisible and made of fundamental particles called electrons, protons and neutrons. Hence Dalton’s theory does not give an explanation regarding further divisibility of atoms and also the internal structure of an atom.
Let us see the contradictions of Dalton’s theory by the modern atomic theory.
|
S.No. |
Dalton’s atomic theory |
Modern atomic theory |
|
1. |
Matter consists of small indivisible particles called atoms. |
Atom is no longer indivisible, but consists of neutrons, protons and electrons. |
|
2. |
Atoms of same element are alike in all respects. |
All atoms have isotopes. It means some of the atoms of same element have different atomic weights. |
|
3. |
Atoms of different elements are different in all respects. |
Atoms of different elements are sometimes similar in some respects. For example, atoms of argon and calcium have same atomic weight. |
|
4. |
Atoms combine in small whole numbers to form compound atoms (molecules). |
Atoms in organic compounds do not combine in small whole number ratio. The molecules of proteins are highly complex. |
|
Similarity : Atom is the smallest unit of matter that takes part in a chemical reaction. |
||
4.4. DISCOVERY OF CATHODE RAYS
Although many of the pioneers of 19th century physics, including Faraday, were convinced on the basis of chemistry and the phenomena observed in electrolysis that electric current consisted of the flow of particles of charge, the nature of these charges was not understood. Even the basic question of whether the charge of the particles was positive or negative remained undetermined. The answers to these questions, and to the basic structure of matter, were resolved by experiments that began with the study of electric discharges in evacuated tubes. Along the way a series of discoveries were made which led to the technological revolution of the 20th Century.
In 1855, The German inventor Heinrich Geissler developed the mercury pump and produced the first good vacuum tubes. These tubes, as modified by Sir William Crookes, became the first to produce cathode rays, leading eventually to the discovery of the electron.
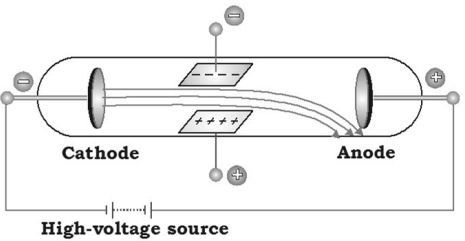
Description of discharge tube
The discharge tube consists of a glass tube from which most of the air has been evacuated having two metal plates sealed at both the ends. These metal plates are called electrodes. These electrodes are connected to positive and negative terminals of a battery. The electrode connected to the positive terminal is known as anode and the electrode connected to the negative terminal is known as cathode.
Experiments in discharge tube
The electrodes are connected to high voltage for the current to flow. The changes that occur in the discharge tube at different pressures were observed. The high voltage provides energy for the atoms of a gas to further split or break up. When both electrodes are connected to high voltage, current starts flowing. At high pressure no electricity flows through the air in the discharge tube, so low pressure is used. Low pressure helps in conduction of electricity.
At high voltage of 10,000 volts and at normal atmospheric pressure there is no effect. But keeping the same voltage if pressure is reduced to 0.01 mm of Hg, a greenish glow was observed at anode. The rays are emitted from the direction of the cathode, and are called cathode rays.
Properties of cathode rays
The experiments on cathode rays by J.J Thomson led to the discovery of electrons. The following are the properties of cathode rays:
(i) Cathode rays travel in straight lines.
Explanation: When an object is placed opposite to the direction of the cathode rays, a sharp shadow is formed.
This concludes that cathode rays travel in straight lines.
(ii) Cathode rays contain some material particles.
Explanation: When cathode rays are allowed to fall on a paddle wheel, it rotates. This is possible only when the rays striking it have some material particles. From this, it can be concluded that cathode rays consist of some material particles.
(iii) Cathode rays are negatively charged and affected by the electric field.
Explanation: When the electric field is applied perpendicularly to the path of cathode rays, they deflect towards positive plate. As opposite charges attract, the cathode ray particles are negatively charged.
(iv) Cathode rays are negatively charged and affected by magnetic field.
Explanation: When the magnetic field is applied perpendicularly to the path of cathode rays, they get deflected towards the north pole of the magnet which is expected of negatively charged particles. This further confirms that cathode rays are negatively charged.
(v) Cathode rays generate heating effect
Explanation: When cathode rays are focused on a thin metal foil, it gets heated up. This proves that cathode rays have some kinetic energy.
(vi) Cathode rays affect ZnS screen.
Explanation: When cathode rays are allowed to strike ZnS screen, they produce a faint greenish fluorescence.
(vii) Cathode rays ionises the gases.
Explanation: When cathode rays are allowed to pass through gases, different glows are seen in the tube. These different glows are due to the ionisation of gases.
(viii) Cathode rays have penetrating power.
Explanation: When cathode rays are allowed to pass through thin metal foils, a glow is seen behind the metal foil indicating that they have good penetrating power.
(ix) Cathode rays produce X-rays
Explanation: When cathode rays are allowed to fall on metals such as tungsten, copper, X-rays are observed.
(x) The e/m ratio of cathode ray particles is constant
When cathode rays were obtained by using different gases and vapours of different materials, the ratio of charge and mass (e/m ratio) of the cathode ray particles of these gases were obtained. It was observed that the e/m ratio of the cathode ray particles remained the same irrespective of the nature of the gas or vapour entering the discharge tube. As the e/m ratios is same, we can conclude that the cathode ray particles obtained from different gases and vapours have the same charge and mass. Further, the cathode ray particles are similar for any gas and vapour of any matter. Thus, a cathode ray-particle is a common constituent of any matter with same mass and charge everywhere. So, it is called the universal constituent of matter. This common constituent of matter was considered as the fundamental particle called electron.
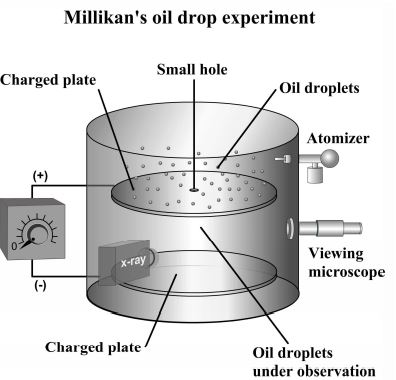
Determination of charge and mass of the electron.
The charge of the electron was determined by R.A Millikan with the help of oil drop experiment. The value was found to be coulomb (C). This negative charges on the electron was regarded as unit negative charge (–1). The mass of the electron was also determined by a suitable method. It was found to be 9.1 × g. This was regarded as negligible and was nearly times the mass of hydrogen atom. The charge (e) of the
electron was divided by its mass (m) to calculate the charge/mass ratio also called e/m
ratio. .
4.5. DISCOVERY OF ANODE RAYS
We know that in science many discoveries and inventions had their origin from small ideas. One such discovery was the existence of proton, by Goldstein.
Goldstein repeated the cathode-ray experiment, using a perforated cathode. He observed that there was a glow on the wall opposite the anode. So, some rays must be travelling in the direction opposite to that of the cathode rays, i.e., from the anode towards the cathode. These rays were called anode rays or canal rays (as they moved through the perforations, or canals, in the cathode). It was found that these rays contained positively charged particles, and so J.J.Thomson called them positive rays.
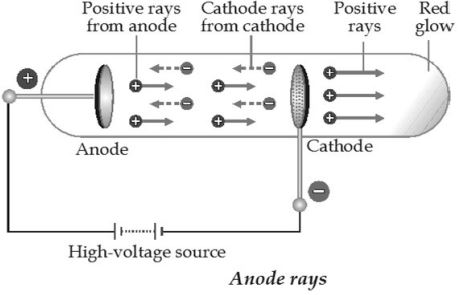
How anode rays are produced
When an electric discharge is passed through the gas, some of the molecules of the gas ionised and produce cathode rays. Cathode rays consist of electrons. These electrons move with high speed towards the anode. As they move, they collide with the remaining molecules of the gas in the tube causing them to electrons. The positive ions formed are attracted by the performed cathode. The stream of these positive ions pass a glow on the glass wall of the discharge tube. (On the other side of the discharge tube, the cathode rays produce a green light). The stream of positive ions so formed constitute the positive or anode rays.
Properties of anode rays
i) Anode rays travel in straight lines.
ii) Anode rays contain positively charged particles and hence cause mechanical motion.
iii) Anode rays are deflected both in electric (towards negative plate) and magnetic fields (towards South pole).
iv) They cause heating effect and have some kinetic energy.
v) The e/m ratio of the particles in the anode rays is not constant and depends on the nature of gas taken in the discharge tube.
Discovery of protons
So the experiments showed that the e/m ratio for positive rays depends on the gas used in the discharge tube.
Since hydrogen is the lightest element, its mass ‘m’ is the lowest and e/m ratio is the highest.
Goldstein gave the name proton for the particles which comprised the positive rays, produced by hydrogen gas.
Thus the proton is a hydrogen ion which is produced from the hydrogen atom. The hydrogen ion is formed by loss of an electron from a hydrogen atom.
Note:
i) The charge of proton e.s.u (electro static unit).
ii) The mass of proton is a.m.u.
Comparison of cathode rays and anode rays
|
Cathode rays |
Anode rays |
|
Cathode rays travel in straight lines from cathode to anode and cast the shadow of the object placed in their path. |
Anode rays travel in straight lines from anode to cathode and cast the shadow of the object placed in their path. |
|
Cathode rays contain material particles (electrons) which are negatively charged. |
Anode rays contain material particles which are positively charged. |
|
These rays are deflected in both magnetic and electric fields. |
These rays are deflected in both magnetic and electric fields. |
|
These rays have kinetic energy and raise the temperature of a metallic object on which they fall. |
These rays have kinetic energy and raise the temperature of a metallic object on which they fall. |
|
Cathode ray particles are common constituents of all matter and their e/m ratio is constant for all gases. |
The e/m ratio of anode ray particles is different for different gases and is maximum for hydrogen. |
4.6. DISCOVERY OF NEUTRONS
By 1920, with the discovery of electron and proton, it was thought that the inner structure of the atom was complete. The mass of the electron is negligible; hence the mass of atom should be equal to the mass of protons concentrated inside the nucleus. Different atoms have different number of protons, hence different atomic masses. However, it was observed that there was a discrepancy between the actual atomic mass and the calculated atomic mass.
For example, an atom of carbon has 6 protons; therefore its mass should be six times the mass of hydrogen atom which has one proton. But, experimentally, it was found that the mass of carbon atoms is twelve times the mass of hydrogen atom. A similar problem was encountered with regard to the mass of other atoms. Then, what was the reason for this discrepancy?
Rutherford was the first scientist to predict the reason for this discrepancy. He predicted that along with protons, there were some other neutral particles present inside the nucleus. These particles were discovered as neutrons by James Chadwick in 1932.
Now, let us study the observations and conclusions that led to the discovery of neutrons.
Chadwick’s observations and conclusions
During an experiment it was found that, when alpha particles bombarded Beryllium nuclei, some radiations were observed. These radiations were found to be undeviated in an electric field. So, the earlier scientist thought these radiations could be electromagnetic radiation and hence discarded it. But Chadwick repeated the experiment and made the following observations.
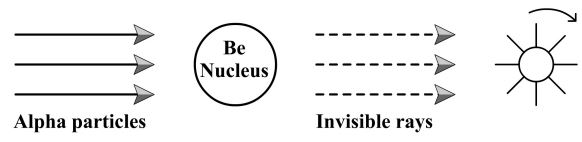
1. A paddle wheel was placed behind the Beryllium nucleus and the nucleus was bombarded with – particles. It was observed that the paddle wheel rotates. From this, it was concluded that the beryllium nucleus emits some invisible radiations having material particles.
2. When these invisible radiations were allowed to pass through an electric field, there was no deviation seen. This confirmed the fact that these rays contained neutral particles. These neutral particles were called neutrons by James Chadwick.
SUMMARY
|
|
Electron |
Proton |
Neutron |
|
Discoverer |
J.J. Thomson |
Goldstein |
James Chadwick |
|
Symbol |
|||
|
Absolute charge |
|||
|
Relative charge Mass in kg |
|||
|
Mass in amu Relative mass (app) |
The Structure of Atom
After the discovery of fundamental particles, scientists started thinking of the arrangement of these fundamental particles inside the atoms. This resulted in the development of various atomic models by different scientists. People are acquainted with the revolving air symbol used to depict the atom. The symbol shows a glowing nucleus enmeshed within the intertwined loops of orbiting electrons. What is the truth behind this symbol? Did the atom ever sit for its portrait?
The truth, however, is that no one has ever seen an atom. Nevertheless, bit by bit, the minute terrain of the atom has been mapped. Taken as a whole, this airy symbol has built up a picture of the atom with proven usefulness.
4.7. THOMSON’S ATOMIC MODEL
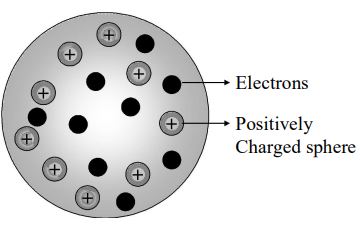
One of the first attempts in predicting the arrangement of subatomic particles with in the atom was made by J.J. Thomson. This model of atom has different names: Water Melon Model (or) Plum Pudding Model (or) Raisin Pudding Model. He believed that an atom was made up of positively charged substances in the form of a sphere in which electrons were embedded. This can be compared to a water melon, which has evenly distributed red spongy mass with black seeds embedded in it. He could not explain how the positively charged particles were shielded from the negatively charged particles without getting neutralized. Hence this model of atom was discarded.
4.8. RUTHERFORD MODEL OF ATOM
Rutherford overturned Thomson’s model in 1911 with his famous gold-foil experiment in which he demonstrated that the atom has a tiny, massive nucleus Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate would make a sharp-edged picture, while alpha particles beamed through a sheet of mica only 20 micrometre (or about 0.002 cm) thick would make an impression with blurry edges. For some particles the blurring corresponded to a two-degree deflection. Remembering those results, Rutherford had his postdoctoral fellow, Hans Geiger, and an undergraduate student, Ernest Marsden, refine the experiment. Let us now know about the experiment.
Experimental Set up
The experimental arrangement was very simple. First, a lead block with a cavity containing the radioactive element, radium was taken. In front of the lead block, a very thin gold foil (0.0004 mm) was kept. At the other end, a screen of zinc sulphide was adjusted. Alpha rays from the radioactive radium passed through the golden foil and hit the ZnS screen. Scintillations were produced when alpha particles struck the zinc sulphide screen.
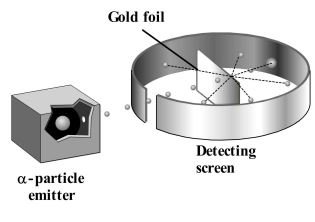
Rutherford’s observations and conclusions
1. Most of the -particles went straight through the foil. This is explained by the fact that they were not attracted to or repelled by any particle. In other words, the atom is mostly empty.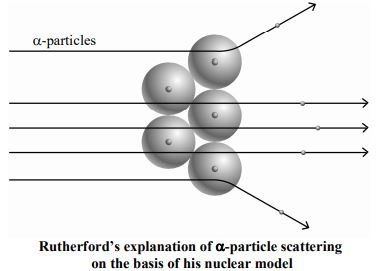
2. Some of these particles deviated slightly from their path. They were repelled to a small extent by a positive charge. Very few of the particles, the ones at the centre, almost retraced their path. This meant that they were strongly repelled by a small positively charged body at the centre of the atom. This positively charged body is called the nucleus. Due to this reason this model of atom is also called Planetary model of atom. The mass of the atom is concentrated in the nucleus.
3. Rutherford also theorised that electrons revolve round the nucleus at large distances from it just like planets round the sun. For this reason, this model of atom is also called Planetary model or solar model. Rutherford estimated the diameter of the nucleus to be of the order of cm and that of the atom to be of the order of cm. Thus, the diameter of the nucleus is about (= 1,00,000) times smaller than that of the atom.
Rutherford’s explanation for the stability of nucleus and atom: After the success of bringing out a successful atomic model, Rutherford was cornered by the other seniors by a question: “Why does the nucleus not disintegrate inspite of repulsion among the protons?”
To explain the stability of the nucleus, Rutherford predicted the presence of neutral particles known as neutron. The presence of these neutrons between the protons neutralises the repulsion among the protons. Rutherford predicted the presence of the neutron even before it was discovered.
Coming to the stability of the atom, he explained that the revolving electron is under the influence of two types of forces.
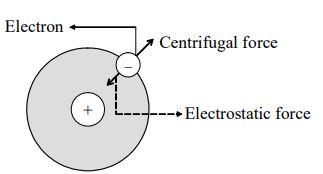
i) The electrostatic force of attraction between the nucleus and the electron and
ii) The centrifugal force directed away from the revolving electron.
These two forces are equal and opposite and hence keep the electron in equilibrium in the path. This is the reason why electrons do not fall into the nucleus in spite of inward nuclear pull.
Drawbacks of Rutherford’s atomic model
1. Spiral path of electron: According to the law of electro-dynamics, a charged particle revolving round another oppositely charged particle should lose energy continuously. So the electron moving round the positively charged nucleus should continuously emit radiation and lose energy. As a result of this, a moving electron will come closer and closer to the nucleus and after passing through a spiral path, it should ultimately fall into the nucleus.
It was calculated that the electron should fall into the nucleus in less than sec.
But it is known that electrons keep moving outside the nucleus.
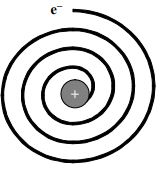
2. Discrete lines in atomic spectra: Experimentally, the atomic spectra consist of
discrete lines. If the electron loses energy continuously, the atomic spectra should be a continuous band. Rutherford could not explain this.
4.9. BOHR’S ATOMIC MODEL
In 1913, Neils’ Bohr presented a model of the atom, called Bohr model. This model was the first in the series of modern concept which explains many properties of an atom. It is based on certain assumptions (usually called postulates) which we shall discuss now.
Important Postulates
i) Circular Orbits:
An atom consists of a dense nucleus situated at the centre with the electron revolving round in circular paths called circular orbits.
ii) Stationary Orbits:
As long as an electron is revolving in an orbit it neither loses nor gains energy. Hence these orbits are called stationary orbits or stationary states.
iii) Energies of the Orbits:
Each stationary state is associated with a definite amount of energy and it is also known as energy l e v e l s. The greater the distance of the energy level from the nucleus, the more the energy associated with it. The different energy levels are numbered as 1, 2, 3, 4, (from nucleus onwards) or K, L, M, N etc.
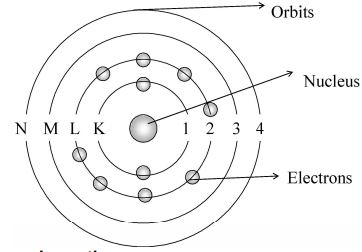
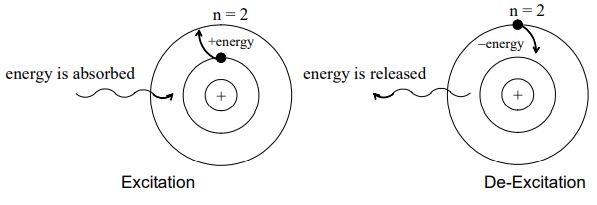
iv) Energy emissions or absorption:
Ordinarily an electron continues to move in a particular stationary state without losing energy. Such a stable state of the atom is called as ground state or normal state. If energy is supplied to an electron, it may jump (excite) instantaneously from lower energy (say 1) to higher energy level (say 2, 3, 4, etc.) by absorbing one quantum of energy. This new state of electron is called as excited state. On the other hand, energy is emitted when an electron jumps from higher orbit to lower orbit. This is called de-excitation.
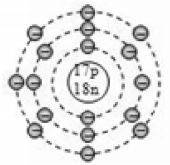
4.10.ATOMIC NUMBER AND MASS NUMBER
The atomic number, or the proton number, of an element is the number of protons present in the nucleus of an atom of the element.
The sum of the numbers of protons and neutrons in an atom is known as the mass number of the atom. You also know that an atom is electrically neutral and so it has the same number of electrons as protons. So, in a neutral atom, the number of electrons is equal to the number of protons (Z) and the number of neutrons = A – Z.
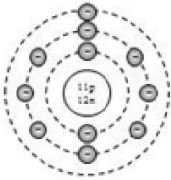
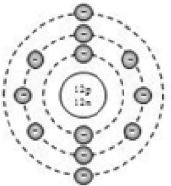
The numbers of fundamental particles in atoms with atomic numbers 1 to 20 are given in the following table
|
Element |
Symbol |
Atomic number (Z) |
Mass number (A) |
No. of electrons |
No. of protons |
No. of neutrons |
|
Hydrogen |
H |
1 |
1 |
1 |
1 |
0 |
|
Helium |
He |
2 |
4 |
2 |
2 |
2 |
|
Lithium |
Li |
3 |
7 |
3 |
3 |
4 |
|
Beryllium |
Be |
4 |
9 |
4 |
4 |
5 |
|
Boron |
B |
5 |
11 |
5 |
5 |
6 |
|
Carbon |
C |
6 |
12 |
6 |
6 |
6 |
|
Nitrogen |
N |
7 |
14 |
7 |
7 |
7 |
|
Oxygen |
O |
8 |
16 |
8 |
8 |
8 |
|
Fluorine |
F |
9 |
19 |
9 |
9 |
10 |
|
Neon |
Ne |
10 |
20 |
10 |
10 |
10 |
|
Sodium |
Na |
11 |
23 |
11 |
11 |
12 |
|
Magnesium |
Mg |
12 |
24 |
12 |
12 |
12 |
|
Aluminium |
Al |
13 |
27 |
13 |
13 |
14 |
|
Silicon |
Si |
14 |
28 |
14 |
14 |
14 |
|
Phosphorus |
P |
15 |
31 |
15 |
15 |
16 |
|
Sulphur |
S |
16 |
32 |
16 |
16 |
16 |
|
Chlorine |
Cl |
17 |
35 |
17 |
17 |
18 |
|
Argon |
Ar |
18 |
40 |
18 |
18 |
22 |
|
Potassium |
K |
19 |
39 |
19 |
19 |
20 |
|
Calcium |
Ca |
20 |
40 |
20 |
20 |
20 |
Nuclide symbol
The nuclide symbol of an atom is the symbol of the element with its atomic number as the subscript and mass number as the superscript, which are set to the left of the symbol of the element.
The nuclide symbol is expressed as
For example, the nuclide symbol represents a chlorine atom, whose atomic number is 17 and mass number is 35.
You can immediately guess that there are 17 electrons, 17 protons and 35 – 17 (= 18) neutrons in the atom.
4.11.ELECTRONIC CONFIGURATION
The arrangement of electrons in the different shells of an atom is called electronic configuration. The electron shells of an atom are not filled arbitrarily. The number of electrons in a shell follows a set of rules called the Bohr-Bury rules.
Bohr-Bury Rules
Of the various Bohr-Bury rules, we need to know the following in order to write the electronic configuration of elements up to calcium (Z = 20).
1. The maximum number of electrons that can be accommodated in a shell is given by
, where n is the orbit number or shell number.
For example, n = 1 denotes the first (K) shell, and so on. The K, L, M, N,… shells can accommodate a maximum of 2, 8,18, 32,… electrons respectively.
2. The outermost shell of an atom cannot contain more than 8 electrons in any case. A new shell is formed as soon as the outermost shell attains 8 electrons.
3. The penultimate shell (last but one) cannot have more than 18 electrons.
Applying Bohr-Bury rules
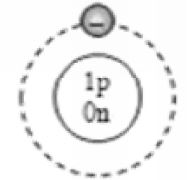
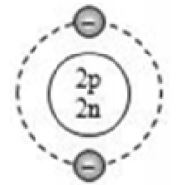
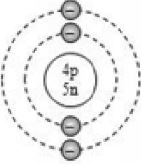
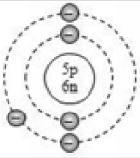
By now, it should be clear to you that the only electron in a hydrogen atom () occupies the K shell, and so do the two electrons in a helium atom (). As the K shell cannot have more than two electrons (Rule 1), the third electron of the lithium atom () must go to the next shell, i.e., the L shell. So, the arrangement of electrons in a lithium atom can be shown as However, there is a convention that the names of the shells are not mentioned in electronic configuration. The order in which the numbers of electrons are mentioned indicate the order of the shell, i.e., K, L, M, N,… respectively.
4.12.GEOMETRIC REPRESENTATION
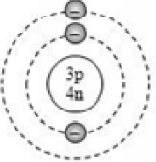
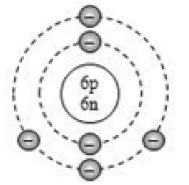
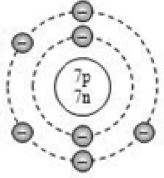
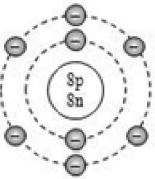
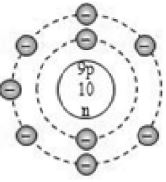
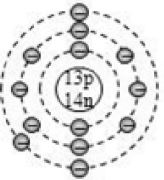
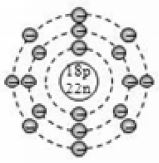
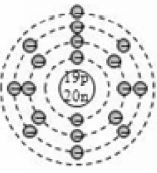
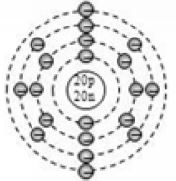
This representation helps us to know the number of electrons present in each orbit round the nucleus. In this representation, the nucleus is shown at the centre with the no. of protons and neutrons it contains. The orbits are shown in form of circles round the nucleus. The circle nearest to nucleus represents first orbit. The next immediate circle represents the second one and so on.
Based on electronic configuration, the electrons are placed in the respective orbits. The electrons are shown with small circles (). Now, let’s see the geometric representation of oxygen .
Mass number of oxygen (A) = 16 ;
Atomic number of oxygen (Z) = 8
Number of protons = Z = 8
Number of electrons = No. of protons = 8
Number of neutrons = A – Z = 16 – 8 = 8
And the electrons in the first shell = 2 [K-shell]
Remaining electrons in second shell = (8 – 2) = 6 [L – shell]
So, the geometric structure of oxygen atom is:
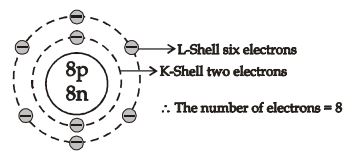
|
Element
|
No.of protons |
No.of electrons |
No. of neutrons |
Geometric representation of atomic structure |
|
Hydrogen |
1 |
1 |
|
|
|
Helium |
2 |
2 |
|
|
|
Lithium () |
3 |
3 |
|
|
|
Beryllium |
4 |
4 |
|
|
|
Boron |
5 |
5 |
|
|
|
Carbon |
6 |
6 |
|
|
|
Nitrogen |
7 |
7 |
|
|
|
Oxygen |
8 |
8 |
|
|
|
Fluorine |
9 |
9 |
|
|
|
Neon
Sodium |
10
11 |
10
11 |
|
|
|
Magnesium |
12 |
12 |
|
|
|
Aluminium |
13 |
13 |
|
|
|
Silicon |
14 |
14 |
|
|
|
Phosphorus |
15 |
15 |
|
|
|
Sulphur |
16 |
16 |
|
|
|
Chlorine |
17 |
17 |
|
|
|
Argon |
18 |
18 |
|
|
|
Potassium |
19 |
19 |
|
|
|
Calcium |
20 |
20 |
|
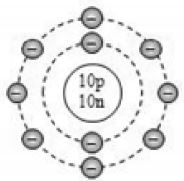
4.13.ISOTOPES
We know that atoms of given elements are similar. But later studies have proved that, for a few elements this similarity is confined to only number of protons and electrons and they differ in number of neutrons. Such atoms are called isotopes.
Isotopes are atoms of the same element having the same atomic number but different mass numbers. Isotopes of an element have same number of protons but differ in the number of neutrons in their nuclei.
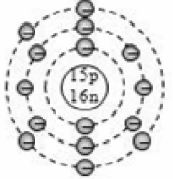
For example the two isotopes of chlorine atoms contain 17 protons. So the atomic number of all the chlorine atoms is 17. But they contain different number of neutrons i.e., one atom has 18 neutrons and another atom has 20 neutrons. Hence they have different mass number of 35 (17 + 18) and 37(17 + 20) the isotopes of chlorine can be written as and .
The complete composition of the two isotopes of chlorine is given below
|
Isotopes |
Protons |
Neutrons |
Electrons |
|
17 |
18 |
17 |
|
|
17 |
20 |
17 |
1. Isotopes of Hydrogen: The hydrogen element has three isotopes having the same atomic number of 1 but different mass numbers of 1, 2 and 3 respectively. The three isotopes of hydrogen can be represented as: The three isotopes of hydrogen, have been given special names like protium, deuterium and tritium respectively
i) Protium is the ordinary isotope of hydrogen with mass number 1. Protium is represented as Protium does not have a special symbol.
ii) Deuterium is a heavy isotope of hydrogen with mass number 2. Deuterium is represented as . The special symbol of deuterium is D.
iii) Tritium is a very heavy isotope of hydrogen with mass number 3. Tritium is represented as . The special symbol of tritium is T.
Thus, we can now say that hydrogen element has three isotopes: protium, deuterium and tritium, having the same atomic number of 1, but different mass number of 1, 2 and 3 respectively.
The complete composition of the three isotopes of hydrogen is given below:
|
Name |
Isotope |
Protons |
Neutrons |
Electrons |
|
Protium |
1 |
0 |
1 |
|
|
Deuterium |
1 |
1 |
1 |
|
|
Tritium |
1 |
2 |
1 |
It is clear from the above table that all the isotopes of hydrogen contain 1 proton and 1 electron each, but they contain 0, 1 and 2 neutrons respectively. It should be noted that the ordinary hydrogen isotope (protium) does not contain any neutron; the heavy hydrogen isotope (deuterium) contains 1 neutron; whereas the very heavy hydrogen isotope (tritium) contains 2 neutrons.
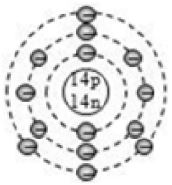
2. Isotopes of Carbon
The carbon element has three isotopes having the same atomic number of 6, but different mass numbers of 12, 13 and 14. The three isotopes of carbon can be written as:
These three isotopes of carbon contain 6 protons and 6 electrons each, but they contain an unequal number of neutrons. The C – 12 isotope contains 6 neutrons, C – 13 isotope contains 7 neutrons, whereas the C – 14 isotope contains 8 neutrons.
3. Isotopes of Oxygen
The oxygen element has three isotopes:
All the isotopes of oxygen have the same atomic number of 8, but they have different mass numbers (or atomic masses) of 16, 17 and 18 respectively.
Chemical properties of isotopes
The chemical properties of an atom of the element depend on the number of protons and electrons, but not on the number of neutrons. Since all the isotopes of an element contain the same number of protons and electrons, they have identical electronic configuration having the same number of valence electrons. Hence, isotopes have similar chemical properties.
For example, the two isotopes of chlorine, , both have the same number of 17 electrons in them due to which both of them have the same electronic configuration of 2, 8, 7. Since both the isotopes of chlorine, Cl – 35 and Cl – 37 have identical electronic configurations (having the same number of 7 valence electrons), they show identical chemical properties.
The physical properties of an element depend on the mass of the atom and its mass number. The isotopes of an element have different mass number due to difference in number of neutrons. Hence, isotopes differ in their physical properties.
For example, the two isotopes of chlorine,, have slightly different physical properties because they have slightly different atomic masses of 35 and 37 respectively
4.14.ISOBARS
Observe the following atoms of different elements:
In the above atoms, the atomic number is different, but their mass number is same. This means, number of protons in the atoms are different, but the total number of nucleons i.e., sum of protons and neutrons number is same. So these nuclides are called isobars.
Isobars are the nucleus with same mass number but different atomic number.
Isobars have different number of protons and neutrons, but the sum of protons and neutrons is same. Isobars differ in all except in mass number.
Some more examples of isobars:
i) ii)
4.15.ISOTONES
Let us understand by observing the number of neutrons in the following species:
|
Species |
Z |
A |
n = A -Z |
|
7 |
17 |
17 – 7 = 10 |
|
|
8 |
18 |
18 – 8 = 10 |
|
|
9 |
19 |
18 – 8 = 10 |
|
|
10 |
20 |
20 – 10 = 10 |
We observe that in the above set of species, the number of neutrons are same. Such species are called isotones.
Isotones are nuclides with different mass numbers and atomic numbers but have same number of neutrons.
Example:
4.16.ISOELECTRONIC SPECIES
How many electrons are present in the species of the following set?
|
Species |
Number of electrons |
|
7 + 3 = 10 |
|
|
8 + 2 = 10 |
|
|
9 + 1 = 10 |
|
|
10 + 0 = 10 |
|
|
11 – 1 = 10 |
|
|
12 – 2 = 10 |
We observe that, each of the species in the above set contain same number of electrons i.e., 10. Such set of species are called isoelectronic species.
The species of different elements or compounds containing the same number of electrons are called isoelectronic species.
Some more examples of isoelectronic species:
i) (each of the species contains 2 electrons)
ii) (each of the species contains 18 electrons)