3.1 INTRODUCTION
In our previous classes and chapters, we had the glimpses of microscopic part of the chemical world. We have known the details of the constituents of the microscope world electrons, protons, neutrons, atoms, molecules, ions etc. Now, there are some basic questions to be answered. How can we find the number of atoms / molecules / ions, etc. present in the given amount of substance?
What is the weight of the given number of atoms, molecules, ions, etc.? And many more such questions related to the reaction. All the above questions are very important in chemistry and for chemists. Practically, they form the foundation for many branches of chemistry and various other fields. Counting the number of atoms is impossible, weighing the number of atoms or molecules or ions is impractical and weighing the products formed in a reaction every time is a tedious job. To simplify all these intricate and challenging tasks, you need to comprehend the following chapter. This chapter helps you to study the relation between the microscopic the macroscopic worlds.
3.2 LAWS OF CHEMICAL COMBINATION
There are three important laws of chemical combination. These are:
1. Law of conservation of mass (or matter),
2. Law of constant proportions, and
3. Law of multiple proportions.
The laws of chemical combination are the experimental laws which have been formulated by scientists after performing a large number of experiments involving various types of chemical reactions. These experimental laws ultimately led to the idea of ‘atoms’ being the “smallest unit” of matter. In fact, the laws of chemical combination played a significant role in the development of Dalton’s atomic theory of matter.
Law of Conservation of Mass
In the 18th century, scientists noticed that if they carried out a chemical reaction in a closed container, then there was no change of mass. This preservation of mass in a chemical reaction led to the formulation of the law of conservation of mass (or law of conservation of matter). Law of conservation of mass was given by Lavoisier in 1774. The law of conservation of mass states that, matter is neither created nor destroyed in a chemical reaction. The substances which combine together (or react) in a chemical reaction are known as ‘reactants’ whereas the new substances formed (or produced) as a result of chemical reaction are called ‘products’.
The law of conservation of mass means that in a chemical reaction, the total mass of products is equal to the total mass of reactants. There is no change in mass during a chemical reaction. Since there is no gain or loss in mass in a chemical reaction, the mass remains conserved. Please note that the term ‘total mass’ of reactants and products includes solids, liquids and gases – including air – that are a part of the reaction.
Example
When calcium carbonate is heated, a chemical reaction takes place to form calcium oxide and carbon dioxide. It has been found by experiments that if 100 grams of calcium carbonate are decomposed completely then 56 grams of calcium oxide and 44 grams of carbon dioxide are formed. This can be written as:
In this example, calcium carbonate is the reactant and it has a mass of 100 g. Calcium oxide and carbon dioxide are the products and they have a total mass of 56 g + 44 g = 100 g. Now, since the total mass of products (100 g) is equal to the total mass of reactant (100 g), there is no change of mass during this chemical reaction. The mass remains the same or conserved. So, this example supports the law of conservation of mass.
Law of Constant Proportions
The law of constant proportions was given by Proust in 1779. He analysed the chemical composition (type of elements present and percentage of elements present) of a large number of compounds and came to the conclusion that the proportion of each element in a compound is constant (or fixed). Based on these observations, Proust formulated the law of constant proportions. The law of constant proportions states that, a chemical compound always consists of the same elements combined together in the same proportion by mass.
This law means that whatever be the source from which it is obtained (or the method by which it is prepared), a pure chemical compound is always made up of the same elements in the same mass percentage.
Example
Water is a compound which always consists of the same two elements, hydrogen and oxygen, combined together in the same constant proportion of 1: 8 by mass (1 part by mass of hydrogen and 8 parts by mass of oxygen). Let us discuss this in a little more detail.
We know that water is a compound. If we decompose 100 grams of pure water by passing electricity through it, then 11 grams of hydrogen and 89 grams of oxygen are obtained. Now, if we repeat this experiment by taking pure water from different sources (like river, sea, well, etc.), the same masses of hydrogen and oxygen elements are obtained in every case. This experiment shows that water always consists of the same two elements, hydrogen and oxygen, combined together in the same constant proportion of 11: 89 or 1: 8 by mass. And this is the law of constant proportions.
Law of Multiple Proportions
This law was discovered by John Dalton (1803). This law states that: When two elements combine with each other to form two or more than two compounds, the masses of one of the elements which combine with fixed mass of the other, bear a simple whole number ratio to one another.
Law of Reciprocal Proportions
This law was proposed by Richter (1792). Law of reciprocal proportions states that: When two elements combine separately with a fixed mass of a third element, then the ratio of their masses in which they do so is either same or some whole number multiple of the ratio in which they combine with each other.
Example
Let us consider three elements hydrogen, sulphur and oxygen. Hydrogen combines with oxygen to form whereas sulphur combines with it to form . Hydrogen and sulphur can also combine together to form .
The formation of these compounds is shown in figure.
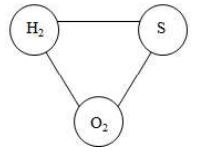
In , the ratio of masses of H and O is 2: 16.
In , the ratio of masses of S and O is 32: 32.
Therefore, the ratio of masses of H and S which combine with a fixed mass of oxygen (say 32 parts) will be 4: 32 i.e., 1: 8……… (1)
When H and S combine together, they form in which the ratio of masses of H and S is
2:32 i.e., 1: 16……… (2)
The two ratios (i) and (ii) are related to each other as: or 2: 1
i.e., they are whole number multiple of each other.
Thus, the ratio of masses of H and S which combines with a fixed mass of oxygen is a whole number multiple of the ratio in which H and S combine together. Sulphur and oxygen combine together to form also. This case can also be worked out in the same way as above and can be shown to follow the law of reciprocal proportions.
3.3 DALTONS ATOMIC THEORY
The laws of chemical combination were the experimental laws. The only logical explanation of the laws of chemical combination is that matter (say, elements) must be made up of minute “unit particles”, which take part in chemical combination in fixed whole numbers. These “unit particles” of matter were called atoms. So, in an attempt to explain the laws of chemical combination, Dalton put forward his atomic theory of matter.
The theory that “all matter is made up of very tiny invisible particles (atoms)” is called atomic theory of matter. Dalton put forward his atomic theory of matter in 1808. The various postulates (or assumptions) of Dalton’s atomic theory of matter are as follows:
1. All the matter is made up of very small particles called “atoms”.
2. Atoms cannot be divided.
3. Atoms can neither be created nor destroyed.
4. Atoms are of various kinds. There are as many kinds of atoms as are elements.
5. All the atoms of a given element are identical in every respect, having the same mass, size and chemical properties.
6. Atoms of different elements differ in mass, size and chemical properties.
7. Chemical combination between two (or more) elements consists in the joining together of atoms of these elements to form molecules of compounds.
8. The “number” and “kind” of atoms in a given compound is fixed.
9. During chemical combination, atoms of different elements combine in small whole numbers to form compounds.
10. Atoms of the same elements can combine in more than one ratio to form more than one compound.
Dalton’s atomic theory was based on the laws of chemical combination. For example, the postulate of Dalton’s atomic theory that “atoms can neither be created nor destroyed” was the result of law of conservation of mass given by Lavoisier. And the postulates of Dalton’s atomic theory that “the elements consist of atoms having fixed mass, and that the number and kind of atoms in a given compound is fixed,” came from the law of constant proportions given by Proust.
Dalton’s atomic theory was the first modern attempt to describe the behaviour of matter (or properties of matter) in terms of atoms. This theory was also used to explain the laws of chemical combination in terms of atoms. Dalton’s atomic theory provides a simple explanation for the laws of chemical combination.
(a) The postulates of Dalton’s atomic theory that “the elements consist of atoms and that atoms can neither be created nor destroyed” can be used to explain the law of conservation of mass.
(b) The postulates of Dalton’s atomic theory that “the elements consist of atoms having fixed mass, and that the number and kind of atoms of each element in a given compound is fixed” can be used to explain the law of constant proportions.
Explanation of the Law of Conservation of Mass
According to Dalton’s atomic theory, atoms can neither be created nor destroyed. Now, since an atom cannot be created or destroyed, therefore, the number of various types of atoms in the products of a chemical reaction is the same as the number of all those atoms in the reactants. The same number of various atoms in products and reactants will have the same mass. So, the total mass of products is equal to the total mass of reactants. The mass remains the same (or conserved) in a chemical reaction. And this is the law of conservation of mass. This explanation will become clearer from the following example of calcium carbonate.
Calcium carbonate () is made up of 1 calcium atom, 1 carbon atom and 3 oxygen atoms. The products of its decomposition, calcium oxide (CaO) and carbon dioxide (), taken together, also contain 1 calcium atom, 1 carbon atom and 3 oxygen atoms. Now, since the number of various types of atoms in the products (CaO and ) and reactant () remains the same, therefore, the mass of products and reactants also remains the same in this reaction. There is no change in mass during the decomposition of calcium carbonate to form calcium oxide and carbon dioxide. The mass remains conserved.
Explanation of the Law of Constant Proportions
According to Dalton’s atomic theory, every element consists of small particles called atoms, each having a fixed mass. It also says that atoms of different elements combine to form compounds, and that the “number” and “kind” of atoms of each element in a compound is fixed. Now, since the “number of atoms”, the “kind of atoms”, and the “mass of atoms” of each element in a given compound is fixed, therefore, a compound will always have the same elements combined together in the same proportion by mass. And this is the law of constant proportions. This explanation will become more clear from the following example of water.
According to Dalton’s atomic theory, hydrogen element consists of hydrogen atoms (H), and oxygen element consists of oxygen atoms (O). It also says that two hydrogen atoms always combine with one oxygen atom to form water molecule (). Since a water molecule always contains the same number of hydrogen and oxygen atoms, each atom having a fixed mass, therefore, the masses of hydrogen and oxygen elements in water will be in constant proportion. And this is the law of constant proportions.
Drawbacks of Dalton’s Atomic Theory
It is now known that some of the statements of Dalton’s atomic theory of matter are not exactly correct. Some of the drawbacks of the Dalton’s atomic theory of matter are given below:
1. One of the major drawbacks of Dalton’s atomic theory of matter is that atoms were thought to be indivisible (which cannot be divided). We now know that under special circumstances, atoms can be further divided into still smaller particles called electrons, protons and neutrons. So, atoms are themselves made up of three particles: electrons,
protons and neutrons.
2. Dalton’s atomic theory says that all the atoms of an element have exactly the same mass. It is, however, now known that atoms of the same element can have slightly different masses.
3. Dalton’s atomic theory said that atoms of different elements have different masses. It is, however, now known that even atoms of different elements can have the same mass.
3.4 ATOM
Atoms are the building blocks of all the matter around us. In chemistry, atom is defined as follows:
Atoms of most of the elements are very reactive and do not exist in the free state. They exist in combination with the atoms of the same element or another element. There are as many kinds of atoms as are elements. Atoms are very, very small in size. An idea of the extremely small size of atoms can be had from the fact that the tip of a needle contain atoms ( 1,000000000000000000000 atoms). The size of an atom is indicated by its radius which is called ‘atomic radius’ (radius of atom). Atomic radius is measured in ‘nanometres’ (which is a very, very small unit of measuring length). The symbol of a nanometre is nm.
Hydrogen atom is the smallest atom of all. The atomic radius of a hydrogen atom is 0.037 nanometre (or 0.037 nm). If we express the radius of a hydrogen atom in metres, it will be metre which means 0.000000000037 metre. If we try to compare it with the radius of a grain sand () and to an ant (), the size of the atom is very
very small. The mass of atoms is very small. For example, an atom hydrogen has a mass nearly . It is infact a dream to imagine how atoms look like. We must thank our scientists for their contribution in promoting scientific knowledge. They have developed sophisticated microscope known as Scanning Tunneling Microscope (STM) in 1981. With the help of this microscope, it has become possible to take the photographs of some atoms. In the adjacent figure, atoms of carbon are shown on the surface of carbon element.
Units of size: Angstrom
Shape: Atoms are assumed to have spherical shape.
3.5 BASIC STRUCTURE OF ATOM
The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms fit together with other atoms to make up matter. It takes a lot of atoms to make up anything. There are so many atoms in a single human body we won’t even try to write the number here. Suffice it to say that the number is trillions and trillions (and then some more). There are different kinds of atoms based on the number of electrons, protons, and neutrons each atom contains. Each different kind of atom makes up an element. There are 92 natural elements and up to 118 when you count in man-made elements.
Atoms last a long time, in most cases forever. They can change and undergo chemical reactions, sharing electrons with other atoms. But the nucleus is very hard to split, meaning most atoms are around for a long time.
Structure of the Atom
At the center of the atom is the nucleus. The nucleus is made up of the protons and neutrons. The electrons spin in orbits around the outside of the nucleus.
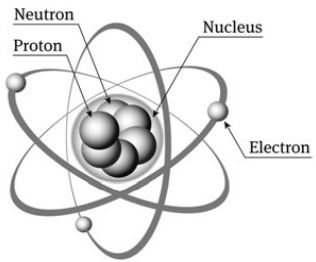
The Proton
The proton is a positively charged particle that is located at the center of the atom in the nucleus. The hydrogen atom is unique in that it only has a single proton and no neutron in its nucleus. The mass of one proton is taken as 1 a.m.u. (atomic mass unit). The charge on one proton is taken as unit positive charge.
It is the number of protons present within the nucleus, which distinguishes atoms of one element from the other elements.
For example, if the number of protons within the nucleus is only one, the atom of an element is hydrogen.
However, if nucleus has two protons, the atom is of an element is helium.
The Electron
The electron is a negatively charged particle that spins around the outside of the nucleus. Electrons spin so fast around
the nucleus, scientists can never be 100% sure where they are located, but scientists can make estimates of where electrons should be. If there are the same number of electrons and protons in an atom, then the atom is said to have a neutral charge.
Electrons are attracted to the nucleus by the positive charge of the protons. Electrons are much smaller than neutrons and protons. About 1800 times smaller!
The Neutron
The neutron doesn’t have any charge. The number of neutrons affects the mass and the radioactivity of the atom. The mass of a neutron is almost equal to the mass of a proton.
For the sake of simplicity, we regard the mass of one neutron equal to 1 a.m.u.
Note: a.m.u is called atomic mass unit. It is the unit of mass used to measure the mass of tinier particles like atoms, electrons, protons, etc.
Other (even smaller!) particles
Quark – The quark is a really small particle that makes up neutrons and protons. Quarks are nearly impossible to detect and it’s only recently that scientists figured out they existed. They were discovered in 1964 by Murray GellMann. There are 6 types of quarks: up, down, top, bottom, charm, and strange.
Neutrino – Neutrinos are formed by nuclear reactions. They are like electrons without any charge and are usually travelling at the speed of light. Trillions and trillions of neutrinos are emitted by the sun every second. Neutrinos pass right through most solids including humans!
3.6 SYMBOLS OF ELEMENTS
We have learnt in our previous classes, that any pure substance which cannot be broken down into two or more simpler substances by any chemical means is called an element.
Chemists have discovered around 118 elements so far. We also know that there are millions of substances present around us.
In fact, everything around us in the Universe is a combination of these hundred odd elements. Writing their names, using the names of the elements, is cumbersome.
Hence, there is a need to write the names of the elements in a short form. This short hand form representation of an element is called a symbol.
We use the symbols like &, and to represent ‘and’, ‘therefore’ and ‘ since’ respectively. Much the same way, in chemistry each element is denoted by a symbol.
Let us travel to know the evolution leading to the modern symbols.
Ancient Symbols
In their pursuit of finding a substance called the philosopher’s stone, which (it was believed) could change base metals into gold, alchemists in ancient times used a set of certain symbols to denote the elements known at that time. They did this to maintain secrecy of their works.
Later, John Dalton devised pictorial symbols to represent elements.
These two types of symbols are shown below.
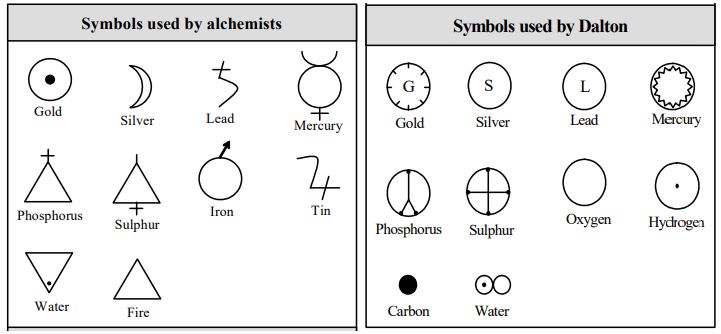
Alchemists considered ‘water and ‘fire’ as elements.
Modern view
In 1814, Baron Jons Jakob Berzelius suggested a simple system of representing elements with symbols.
Berzelius put forward some guidelines for representation of symbols:
1. For most of the elements, the first letter from the name of the element is taken as a symbol for that element. The letter is to be written in capital form. Example: Carbon (C), Nitrogen (N), etc.
2. In case where the first letter is already adopted, the symbol is written by associating another prominent letter (in small form) with the first letter (capital) of its name.
Example: Calcium (Ca), Barium (Ba), Chlorine (Cl) etc.
Note: In most of the cases, this prominent letter is the second letter.
3. The symbols for some elements are derived from their Latin names.
Example: The Latin name of Sodium is Natrium. So, its symbol is Na.
The Latin name of Gold is Aurum. So its symbol is Au.
Symbols of elements based on various criteria:
I) Symbols based on first letter
|
S.NO |
Name |
Symbol |
|
1. |
Hydrogen |
H |
|
2. |
Carbon |
C |
|
3. |
Nitrogen |
N |
|
4. |
Oxygen |
O |
|
5. |
Fluorine |
F |
|
6. |
Sulphur |
S |
|
7. |
Boron |
B |
|
8. |
Phosphorus |
P |
|
9. |
Iodine |
I |
II) Symbols based on two letters
|
S.NO |
Element |
Symbol |
|
1. |
Calcium |
Ca |
|
2. |
Cadmium |
Cd |
|
3. |
Chlorine |
Cl |
|
4. |
Platinum |
Pt |
|
5. |
Palladium |
Pd |
|
6. |
Barium |
Ba |
|
7. |
Bromine |
Br |
|
8. |
Phosphorus |
Be |
III) Symbols based on Latin name
|
S.NO |
Element |
Latin Name |
Symbol |
|
1. |
Sodium |
Natrium |
Na |
|
2. |
Potassium |
Kalium |
K |
|
3. |
Iron |
Ferrum |
Fe |
|
4. |
Copper |
Cuprum |
Cu |
|
5. |
Silver |
Argentum |
Ag |
|
6. |
Gold |
Aurum |
Au |
|
7. |
Mercury |
Hydrargyrum |
Hg |
|
8. |
Lead |
Plumbum |
Pb |
|
9. |
Tin |
Stannum |
Sn |
|
10. |
Antimony |
Stibium |
Sb |
|
11. |
Tungsten |
Wolfram |
W |
IV) Symbols based on scientist’s name
|
Element |
Scientist’s |
NameSymbol |
|
Curium |
Madam Curie |
Cm |
|
Einsteinium |
Albert Einstein |
Es |
|
Fermium |
Enrico Fermi |
Fm |
|
Nobelium |
Alfred Nobel |
No |
|
Mandelevium |
Mendeleev |
Md |
V) Symbols based on names of the countries or Laboratories
|
Element |
Country or laboratory |
Symbol |
|
Berkelium |
City of Berkeley |
Bk |
|
Californium |
University of California |
Cf |
|
Polonium |
Poland |
Po |
|
Americium |
America |
Am |
|
Ruthenium |
Russia |
Ru |
|
Germanium |
Germany |
Ge |
VI) Symbols based on names of the Planets
|
Element |
Planet Names |
Symbol |
|
Uranium |
Uranus |
U |
|
Neptunium |
Neptune |
Np |
|
Plutonium |
Pluto |
Pu |
Significance of a symbol
A symbol of an element has a qualitative and quantitative significance.
a) Qualitative significance: Qualitatively, it represents the name of the element.
For example O stands for oxygen, N stands for nitrogen.
b) Quantitative significance: Quantitatively it represents.
i) One atom of an element.
ii) The number of parts by weight (Atomic weight) of an element.
iii) One gram atom of an element.
For example the symbol O represents
i) One atom of oxygen.
ii) 16 parts by weight of oxygen.
iii) One gram atom of oxygen i.e., 16 grams.
|
AN Element |
Symbol |
AN |
Element |
Symbol |
AN |
Element |
Symbol |
|
1 Hydrogen |
H |
41 |
Niobium |
Nb |
81 |
Thallium |
TI |
|
2 Helium |
He |
42 |
Molybdenum |
Mo |
82 |
Lead |
Pb |
|
3 Lithium |
Li |
43 |
Technetium |
Tc |
83 |
Bismuth |
Bi |
|
4 Beryllium |
Be |
44 |
Ruthenium |
Ru |
84 |
Polonium |
Po |
|
5 Boron |
B |
45 |
Rhodium |
Rh |
85 |
Astatine |
At |
|
6 Carbon |
C |
46 |
Palladium |
Pd |
86 |
Radon |
Rn |
|
7 Nitrogen |
N |
47 |
Silver |
Ag |
87 |
Francium |
Ft |
|
8 Oxygen |
O |
48 |
Cadmium |
Cd |
88 |
Radium |
Ra |
|
9 Fluorine |
F |
49 |
Indium |
In |
89 |
Actinium |
Ac |
|
10 Neon |
Ne |
50 |
Tin |
Sn |
90 |
Thorium |
Th |
|
11 Sodium |
Na |
51 |
Antimony |
Sb |
91 |
Protactinium |
Pa |
|
12 Magnesium |
Mg |
52 |
Tellurium |
Te |
92 |
Uranium |
U |
|
13 Aluminum |
Al |
53 |
Iodine |
I |
93 |
Neptunium |
Np |
|
14 Silicon |
Si |
54 |
Xenon |
Xe |
94 |
Plutonium |
Pu |
|
15 Phosphorus |
P |
55 |
Cesium |
Cs |
95 |
Americium |
Am |
|
16 Sulfur |
S |
56 |
Barium |
Ba |
96 |
Curium |
Cm |
|
17 Chlorine |
Cl |
57 |
Lanthanum |
La |
97 |
Berkelium |
Bk |
|
18 Argon |
Ar |
58 |
Cerium |
Ce |
98 |
Californium |
Cf |
|
19 Potassium |
K |
59 |
Praseodymium |
Pr |
99 |
Einsteinium |
Es |
|
20 Calcium |
Ca |
60 |
Neodymium |
Nd |
100 |
Fermium |
Fm |
|
21 Scandium |
Sc |
61 |
Promethium |
Pm |
101 |
Mendelevium |
Md |
|
22 Titanium |
Ti |
62 |
Samarium |
Sm |
102 |
Nobelium |
No |
|
23 Vanadium |
V |
63 |
Europium |
Eu |
103 |
Lawrencium |
Lr |
|
24 Chromium |
Cr |
64 |
Gadolinium |
Gd |
104 |
Rutherfordium |
Rf |
|
25 Manganese |
Mn |
65 |
Terbium |
Tb |
105 |
Dubnium |
Db |
|
26 Iron |
Fe |
66 |
Dysprosium |
Dy |
106 |
Seaborgium |
Sg |
|
27 Cobalt |
Co |
67 |
Holmium |
Ho |
107 |
Bohrium |
Bh |
|
28 Nickel |
Ni |
68 |
Erbium |
Er |
108 |
Hassium |
Hs |
|
29 Copper |
Cu |
69 |
Thulium |
Tm |
109 |
Meitnerium |
Mt |
|
30 Zinc |
Zn |
70 |
Ytterbium |
Yb |
110 |
Darmstadtium |
Ds |
|
31 Gallium |
Ga |
71 |
Lutetium |
Lu |
111 |
Roentgenium |
Rg |
|
32 Germanium |
Ge |
72 |
Hafnium |
Hf |
112 |
Copernicium |
Cn |
|
33 Arsenic |
As |
73 |
Tantalum |
Ta |
113 |
Ununtrium |
Uut |
|
34 Selenium |
Se |
74 |
Tungsten |
W |
114 |
Flerovium |
Fi |
|
35 Bromine |
Br |
75 |
Rhenium |
Re |
115 |
Ununpentium |
Uup |
|
36 Krypton |
Kr |
76 |
Osmium |
Os |
116 |
Livermorium |
Lv |
|
37 Rubidium |
Rb |
77 |
Iridium |
Ir |
117 |
Ununseptium |
Uus |
|
38 Strontium |
Sr |
78 |
Platinum |
Pt |
118 |
Ununoctium |
Uuo |
|
39 Yttrium |
Y |
79 |
Gold |
Au |
|
|
|
|
40 Zirconium |
Zr |
80 |
Mercury |
Hg |
AN = Atomic Number |
||
3.7 ATOMIC NUMBER
Though every human being is apparently similar to fellow human beings, yet certain unique features are exclusively associated with an individual. No two individuals can have these features in common. Do you know what these unique features are? They are the DNA and fingerprints. Similarly, elements too have a unique feature. No two elements have
this unique feature. Can you name this unique feature?
This unique feature is atomic number. What DNA and fingerprints are to human beings, the atomic number is to elements.
The atomic number of an element is defined as the number of electrons present in the neutral atom of the element. It is represented by the symbol ‘Z’.
If the atomic number of an element is equal to ‘X’, it means that the number of electrons in a neutral atom of that element are equal to ‘X’ each (or) the number of protons in the nucleus of an atom of that element are equal to ‘X’.
Eg: The atomic number of ‘Phosphorus’ is 15. This means that the number of protons in the nucleus of the Phosphorus atom are equal to 15 and also the number of electrons in the neutral atom of Phosphorus are equal to 15.
3.8 MASS NUMBER
We know that an atom consists of protons, neutrons and electrons and the mass of the electrons is negligible. Therefore, the real mass of an atom is determined by the total number of protons and neutrons it contains.
The total number of protons and neutrons present in the atom of an element is known as its mass number. It is
represented by ‘A’.
Mass number (A) = Number of protons + Number of neutrons (n)
Mass number (A) = Atomic number (Z) + Number of neutrons (n) A = Z + n
Number of Neutrons in Nucleus:
We know, A = Z + n n = A – Z
Number of neutrons = Mass number – Atomic number
|
Name |
Atomic number (Z) |
Mass number(A) |
No. of neutrons (A-Z) |
|
Hydrogen |
1 |
1 |
0 |
|
Helium |
2 |
4 |
2 |
|
Lithium |
3 |
7 |
4 |
|
Beryllium |
4 |
9 |
5 |
|
Boron |
5 |
11 |
6 |
|
Carbon |
6 |
12 |
6 |
|
Nitrogen |
7 |
14 |
7 |
|
Oxygen |
8 |
16 |
8 |
|
Fluorine |
9 |
19 |
10 |
|
Neon |
10 |
20 |
10 |
|
Sodium |
11 |
23 |
12 |
|
Magnesium |
12 |
24 |
12 |
|
Aluminium |
13 |
27 |
14 |
|
Silicon |
14 |
28 |
14 |
|
Phosphorus |
15 |
31 |
16 |
|
Sulphur |
16 |
32 |
16 |
|
Chlorine |
17 |
35 |
18 |
|
Argon |
18 |
40 |
22 |
|
Potassium |
19 |
39 |
20 |
|
Calcium |
20 |
400 |
20 |
3.9 COMPLETE REPRESENTATION OF AN ELEMENT
We know that symbol is that shorthand form of representing an element. All the details of an element cannot be conveyed by a symbol. Hence, there is a need for representation that conveys all the possible details of an element.
One such representation of an element is as follows:
The atomic number (Z) is written on the lower left side of the symbol. The mass number (A) is written on either the upper left side or upper right side of the symbol (x).
For example: Oxygen is represented as This means that:
i) The element is oxygen
ii) Its atomic number is 8.
iii) Its mass number is 16.
iv) Number of protons in it are 8.
v) Number of electrons in it are 8.
vi) Number of neutrons in it are 16 – 8 = 8
Hence, we can draw the maximum details of an element, if its complete representation is known.
3.10 MOLECULES
A molecule is a group of two or more atoms that stick together. Molecules (MOLL-uh-cyools) are so small that nobody can see them, except with an electron microscope. Pretty much everything on Earth and other planets is made of molecules, and so is some of the dust in space.
When atoms of different types of elements join together, they make molecules called compounds. Water consists of compound molecules made up of 2 hydrogen atoms and 1 oxygen atom. This is why it’s called . Water will always have 2 times the number of hydrogen atoms as oxygen atoms.
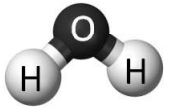
Water Molecule showing 1 Oxygen atom and 2 hydrogen atoms
There are only just over 100 types of atoms, but there are millions and millions of different types of substances out there. This is because they are all made up of different types of molecules. Molecules are not only made up of different types of atoms but also different ratios. Like in THE WATER example above, a water molecule has 2 hydrogen atoms and 1 oxygen atom. This is written as . Other examples are carbon dioxide (), sodium chloride (NaCl), and sugar or glucose ().Some formulas can get quite long and complex.
Let’s look at the molecule for sugar (glucose):
carbon atoms, hydrogen atoms, oxygen atom
It takes these specific atoms in these specific numbers to make up a sugar molecule.
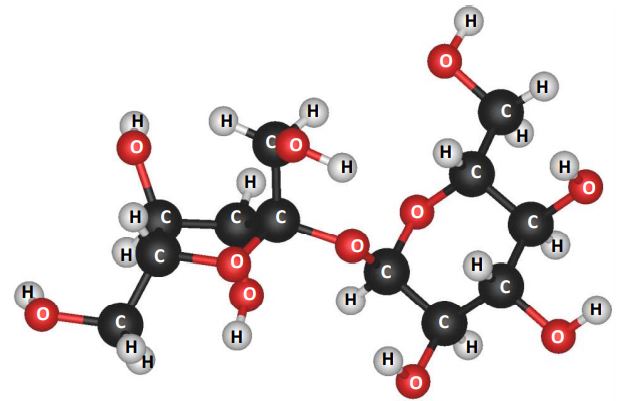
It was the Italian chemist, Amedo Avogadro (1776-1856 A.D), who introduced the word ‘molecule’
The smallest particle of an element or a compound that can exist independently is called a molecule.
Bonds
Molecules and compounds are held together by forces called chemical bonds. There are two main types of bonds that hold most compounds together: covalent bonds and ionic bonds. Some compounds can have both types of bonds.
Both main types of bonds involve electrons. Electrons orbit atoms in shells. They want these shells to be full. When they aren’t they will try to bond with other atoms to try and fill their shells.
Covalent Bonds – Covalent bonds share electrons between atoms. This happens when it works out for atoms to share their electrons in order to fill their outer shells.
Ionic Bonds – Ionic bonds form when one electron is donated to another. This happens when one atom gives up an electron to another in order to form a balance and, therefore, a molecule or compound.
Fun Facts about Molecules
· Oxygen gas normally is the molecule , but it can also be which we call ozone.
· 66% of the mass of the human body is made up of oxygen atoms.
· Molecules can have different shapes. Some are long spirals while others may be pyramid shaped.
· Organic compounds are compounds that contain carbon.
· A perfect diamond is a single molecule made of carbon atoms.
· DNA is a super long molecule that has information uniquely describing every human being.
History of Molecules
The first molecules formed about 300,000 years after the Big Bang, or just under 15 billion years ago. They were the smallest kind of molecule – two hydrogen atoms joined together. As time went on, and supernovas from exploding stars shot out different kinds of atoms, different kinds of molecules formed and floated around in space. Because most of the
atoms in space were hydrogen atoms, many of these molecules combined hydrogen with another kind of atom. So hydrogen combined with oxygen to make water molecules. Hydrogen combined with carbon to make hydrocarbons (what living things are built out of). Even before there were any planets, water and hydrocarbons were floating around in space on their own. Other molecules were made of heavier atoms, like silicon or gold. Still out in space, some hydrocarbons got together and formed bigger molecules called amino acids.
When the planets did form, the ones that were further away from stars, like Jupiter and Neptune, were made mostly of lighter molecules like water and hydrocarbons. Earth, which formed about 4.5 billion years ago, was closer to the Sun, and made mostly of heavier molecules like iron. A lot of silicon and other minerals also got to Earth, where they make
up the rocks of the Earth’s crust.
We’re not sure how or when the water, hydrocarbons, and amino acids got to Earth. But once they were on Earth, the amino acids got together to make more and more complicated molecules – maybe first ribonucleic acids, then proteins. The biggest organic molecule today is DNA. Each molecule of DNA has more than two billion carbon atoms in it (plus a lot of other kinds of atoms too).
Types of Molecules
Molecules like, are formed by the combination of atoms of the same element and molecules like are formed by the combination of atoms of different elements. Thus, molecules can be categorised as follows:
Homogeneous molecules: Molecules that are formed by the combination of atoms of the same element are called homogeneous molecules.
Eg:
Heterogeneous molecules: Molecules that are formed by the combination of atoms of different elements are called heterogeneous molecules.
Eg:
Atomicity
The number of atoms present in a homogeneous molecule is known as Atomicity
|
Molecule |
No. of atoms |
No. of atoms |
|
He, Ne, Ar, Kr, Xe |
1 |
Monoatomic |
|
2 |
Diatomic |
|
|
3 |
Triatomic |
|
|
4 |
Tetra atomic |
|
|
More than 4 |
Poly atomic |
Highest atomicity: The element with the highest atomicity is Carbon. Carbon has the property of self-linkage, known as Catenation. A group of Carbon atoms can interlink in a network, resulting in the formation of a spherical football-like structure. The most famous one is C–60 molecule called Buckminster fullerene. This C–60 molecule looks like a soccer ball and is called “Bucky ball”. Similar fullerenes like may also be formed.
3.11 IONS
So far we have discussed atoms and molecules. The atoms are represented by symbols. Molecules are also shown by certain symbols but these are known as formulae. We shall discuss them a little later. are the formulae of the molecules that we have mentioned. These molecules are neutral in nature. This means that they do not
have any charge. But in some compounds, certain charged species are present. These are known as ions. Let’s discuss about them now.
An ion is a positively or negatively charged atom (or group of atoms).
Removal of electron from an atom or addition of electron to the atom leads to the formation of a charged particle called ion.
Types of ions
Based on the mode of formation of ion, they are classified into electron negative and electropositive ions. There are two types of ions: Electronegative ion (Anions) and Electropositive (cations).
Electronegative ion(Anion): The atom that gains electron(s) and forms a negative ion is called an anion. Anion bears the same charge as the number of electrons gained.
Electropositive(Cation): The atom that loses electron(s) and forms a positive ion is called a cation. Cation bears the same charge as the number of electrons lost.
Based on the kind of atom that form an ion, they are classified into simple ion and compound ion.
Simple ions: Ions which are formed from single atoms are called simple ions. Simple ions are also known as monoatomic ions.
Compound ions: Ions which are formed from groups of joined atoms are called compound ions (or polyatomic ions).
3.12 VALENCY
Old Concept
Valency of an element may be defined as the number of hydrogen atoms or the number of chlorine atoms or double the number of oxygen atoms with which one atom of that element combines.
Examples:
i) One atom of nitrogen combines with three atoms of hydrogen to form . Thus, the valency of nitrogen is 3.
ii) is formed when one atom of aluminium combines with three atoms of chlorine. Thus, the valency of Al is 3.
iii) ZnO is formed when one atom of Zn combines with one atom of oxygen. Thus double the number of oxygen atoms is the valency of Zn. The valency of zinc is 2.
Now let us consider a few compounds of C and H, viz:
i) Methane ()
ii) Ethane ()
iii) Ethene () and iv) Ethyne ().
i) In methane (), the valency of carbon is 4.
ii) In , the valency of carbon is 3, as three hydrogen atoms combine with one carbon atom.
iii) In , the valency of carbon is 2.
iii) In , the valency of carbon is 1.
In the above compounds, we see that the valency of carbon is 4, 3, 2 and 1. But we know that the valency of carbon is always 4. Thus, the above definition is not perfect.
Modern Concept
According to the modern concept, the valency may be defined as the number of electrons lost, gained or shared with one atom of the element in order to acquire the stable configuration of the nearest inert gas element.
Variable Valency: Observe the valency of the elements in the following compounds:
|
Element |
Compound formed |
Valency |
|
Copper |
||
|
Iron |
||
|
Mercury |
From the table, we can see that the atom of same element combine differently with atoms of other element, to form different compounds. By applying modern concept of valency, different values of valencies of same element are obtained. Each of the above elements are exhibiting more than one valency. In such case, we say that, an element exhibits variable valency.
Reason for variable valency:
Let us understand it from the behaviour of copper during the formation of Copper in :
The copper in is in form with valency of 1. The is formed by the loss of one electron from the outermost shell of copper as shown below.
Copper in CuO:
The copper in CuO is in form with valency of 2. The is formed due to the lose of two electrons from copper. One electron is lost from the outer most shell and the other from inner shell.
Hence, bivalency of copper is due to the loss of electron from its inner shell. Therefore, we can say that the participation of electrons present in the inner shells (penultimate shell) during reaction, is responsible for variable valency.
Naming an element with variable valencies:
If an element exhibits two different valencies, the suffix ‘–ous’ is attached to the lowest valency ion/radical and the suffix ‘ic’ is attached to the highest valency ion/radical
Examples: The lowest valency exhibited by iron is 2 and highest valency is 3. Therefore,
i) is named as ferrous and is called Ferrous chloride.
ii) is named as ferric and is called Ferric chloride.
|
Element |
Valency |
Compound formed |
Valency Chart
|
Ion |
Symbol |
Charge |
|
Hydrogen Lithium Sodium Potassium Rubidium Copper Silver Gold Mercury
Ammonium Phosphonium |
Cuprous or Copper (I) Aurous or gold (I) Mercurous or Mercury (I) |
+ 1 |
II. MONOVALENT ELECTRONEGATIVE IONS
|
Ion |
Symbol |
Charge |
|
Acetate Formate Bicarbonate (or) Hydrogen carbonate Bisulphate Bisulphite (or) Hydrogen sulphite Hydrogen Sulphide (or) Bisulphide Fluoride |
– 1 |
Such radicals are called _______
|
Ion |
Symbol |
Charge |
|
Chloride Bromide Iodide Hypochlorite Hypobromite Hypoiodite Chlorite Bromite Iodite Chlorate Bromate Iodate Perchlorate Perbromate Periodate Nitrite Nitrate Hypophophite Dihydrogen phosphate or Cyanide Cyanate Thiocyanate Permanganate Hydride Hydroxide Superoxide Hydrogen peroxide |
Sulphocyanide |
– 1 |
III. Divalent Electropositive Ions
|
Ion |
Symbol |
Charge |
|
Beryllium Magnesium Calcium Strontium Barium Radium Copper Mercury Iron Chromium Cobalt Nickel Manganese Cadmium Zinc Lead Tin |
+ 2 |
IV. Divalent Electronegative Ions
|
Ion |
Symbol |
Charge |
|
Carbonate Chromate Dichromate Manganate Sulphide Sulphite Thiosulphate Tetrathionate Sulphate Perdisulphate Oxide Peroxide Stannite Stannate Silicate
Oxalate
Molybdate Plumbite Plumbate Pyroantimonate Tetraborate Mono hydrogen phosphite Hydrophosphate (monohydrogen phosphate) Zincate Tartarate Tungstate Titanate Fluorosilicate |
|
– 2 |
V. Trivalent Electropositive Ions
|
Ion |
Symbol |
Charge |
|
Iron Manganese Aluminium Gold Antimony Arsenic Chromium Cobalt Boron |
+ 3
|
VI. Trivalent Electronegative Ions
|
Ion |
Symbol |
Charge |
|
Aluminate Arsenite Arsenate Arsenide Boride Borate Nitride Phosphide Phosphite Phosphate Ferricyanide Cobaltnitrite Oxychloride Oxybromide Oxyiodide |
– 3
|
VII. Tetravalent Electropositive Ions
|
Ion |
Symbol |
Charge |
|
Platinum Lead Tin |
+ 4 |
VIII. TETRAVALENT ELECTRONEGATIVE IONS
|
Ion |
Symbol |
Charge |
|
Ferrocyanide Pyrophosphate Pyroarsenate Pyroantimonate Carbide |
– 4 |
IX. PENTAVALENT ELECTROPOSITIVE IONS
|
Ion |
Symbol |
Charge |
|
Arsenic Antimony |
|
3.13 CHEMICAL FORMULA
The chemical formula of a compound represents the composition of a molecule of the compound in terms of the symbols of the elements present in it.
In other words, the formula of a compound tells us ‘the kind of atoms’ as well as ‘the number of atoms’ of various elements present in one molcule of the compound. In the chemical formula of a compound, the elements present are represented by their symbols and the number of atoms of each element are indicated by writing the digits 2, 3, 4, etc.,
as subscripts (lower figures) on the right hand side bottom of the symbol. For example, water is a compound made up of 2 atoms of hydrogen element and 1 atom of oxygen element, so the formula of water is written as H2O. In the formula , the subscript 2 indicates 2 atoms of hydrogen. In the formula of water, oxygen O is written without a subscript and it indicates 1 atom of oxygen. Please note that 1 atom of oxygen is written just as O and not as O1.
Another point to be noted is that in the case of molecular compounds, the chemical formula represents the composition of molecule which makes up the compound. But in the case of ionic compounds, the chemical formula represents the simplest ratio of ions present in the compound. We will now discuss the naming of simple ionic compounds and simple molecular compounds.
Writing of formulae of molecular compounds – Crisscross Method
If we know the valencies of elements, then we can work out the formulae of their compounds by balancing the valencies of the different atoms which occur in the compound. Let’s understand the steps for writing the chemical formula of a molecular compound. This is known as Crisscross method.
Step-1: We first write the symbols of the elements which form the compound.
Step-2: Below the symbol of each element, we write down its valency.
Step-3: Finally, we cross-over the valencies of the combining atoms. That is, with first atom we write the valency of second atom (as a subscript); and with the second atom we write the valency of first atom (as subscript). This will give us the required formula.
This method of writing the formulae will become more clear from the following example. Let us work out the formula of hydrogen sulphide by this method.
Step-1: Hydrogen sulphide compound is made up of hydrogen and sulphur elements. So, first we write down the symbols of hydrogen and sulphur.
Step-2: The valency of hydrogen is 1 and the valency of sulphur is 2. So, below the symbol H we write 1 and below the symbol S we write 2:
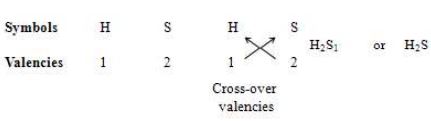
Step-3: We now cross-over the valencies of H and S atoms. With H atom we write the valency of S (which is 2) as a subscript so that it becomes . With S atom we write the valency of H (which is 1) as a subscript so that it becomes S,. Now, joining together and S, the formula of hydrogen sulphide becomes , or (This is because we do not write the subscript 1 with an atom in a formula).
Writing of Formulae of Ionic Compounds
We will now describe the method of writing the chemical formulae of ionic compounds. First of all we write down the name of compound whose formula is required. Then we write down the symbols of its ions. As a convention, the cation (positive ion) is written on the left hand side and the anion (negative ion) is written on the right hand side. The number of cations and anions is adjusted in such a way that the total number of positive valencies of cations becomes equal to the total number of negative valencies of anions. In other words, the number of cations and anions is adjusted in such a way that the total number of positive charges of cations becomes equal to the total number of negative charges of anions (because an ionic compound is electrically neutral, having no over-all charge). The number of cations required (2, 3, 4, etc.) is written on the right side bottom of the symbol of cation but without showing the charge on the cation. For example, if 2 aluminium ions are required to balance the charges in a compound, then is written as Al2 in the formula of the compound. Similarly, the number of anions required (2, 3, 4, etc.) is written on the right side bottom of the symbol of the anion but without showing the charge on the anion. For example, if three sulphate ions are needed to balance the charges in a compound, then is written as in the formula of the compound. Please note that if only 1 cation or anion is required to write the formula of a compound
then the digit 1 is not written with the symbol of the ion. For example, if 1 sodium ion is needed to write the formula of a compound, then we write just Na for it (and not ). Similarly, if one chloride ion is required, then we write just Cl for it (and not ). Another point to be noted is that the final formula of an ionic compound is written
without showing the charges on the ions involved in it.
Example for writing the formula of sodium carbonate
First Method
1. Sodium carbonate is made up of two types of ions: sodium ion, , and carbonate ion,
2. We find that the sodium ion, , has 1 unit of positive charge whereas carbonate ion, , has 2 units of negative charge. This means that two ions are needed to balance the two negative charges (or valencies) of a carbonate ion . So, the sodium carbonate compound is made up of ions and one ion, that is,
3. When we write the formula of sodium carbonate then is written as and is written as CO3 (because charges are not shown in the formula), so that the formula of sodium carbonate becomes .
Second Method – By Crisscross
1. We write down the symbols of sodium ion and carbonate ion (without showing the charges on them).
2. Below the symbol of sodium ion we write the valency (or charge) of sodium ion which is 1+.
3. And below the carbonate ion we write the valency (or charge) of carbonate ion which is 2-. This is shown below:

4. We now cross-over the valencies (or charges) of the sodium ion and carbonate ion.
5. The crossed-over valencies are written as subscripts with the ions (but without their charges). In this way we get which on joining give . This is the formula of sodium carbonate.
Naming chemical compounds from their formula
Usually the elements present in a compound are named in order of symbols appearing in formula. Following rules are applied in naming a compound.
Rule 1
If a compound contains only two elements (binary compounds) such that one fo them is metal, the metal is named first. Non-metallic part is given a suffix ‘ide’ at the end.
Example:
i) Compound of sodium and chlorine is sodium chloride (NaCl)
ii) Compound of magnesium and nitrogen is magnesium nitride
iii) Compound of aluminium and oxygen is aluminium oxide
iv) Compound of calcium and sulphur is calcium sulphide (CaS)
Rule 2
The compound containing two non-metals are named by using Greek prefix like mono, di, tri, tetra, penta, which denotes the number of atoms present in the compound.
Examples:
(i) CO stands for carbon monoxide.
(ii) stands for carbon dioxide.
(iii) stands for nitrogen dioxide.
(iv) stands for phosphorous pentachloride.
(v) stands for sulphur trioxide.
Rule 3
Compounds containing three elements (tertiary compounds), one of which is oxygen, are named with suffix-ate at the end, provided there is only one such compound. If there are two compounds, the one with more oxygen is named with suffix ‘ate’ ending and one with less oxygen is named with ‘ite’ ending.
Examples:
(i) (a) Sodium nitrate
(b) Sodium nitrite
(ii) (a) Calcium sulphate
(b) Calcium sulphite
Rule 4
If in a compound oxygen is less than the oxygen present in a compound ending withite, then it is given the prefix hypo- in the beginning and if oxygen present in a compound ending with -ate is more, then it is given the prefix per-in the beginning
Examples:
(i) KClO is named potassium hypo-chlorite as it contains less oxygen than potassium chlorite .
(ii) is named potassium per-chlorate as it contains more oxygen than potassium chlorate ().
Naming of Acids
(a) The names of binary acids (acids containing hydrogen and one more element) are given by adding prefix hydro and suffix -ic to the name of second element.
Examples:
(i) Acid of hydrogen and chlorine is (HCl) hydrochloric acid,
(ii) Acid of hydrogen and fluorine is (HF) hydrofluoric acid.
(b) The names of acids containing radicals or polyatomic groups are given on the basis of second element, but prefix hydro- is not used.
Examples:
(i) Sulphuric acid
(ii) Carbonic acid
(iii) Sulphurous acid [-ous is used because of less number of oxygen atoms.]
Naming of Bases
Bases containing -OH radical are named as hydroxides, after the name of metal.
Examples:
(i) Sodium hydroxide
(ii) Ammonium hydroxide.
Trivial Names or Common Names
There are certain names of compounds which do not follow any systematic rule. Such names are called trivial names or common names. Chemists have not considered wise to replace these names by systematic names as they are widely understood by the common man.
Examples:
(i) Common name for nitrogen trihydride is ammonia []
(ii) Common name for sodium chloride is table salt []
(iii) Common name for hydrogen hydroxide is water []
Significance of the Formula of a Substance
1. Formula represents the name of the substance.
2. Formula represents one molecule of the substance.
3. Formula also represents one mole of molecules of the substance. That is, formula also represents molecules of the substance.
4. Formula gives the names of all the elements present in the molecule.
5. Formula gives the number of atoms of each element present in one molecule.
6. Formula represents a definite mass of the substance (equal to molecular mass expressed in grams).
As an example, let us give the significance of the formula .
1. represents water.
2. represents one molecule of water.
3. also represents one mole of molecules of water. That is, also represents molecules of water.
4. tells us that water contains two elements, hydrogen and oxygen.
5. tells us that one molecule of water contains 2 atoms of hydrogen and 1 atom of oxygen.
6. represents 18 grams of water (which is equal to the molecular mass of water expressed in grams)
|
AN |
Element |
Symbol |
Atomic Mass |
|
1 |
Hydrogen |
H |
1.0 |
|
2 |
Helium |
He |
4.0 |
|
3 |
Lithium |
Li |
7.0 |
|
4 |
Beryllium |
Be |
9.0 |
|
5 |
Boron |
B |
10.8 |
|
6 |
Carbon |
C |
12.0 |
|
7 |
Nitrogen |
N |
14.0 |
|
8 |
Oxygen |
O |
16.0 |
|
9 |
Fluorine |
F |
19.0 |
|
10 |
Neon |
Ne |
20.2 |
|
11 |
Sodium |
Na |
23.0 |
|
12 |
Magnesium |
Mg |
24.3 |
|
13 |
Aluminium |
Al |
27.0 |
|
14 |
Silicon |
Si |
28.1 |
|
15 |
Phosphorus |
P |
31.0 |
|
16 |
Sulfur |
S |
32.1 |
|
17 |
Chlorine |
Cl |
35.5 |
|
18 |
Argon |
Ar |
39.9 |
|
19 |
Potassium |
K |
39.1 |
|
20 |
Calcium |
Ca |
40.1 |
|
21 |
Scandium |
Sc |
45.0 |
|
22 |
Titanium |
Ti |
47.9 |
|
23 |
Vanadium |
V |
50.9 |
|
24 |
Chromium |
Cr |
52.0 |
|
25 |
Manganese |
Mn |
54.9 |
|
26 |
Iron |
Fe |
55.8 |
|
27 |
Cobalt |
Co |
58.9 |
|
28 |
Nickel |
Ni |
58.7 |
|
29 |
Copper |
Cu |
63.5 |
|
30 |
Zinc |
Zn |
65.4 |
|
31 |
Gallium |
Ga |
69.7 |
|
32 |
Germanium |
Ge |
72.6 |
|
33 |
Arsenic |
As |
74.9 |
|
34 |
Selenium |
Se |
79.0 |
|
35 |
Bromine |
Br |
79.9 |
|
36 |
Krypton |
Kr |
83.8 |
|
37 |
Rubidium |
Rb |
85.5 |
|
38 |
Strontium |
Sr |
87.6 |
|
39 |
Yttrium |
Y |
88.9 |
|
40 |
Zirconium |
Zr |
91.2 |
|
41 |
Niobium |
Nb |
92.9 |
|
42 |
Molybdenum |
Mo |
95.9 |
|
43 |
Technetium |
Tc |
98.0 |
|
44 |
Ruthenium |
Ru |
101.1 |
|
45 |
Rhodium |
Rh |
102.9 |
|
46 |
Palladium |
Pd |
106.4 |
|
47 |
Silver |
Ag |
107.9 |
|
48 |
Cadmium |
Cd |
112.4 |
|
49 |
Indium |
In |
114.8 |
|
50 |
Tin |
Sn |
118.7 |
|
51 |
Antimony |
Sb |
121.8 |
|
52 |
Tellurium |
Te |
127.6 |
|
53 |
Iodine |
I |
126.9 |
|
54 |
Xenon |
Xe |
131.3 |
|
55 |
Cesium |
Cs |
132.9 |
|
56 |
Barium |
Ba |
137.3 |
|
57 |
Lanthanum |
La |
138.9 |
|
58 |
Cerium |
Ce |
140.1 |
|
59 |
Praseodymium |
Pr |
140.9 |
|
60 |
Neodymium |
Nd |
144.2 |
|
61 |
Promethium |
Pm |
145.0 |
|
62 |
Samarium |
Sm |
150.4 |
|
63 |
Europium |
Eu |
152.0 |
|
64 |
Gadolinium |
Gd |
157.3 |
|
65 |
Terbium |
Tb |
158.9 |
|
66 |
Dysprosium |
Dy |
162.5 |
|
67 |
Holmium |
Ho |
164.9 |
|
68 |
Erbium |
Er |
167.3 |
|
69 |
Thulium |
Tm |
168.9 |
|
70 |
Ytterbium |
Yb |
173.0 |
|
71 |
Lutetium |
Lu |
175.0 |
|
72 |
Hafnium |
Hf |
178.5 |
|
73 |
Tantalum |
Ta |
180.9 |
|
74 |
Tungsten |
W |
183.8 |
|
75 |
Rhenium |
Re |
186.2 |
|
76 |
Osmium |
Os |
190.2 |
|
77 |
Iridium |
Ir |
192.2 |
|
78 |
Platinum |
Pt |
195.1 |
|
79 |
Gold |
Au |
197.0 |
|
80 |
Mercury |
Hg |
200.6 |
|
81 |
Thallium |
Ti |
204.4 |
|
82 |
Lead |
Pb |
207.2 |
|
83 |
Bismuth |
Bi |
209.0 |
|
84 |
Polonium |
Po |
210.0 |
|
85 |
Astatine |
At |
210.0 |
|
86 |
Radon |
Rn |
220.0 |
|
87 |
Francium |
Fr |
223.0 |
|
88 |
Radium |
Ra |
226.0 |
|
89 |
Actinium |
Ac |
227.0 |
|
90 |
Protactinium |
Pa |
231.0 |
|
91 |
Thorium |
Th |
232.0 |
|
92 |
Neptunium |
Np |
237.0 |
|
93 |
Uranium |
U |
238.0 |
|
94 |
Americium |
Am |
243.0 |
|
95 |
Plutonium |
Pu |
244.0 |
|
96 |
Curium |
Cm |
247.0 |
|
97 |
Berkelium |
Bk |
247.0 |
|
98 |
Californium |
Cf |
251.0 |
|
99 |
Einsteinium |
Es |
252.0 |
|
100 |
Fermium |
Fm |
257.0 |
|
101 |
Mendelevium |
Md |
258.0 |
|
102 |
Nobelium |
No |
259.0 |
|
103 |
Lawrencium |
Lr |
262.0 |
|
104 |
Rutherfordium |
Rf |
261.0 |
|
105 |
Dubnium |
Db |
262.0 |
|
106 |
Seaborgium |
Sg |
266.0 |
|
107 |
Bohrium |
Bh |
264.0 |
|
108 |
Hassium |
Hs |
277.0 |
|
109 |
Meitnerium |
Mt |
268.0 |
|
110 |
Darmstadtium |
Ds |
271.0 |
|
111 |
Roentgenium |
Rg |
272.0 |
|
112 |
Ununbium |
Uub |
285.0 |
|
113 |
Ununtrium |
Uut |
284.0 |
|
114 |
Ununquadium |
Uug |
289.0 |
|
115 |
Ununpentium |
Uup |
288.0 |
|
116 117 |
Ununhexium Ununoctium |
Uuh Uuo |
292.0 293.0 |
The relative atomic mass of an element indicates the number of times one atom of that element is heavier than mass of C- 12 isotopes atom. For example, the atomic weight of calcium is 40. This means that an atom of calcium is on average is 40 times the mass of 1/12 the mass of C- 12 isotope’s atom.
Atomic weights of many elements are not whole numbers due to the presence of stable isotopes.
The number of atoms of a particular isotope present in 100 atoms of a natural sample of that element is called its relative abundance which always remains constant for a given element.
Natural chlorine is a mixture of two isotopes with relative abundances 75% (Cl-35) and 25% (Cl-37) approximately.
Then, the atomic weight of chlorine is
Assume an element ‘E’ containing three isotopes. If x, y and z are the percentage abundance of these three isotopes respectively, then
The average atomic weight of ‘E’
3.15 ATOMIC MASS UNIT (A.M.U)
It is the smallest unit of mass and is used to measure the masses of atoms and subatomic particles. The mass of one a.m.u. is equal to the mass of the mass of C-12 atom. The other names of a.m.u. are Aston, Dalton and Avogram.
Note:
Relation of a.m.u with Atomic weight
Consider an element with atomic weight ‘x’. It means that one atom of the element is ‘x’ times heavier than 1/12th of C -12 isotope’s atom. That is, mass ‘of one atom of element = x × mass of 1/12th of C-12 isotope’s atom = x a.m.u (1 a.m.u = Mass of 1/12th of C – 12 isotope’s atom) If the atomic weight of an element is ‘x’, then the weight of one atom of that element is ‘x’ a.m.u.
Examples to understand further:
1. The atomic weight of nitrogen is 14. So, the mass of an atom of nitrogen is 14. a.m.u (its atomic weight expressed with a.m.u).
2. The atomic weight of chlorine is 35.5. So, the mass of an atom of chlorine is 35.5 a.m.u (its atomic weight expressed with a.m.u).
Table showing the weights of first 10 elements
|
At. no |
Element |
Symbol |
At.wt(appr) |
Weight of single atom ( in amu and grams) |
|
1 |
Hydrogen |
H |
1 |
|
|
2 |
Helium |
He |
2 |
|
|
3 |
Lithium |
Li |
7 |
|
|
4 |
Beryllium |
Be |
9 |
|
|
5 |
Boron |
B |
11 |
|
|
6 |
Carbon |
C |
12 |
|
|
7 |
Nitrogen |
N |
14 |
|
|
8 |
Oxygen |
O |
16 |
|
|
9 |
Fluorine |
F |
19 |
|
|
10 |
Neon |
Ne |
20 |
3.16 MOLECULAR WEIGHT
Molecular weight or relative molecular weight is defined as the number of times a molecule is heavier than the mass of C-12 isotope’s atom.
Relative molecular mass or molecular weight has no units. The molecular weight of an element or compound indicates the number of times a molecule is heavier than the mass of C-12 isotope’s atom.
For example, the molecular weight of calcium carbonate is 100, it implies that mass of one molecule of calcium carbonate is 100 times heavier than the mass of C-12 isotope’s atom.
Relation between molecular weight and amu
Consider an element/compound with molecular weight ‘y’. It means that one molecule of the element/compound is ‘y’ times heavier than 1/12th of C -12 isotope’s atom. That is, Mass of one molecule of element/compound = y × mass of 1/12th of C-12 isotope’s atom = y a.m.u ( a.m.u -Mass of 1/12th of C-12 isotope’s atom)
If the molecular weight of an element/compound is ‘y’, then the weight of one molecule of that element/compound is ‘y’ a.m.u.
Examples to understand further:
1. The molecular weight of water is 18. So, the mass of a molecule of water is 18 a.m.u (its molecular weight expressed with a.m.u).
2. The molecular weight of Sulphur dioxide is 64. So, the mass of a molecule of Sulphur dioxide is 64 a.m.u (its molecular weight expressed with a.m.u).
Table showing molecular weights and weight of single of molecule of some compounds in a.m.u
|
S.NO |
Element |
Symbol |
Molecular weight |
Weight of single atom ( in amu and grams) |
|
1 |
Hydrogen |
2 |
||
|
2 |
Nitrogen |
28 |
||
|
3 |
Oxygen |
32 |
||
|
4 |
Carbon dioxide |
44 |
||
|
5 |
Carbon monoxide |
28 |
||
|
6 |
Ammonia |
17 |
||
|
7 |
Methane |
16 |
||
|
8 |
Nitrogen dioxide |
46 |
||
|
9 |
Water |
18 |
||
|
10 |
Hydrochloric acid |
36.5 |
||
|
11 |
Sulphuric acid |
98 |
||
|
12 |
Nitric acid |
63 |
3.17 GRAM ATOMIC WEIGHT
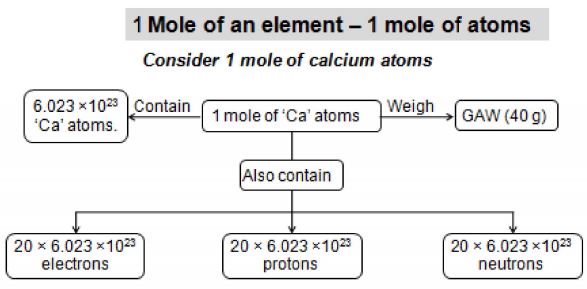
Atomic weight of an element expressed in grams is known as its gram atomic weight. For example, the atomic weight of hydrogen is 1.008. So, the gram-atomic weight of hydrogen is 1.008 g. Gram atomic weight of any substance is also called its gram atom. For example, 1 gram atom of carbon weighs 12 gram and 1 gram atom of nitrogen weighs 14 grams.
For example, the number of gram atoms in 5 g of hydrogen =5/1 = 5.
Note:
1) Weight of x gram atoms = x Gram atomicweight.
2) 1 gram atom or gram atomic weight of an element contain = atoms.
3) Number of atoms in a given substance ( given element) = Number of gram atoms
4) Number of atoms in 1 gram of an element
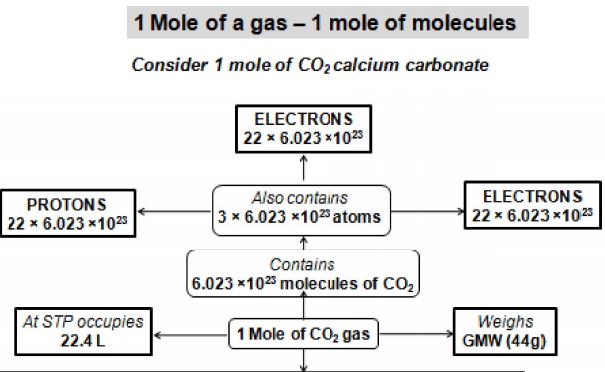
3.18 GRAM MOLECULAR WEIGHT
It is the molecular weight of an element or compound expressed in grams. For example, the molecular weight of hydrogen gas is 2. So, the gram molecular weight of hydrogen is 2 g. Gram molecular weight of a substance is also called its gram molecule or mole molecule. For example, the weight of 1 gram molecule or mole molecule of is 18
grams and the weight of 1 gram molecule of is 44 grams.
Note:
1. Weight of x moles of any compound = x Gram molecular weight.
2. Number of molecules in a given substance= Number of gram molecules.
3. Weight of substance in grams = Number of gram molecules GMW.
4. Gram atomic mass of an element and Molar mass of an element are just the same.
5. Gram molecular weight of a substance and Molar mass of a substance are also just the same.
3.19 AVOGADRO NUMBER
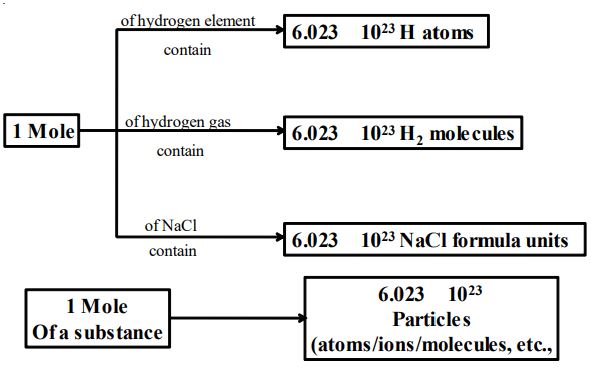
Number of atoms present in gram atomic weight of an element is called Avogadro’s number. Avogadro number= .It is denoted by N or NA.
Similarly, the number of molecules present in gram molecular weight of nay substance is equal to Avogadro number .
Thus,
Avogadro number of atoms = atoms.
Avogadro number of molecules = molecules.
Avogadro number of ions = ions.
3.20 MOLE
In general, we buy groceries in several ways. If we buy eggs or bananas, we buy them by the dozens – dozens – that is by number, by counting them. Eggs or bananas are easy to count. So are the oranges or apples. But there are the other types of items, though countable, are more conveniently sold by mass. A dozen peanuts is too small a number to buy by counting and several hundreds are difficult to count. So we buy them by weighing them. In the first case, it is more convenient to buy the eggs or bananas by counting rather than weighing them. Unknowing the information about the weight of eggs doesn’t affect anything. In the second case, it is more convenient to buy the peanuts by weighing, rather than counting them, Unknowing the information about the number of peanuts doesn’t affect anything. Now, let’s turn our attention from this everyday physical world to the chemical world of Scientists (Chemical world). Unlike the above two cases, both knowing weight and number of particles (atoms/molecules/ions/chemical units) present in the compounds is very important. Since this is of vital importance, ignorance or such knowledge may at times even lead to devastation and destruction. In fact, life itself would come to a standstill if such facts were not known. Thus, we require a standard of measurement which is all embracing dealing at once with macroscopic world and the microscopic world.
In other words, such a system should give us a comprehensive view of the number of particles in any given substance. For instance, units like grams or kilograms, which we use in everyday life, would be inadequate when dealing with two different substances of the same weight containing the same number of particles. Hence the conventional units of measurement would not serve our purpose here.
But our ever thoughtful scientists have come to our rescue. They have come out with a solution by identifying a unit which is not only easy to use but also appropriate to its purpose. It gives just what is required – A magnified view of the anatomy of microscopic world, acting as a bridge between the tangible and the intangible worlds.
Can you guess what this unit is?
It is nothing but mole. Let’s have a detailed understanding of mole.
Understanding of mole
In Latin, the term mole literally means heap, pile or mass. In represents a definite number as century which means 100. it is used as the bridge in chemistry between the atomic and macroscopic scales. For example, in banks they do not count the coins, but weight them, as they know that a fixed number of a particular coin will always have the same mass. Similarly, chemists count atoms and molecules by weighing them. One mole of each of the different substances contains the same number of elementary units.
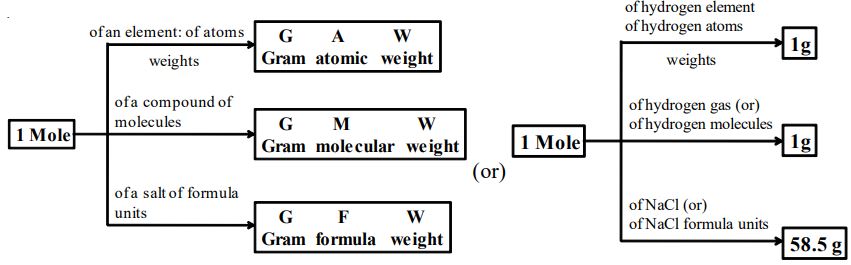
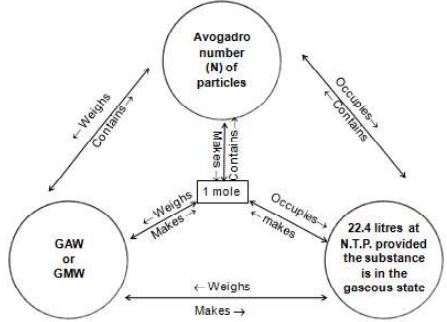
Mole is the mass of the substance containing particles equal to Avogadro number
1. mole of any substance = Weight of
2. mole of any substance contains particles
Mole and Weight relation
What is the weight of 1 mole of carbon atoms and 1 mole of water molecules?
|
Wt. of 1 mole of 1 ‘C’ atoms |
Wt. of 1 mole of 1 molecules |
What do you observe?
The weight of 1 mole of ‘C’ atoms = 12 grams = GAW of ‘C’
The weight of 1 mole of molecules = 18 grams = GMW of
Conclusion: The weight of 1 mole of any substance is equal to its gram atomic weight or gram molecular weight.
MOLE AND PARTICLES RELATION
How many particles are present in 1 mole of any substance?
We know that 1 mole of any substance contains respective particles. Particles can be: Atoms, molecules, ions, etc., Let us understand it with examples …
Mole and Volume relation for a gas
1 mole of any gas at STP or NTP occupies 22.4 litres. This volume occupied any gas at STP is also called Gram Molar Volume (GMV)
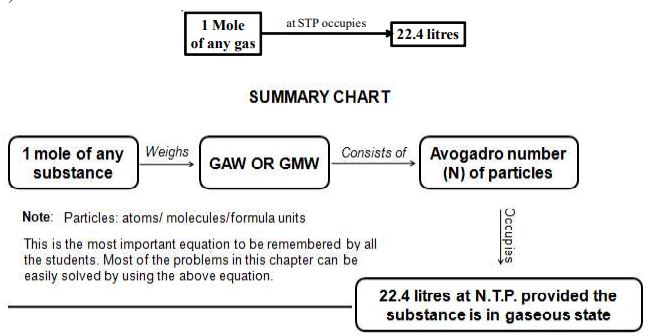
Few more relations
Weight – Particles relation:
‘GAW’ or ‘GMW’ of any substance consists of ‘N’ (Avogadro number) particles or ‘N’ particles of any substance weighs its GAW or GMW respectively.
For example, 32 grams of Oxygen () gas consists of ‘N’ molecules or ‘N’ molecules of Oxygen gas weighs 32 grams.
Weight – Volume relation (only for gases):
‘GAW’ or ‘GMW’ of any gas occupies 22.4 litres at S.T.P. or 22.4 litres of any gas weighs ‘GAW’ or ‘GMW’ at S.T.P.
For example, 2 grams of Hydrogen () gas occupies 22.4 litres at S.T.P or 22.4 litres of Hydrogen gas at S.T.P weighs 2 grams
Volume – Particles relation (only for gases):
22.4 litres of any gas at S.T.P consists of molecules or molecules of any gas at S.T.P. occupies 22.1 litres of a gas.
For example, 2 grams of Hydrogen () gas occupies 22.4 litres at S.P.T. or 22.4 litres of Hydrogen gas at S.T.P. weighs 2 grams.
Summary of Relations
BETTER UNDERSTANDING OF MOLE
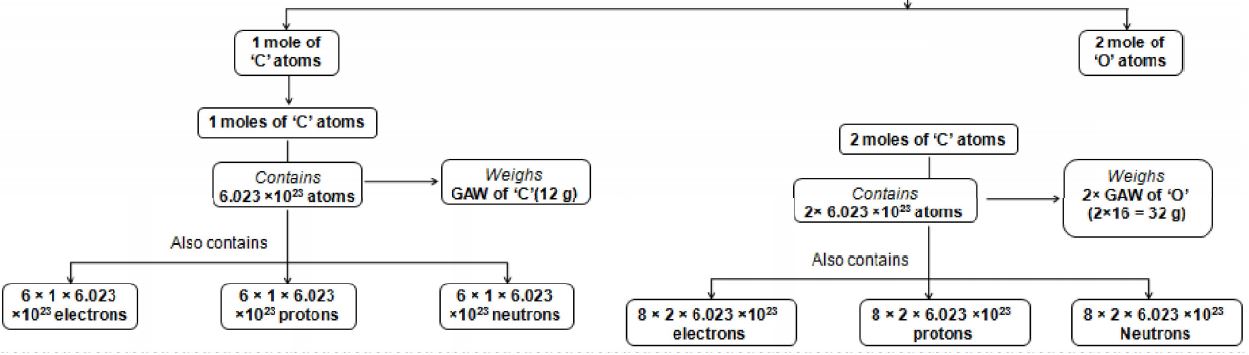
Some of Important Formulae
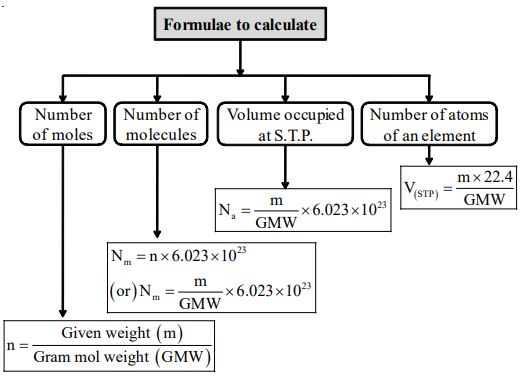
Some more important relations
1. No of gram atoms or mole atoms
2. Number of moles (n) =
3. Weight of x gram atoms = x Gram atomic weight
4. Weight of x moles of any compound = x Gram molecular weight
5. 1 gram atom or gram atomic weight of an element contains atoms.
6. 1 gram molecule or gram molecular weight of a substance contains molecules.
7. Number of atoms in a given substance ( given element) = Number of gram atoms ()
8. Number of molecules in a given substance ( ) = Number of moles (n)
9. Number of atoms in 1 gram of an element =
10. Number of molecule in 1 gram of a substance =
11. Weight of an element in grams = Number of gram atoms GAW
12. Weight of substance in grams = Number of moles GMW
13. Number of atoms of an element per molecule can be calculated if MW and percentage mass of that element are given by using the formula.
14. [ Note: Number of atoms is always is a whole number]
15. No. of atoms present in given amount of substance ()
= No. of molecules () × No. of atoms present in 1 molecule of the substance.
= No. of moles (n) × × No. of atoms present in 1 molecule of the substance.
16. No. of subatomic particles (electrons / protons, etc) present in given amount of substance ()
= No. of molecules () × No. of subatomic particles present in 1 molecule of the substance.
= No. of moles (n) × × No. of subatomic particles present in 1 molecule of the substance.








