1. INTRODUCTION
All substances around us undergo changes. In some cases, the changes are small and difficult to detect. In other cases, the changes are obvious and easy to detect. These changes generally get accelerated, if we heat the substances.
Most of these changes can be classified under two headings:
(i) Physical changes
(ii) Chemical changes
Physical changes are generally temporary in nature and no new substances are formed. Chemical changes are generally permanent in nature and new substances are formed, which have entirely new properties.
2. PHYSICAL CHANGES
The changes are temporary in nature and can be reversed. Such changes are called physical changes. Let’s see few experiments showing physical changes.
Experiment 1
Take a dry hard glass test tube and put in it about 2 g of candle wax. Meat the test tube gently on a Bunsen flame. What do you observe?
The wax melts to form a colourless liquid. Now cool the tube by holding it in cold water. What do you observe?
The molten wax solidifies. Thus, we can say that on heating wax melts and on cooling, the liquid wax solidifies, but no new products are formed.
Similarly, ice, ghee, butter, etc., melt on heating and solidify on cooling.
Experiment 2
Zinc oxide is a white powder. Place about 2 g of zinc oxide in a dry test tube. Heat the test tube strongly. What do you notice after 2 minutes of heating?
The zinc oxide changes to yellow colour. Cool the test tube. In a few minutes, the colour of zinc oxide changes to white colour.
Thus, we can say that the change in zinc oxide was temporary in nature.
Similarly, if we heat lead monoxide powder, which is yellow in colour, its colour changes to reddish brown. However, on cooling, its colour changes back to yellow.
Experiment 3
Take about 50 Cm3
of water in a beaker. Add a spoonful of common salt in it and stir. You will observe
that salt dissolves in water. Now evaporate the salt solution on heated sand bath. It is observed that water disappears leaving behind common salt. Furthermore, the mass of common salt remains same.
Thus, we can say that the above changes are
(i) temporary in nature,
(ii) no new products are formed, and
(iii) there is no change in weight when change takes place.
Experiment 4
Hold a short length of platinum wire or nichrome wire with the help of tongs. Hold the end of wire in a non-luminous Bunsen flame. What do you observe?
The end of wire becomes red hot. Take out the end of wire from the Bunsen flame and allow it to cool. What do you observe? The wire regains its original colour.
Thus, we can say that the change brought about in the appearance of the wire is temporary in nature. Similarly, when electric current flows through a bulb, its filament gets white hot and emits light. When current is switched off, the filament does not give off any light. Thus, the change in the appearance of the bulb is temporary in nature.
Experiment 5
Take a bicycle spoke (wire fitted in wheel of bicycle) and rub it with a permanent bar magnet. Roll the iron wire in the iron filings. What do you observe? Iron filings stick to the wire. Thus, we can conclude that wire has changed into a magnet.
Now hit the wire against the table for 50 times. Again roll the wire in iron filings. It is observed that iron filings no longer stick to the iron wire. Thus, we can conclude that wire has lost its magnetic property.
Thus, the changing of iron wire into a magnet is a temporary change and no new products are formed
3. CHEMICAL CHANGES
The changes are permanent in nature and cannot be reversed. Such changes are called chemical changes. Let’s see few experiments showing chemical changes.
Experiment 1
Hold a short length (about 50 cm) of magnesium ribbon in the fire tongs. Heat magnesium ribbon in the non-luminous flame for about 2 minutes.
The magnesium ribbon burns with a brilliant white flame producing a large amount of heat and light. It leaves behind a white ash, which is commonly called magnesium oxide.
The magnesium oxide, so formed, does not change to magnesium ribbon on cooling. Thus, we can conclude :
(i) the change brought about in magnesium is permanent in nature;
(ii) a new product is formed which has entirely different properties; and
(iii) a large amount of heat and light energy are evolved.
Experiment 2
Fix a candle on the table and light it. What do you observe after 10 minutes?
(i) The candle burns to give heat and light energy.
(ii) The candle does not regain its original size when put off.
Thus, the burning of a candle is a permanent change which cannot be reversed. Actually, the candle burns to form new products, i.e., carbon dioxide gas and water vapour. Glowing splint bursts into flame
Experiment 3
Take a hard glass test tube and put in it 2 g of red coloured mercuric oxide. Clamp the test tube on an iron stand. Heat the test tube strongly. You will notice that first of all the colour of mercuric oxide changes to black. On further heating, the test tube is filled with fumes. At this moment hold a glowing wooden splint in the test tube. The wooden splint of mercury bursts into flame. Also, tiny silvery droplets arc seen sticking to the cooler parts of test tube. On cooling the test tube, the change is not reversed.
Actually, on heating, mercuric oxide decomposes to form mercury and oxygen, i.e., two new products arc formed. The mercury vaporises and liquefies on the cooler parts of the test Heating mercuric oxide brings tube. The oxygen is responsible for the glowing splint to burst into flame, because it supports combustion.
Thus, strong heating of mercuric oxide is a permanent change, which cannot be reversed. Furthermore, new products are formed.
Experiment 4
Take a hard glass test tube and put in it 2 g of sugar. Heat the test tube strongly. What do you observe? The sugar melts and then turns brown. On further heating, it gives off steam which condenses on the cooler parts of the test tube. The residue left in the test tube is black in colour. On cooling, the change does not reverse itself.
Thus, heating of sugar is a permanent change. Actually, sugar decomposes to form charcoal which is a form of carbon and is black in colour. It also gives off water in the form of steam.
From the above experiments, it is clear that there are two types of changes which take place in the substances on heating.
4. CHARACTERISTICS OF A PHYSICAL CHANGE
(i) No new substances are formed during physical change:
Ice, on heating, melts to form water. The water on further heating changes into steam. On cooling, the steam changes into water. On further cooling, the water solidifies to form ice.
However, the molecules of ice, water or steam always contain two atoms of hydrogen and one atom of oxygen. Thus, no new substances are formed.
Similarly, when we add common salt to water, salt solution is formed but no change takes place in its molecules. On evaporation, the water evaporates leaving behind salt.
(ii) Physical changes can be generally reversed:
The zinc oxide, on heating, changes to yellow colour. However, on cooling its colour changes to white.
Similarly, when a piece of iron is stroked with permanent magnet, it gets magnetised. However, if magnetised iron is hammered, it loses its magnetism. The wax on heating changes into liquid state. However, liquid wax changes into solid on cooling.
(iii) There is no change in weight during physical change:
10 g of solid ‘wax on melting will form 10 g of molten wax.
If a salt solution is prepared by dissolving 20 g of salt in water, then on the evaporation of water, 20 g of salt is left behind.
(iv) Only a little heat (if any) is absorbed or given off during physical change.
If water changes into steam by absorbing a certain amount of heat energy, then steam will change into water by giving off the same amount of heat energy. The heat energy supplied during physical change, is in no way, utilised to change the composition of the molecules of a substance.
5. EXAMPLES OF PHYSICAL CHANGES
Some of the very common examples of physical changes are given below :
(i) Melting of ice or wax or butter or ghee.
(ii) Freezing of water to ice or solidification of liquid wax to solid wax.
(iii) Changing of water into steam by boiling.
(iv) Evaporation of water by the heat of sun.
(v) Condensation of water vapours, such as formation of clouds, mist, fog, etc.
(vi) Glowing of an electric bulb on the passage of electric current,
(vii) Magnetization of iron.
(viii) Production of sound when two materials are hit together.
(ix) Expansion or contraction of metals on heating,
(x) Formation of solutions of soluble substances in water,
(xi) Crystallisation of salts from their solutions.
(xii) Change of colour due to heat as in case of zinc oxide or lead monoxide.
(xiii) Beating of metals into sheets or drawing metals into wires,
(xiv) Shaping of glass by heat.
6. CHARACTERISTICS OF A CHEMICAL CHANGE
(i) When a chemical change occurs new substances, with entirely new properties are formed.
Candle wax on burning forms entirely new substances, i.e., carbon dioxide gas and steam.
Mercuric oxide (red in colour) on strong heating forms new substances, i.e., mercury and oxygen gas. Sugar on strong heating forms new substances, i.e., carbon and steam.
Magnesium on burning forms entirely new substance, i.e., magnesium oxide.
(ii) Chemical change cannot be easily reversed
The carbon dioxide and steam formed during the burning of the candle, cannot be converted into wax by altering the conditions of experiment. Carbon and water vapour, formed during the heating of sugar, cannot be recombined to form sugar.
Magnesium oxide formed during the burning of magnesium cannot be easily changed to original metal and oxygen.
(iii) There is usually a change in weight during chemical reaction
When magnesium is burnt in air, then the weight of white ash (magnesium oxide) is more than magnesium metal. For every 3 g of magnesium metal, 5 g of magnesium oxide is formed.
When sugar is burnt, for every 7 g of sugar approximately 3 g of sugar charcoal is left.
Similarly, when iron rusts, the weight of rusted iron is more than that of original metal, because the oxygen combines with iron.
(iv) Lot of heat is usually given off or absorbed during, a chemical change
When magnesium burns in air, it produces a large amount of heat and light energy.
When mercuric oxide decomposes to form mercury and oxygen, it absorbs a large amount of heat energy.
When sugar decomposes to sugar charcoal and steam, it absorbs large amount of heat energy.
7. EXAMPLES OF CHEMICAL CHANGES
Following are the common examples of chemical changes :
(i) Cooking of food,
(ii) Food turning bad after a few days,
(iii) Curdling of milk.
(iv) Fading the colours of clothes.
(v) Germination of seeds,
(vi) Ripening of fruits,
(vii) Lighting of a match stick by striking match head with the side of match box.
(viii) Digestion of food within our bodies,
(ix) Respiration by humans, plants and animals.
(x) Decaying of old pieces of wood,
(xi) Rusting of iron,
(xii) Blackening of silverware,
(xiii) Hardening of cement to form concrete block.
(xiv) Fermentation of sugar solution to alcohol.
(xv) Burning of wood, coal, kerosene oil,petrol or liquefied petroleum gas
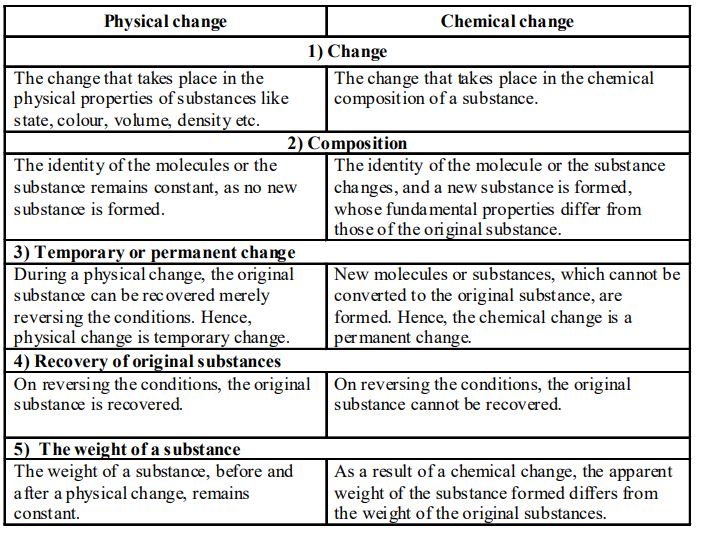
DIFFERENCES BETWEEN PHYSICAL AND CHEMICAL CHANGES
9. RUSTING OF IRON
Deposition of a brown layer on iron is called rusting. In rusting, a new substance is formed. The chemical structures of iron and rust are completely different. Rust is iron oxide. Iron is a grey-black material while rust is reddish brown
Thus, this is a chemical and irreversible change. Reaction in rusting can be written as follows:
When articles made of iron come in contact with moisture present in air, they get rusted. Iron is converted into iron oxide, i.e. rust. The iron article becomes weak in due course as all the iron slowly turns into rust. This is corrosion of iron. Rusting gives a huge monetary loss to the people and nation.
Prevention of rusting
For rusting, both water and oxygen should come in contact with iron. If anyone of these is prevented to come in contact with iron, rusting can be prevented. So, rusting is prevented using following methods:
1. Painting: Articles such as; iron gates, grills, etc. are painted at regular intervals of time.
2. Applying of layer of grease: Applying a layer of grease prevents the iron articles from coming in contact with moist air. This prevents rusting. That is why grease is applied over the chain of bicycle and also over many machine parts.
3. Galvanisation: In the process of galvanization; a layer of non-reactive metal, such as zinc is deposited over iron articles. The layer of non-reactive metal prevents the iron articles from coming in contact with moisture. Thus it prevents rusting. Water pipes, which are made of iron, are galvanized to prevent rusting.
10. CRYSTALLISATION
Common salt is obtained by the vapourisation of sea water, but crystals of common salt are very small. When a small crystal of common salt is left dipped in the saturated solution of common salt for some time, big crystal of common salt is obtained. Formation of big and pure crystal of a substance from the saturated solution is called CRYSTALLISATION.








