1. INTRODUCTION
We use variety of objects in our day to day lives. Objects are made up of different materials.
(i) An object can be made from more than one material
Example: Cake, Soaps
(ii) Different objects can be made from the same material
Eample: Table, Chair, and Door are made from wood.
(iii) An object can be made from different materials
Example: Bottles from glass, Plastic, metals.
2. ADVANTAGES OF CLASSIFICATION
(a) helps in identification of objects
(b) helps in sorting of objects
(c) helps in locating things
(d) makes study of different objects easy and more meaningful rather than studying each other separately.
(e) helps in understanding similarities and dissimilarities among objects.
3. GROUPING OF OBJECTS ON THE BASIS OF COMMON PROPERTIES
Objects are grouped on the basis of properties like lustre, hard/softness, transparency, solubility, floatation, attraction towards magnet, conduction of heat and conduction of electricity.
I. Lustre
Materials can be grouped as lustrous and non-lustrous on the basis of lustre/shine possessed by them.
(i) Lustrous materials are those that have a shine on them. Metals are lustrous. Due to this property metals are widely used for making jewellery.
Example: Gold, silver and most metals are lustrous in nature.
(ii) Non-lustrous materials are dull in appearance. In general, nonmetals are non – lustrous.
Example: Wood, Plastic, etc.
II. Hard/Softness
(i) Materials that can be compressed or scratched easily are called soft materials.
Example: Cotton, Sponge.
(ii) Some materials that are difficult to compress are called hard. Example: Iron and most of the other metals.
III. Transparency
Materials can be classified as transparent, opaque and translucent on the basis of transmittance of light by them.
(i) Transparent Materials
Transparent materials allow light to pass through them completely. One can see through such materials
Example: Glass, water, Air and Some plastics
(ii) Opaque Materials
Opaque materials do not allow light to pass through them. You cannot see through them.
Example: Wood, cardboard and metals.
(iii) Translucent Materials
Translucent materials allow light to pass through them only partially. They are partially transparent and partially opaque.
Example: Butter paper, Frosted glass.
IV. Conductivity
The process of transmission of heat or electricity or sound is called conductivity.
(i) Conductors
Substances that allows the transmission of heat or electricity or sound through them are called conductors.
Example: Metals like silver, copper, gold, etc are good conductors of electricity and heat.
(ii) Insulators
Substances that does not allow the transmission of heat or electricity or sound through them are called nonconductors or insulators.
Example: Plastic, glass, distilled water, etc are insulators.
(iii) Semi-conductors
Substances called semiconductors act as good conductors under some conditions and poor conductors under other conditions.
Example: Silicon, germanium, and various metal oxides are examples of semiconductor materials.
V. Solubility
Solubility is the ability to dissolve into (become a part of) another substance. Something that dissolves, like the sugar in our example, is called a solute. The substance that it dissolves into, like the tea, is called the solvent. When a solute dissolves into the solvent, the end product is called a solution.
(i) Soluble substances We have a word for substances that form solutions when they are mixed with water. These substances are called soluble substances. Example: Sugar, salt are common examples of soluble substances.
(ii) Insoluble substances Substances that do NOT form solutions when they are mixed with water are called insoluble substances.
Example: Sand, gravel, flour, plastic, soap, etc. are examples of insoluble substances.
VI. Volatility
Volatility is the tendency of a substance to vapourize.
(i) Volatile liquids
Volatile substances have the capability to go into the vapour phase. This may happen during heating or without heating.
Example: Acetone, hexane, chloroform are volatile liquids, which evaporates rapidly.
(ii) Non-volatile liquids
Non-volatile substances are substances which do not vapourize rapidly. Nonvolatile substances will mostly be as solids in the room temperature.
Example: Sodium chloride, silver nitrate are nonvolatile compounds.
VII. Miscibility
Miscibility refers to the ability of a liquid to completely dissolve in another liquid solution. A distinct layer between two liquids will not form when you have a solution that is labeled miscible. When a distinct layer does form in a mixed solution this is called immiscibility.
(i) Miscible liquids
When two liquids can be mixed to form a solution they are called Miscible.
Example: Alcohol and water, Vinegar [acetic acid + water]
(ii) Immiscible liquids
If two liquids cannot be mixed to form a solution they are called “immiscible.”
Example: oil and water
VIII. Combustibility
Combustibility is a measure of how easily a substance will set on fire, through fire or combustion.
(i) Combustible substances
A substance that can be burned to provide heat or power are called combustible substances.
Example: Wood, coal, kerosene, petrol, etc.
(ii) Non-Combustible substances
A non- combustible material is a substance that will not ignite, burn, support combustion.
Example: Water, iron, steel, stone, etc.
4. MATTER
The moment we open our eyes and look at the surroundings, our eyes capture different things, varying from very minute to extremely large sizes, which differ in shape, size, appearance and texture. But, a question strikes every mind momentarily as to “What are the constituents, with which this Universe is made of?
And, our eminent and learned scientists have come out with an answer and have put an end to this ever pricking question.
Matter
Anything that occupies space and has mass is called Matter.
Air and water, gold and silver, table and chair, milk and oil etc., are all different kinds of matter, because all of them occupy space and have mass.
Characteristics of matter
i) All matter is composed of particles. These particles have intermolecular spaces between them and attract each other with a force and are in continuous random motion.
ii) All material bodies have weight and hence have mass.
iii) All material bodies occupy space.
5. CLASSIFICATION OF MATTER
On the basis of physical state, matter is classified into three types – solids, liquids and gases.
I. Solids
A solid object characterized by resistance to deformation and changes of volume. At the microscopic scale, solids have the following properties.
Properties
i) Shape and volume: Solids have definite shape and volume.
Reason: The definite shape and volume of solids can be explained on the basis of kinetic theory of matter. In the cases of solids, the kinetic energy of molecules is least and the force of attraction between the molecules is highest. The molecules of the solids can just vibrate about their mean position, but cannot migrate from one position to another. Thus, the solids have definite shape and volume.
ii) Rigidity: Solids are generally rigid.
Reason: The constituent molecules i.e., in solids, they cannot be deformed on application of force, have fixed positions in space relative to each other. This accounts for the solid’s rigidity.
(In some solids like rubber, the shape changes on the application of external force and it regains its shape, on removal of the external force.)
iii) Free surfaces: Solids have several free surfaces.
iv) Intermolecular spaces and forces: In solids, the molecules are very close to one an other. Thus, they have minimum intermolecular spaces. Due to this, they have large intermolecular forces of attraction.
The density of the solids is generally high. This is due to the compact arrangement of the molecules.
v) Effect of heating: Solids expand on heating. But the dimensions of solids do not increase or decrease in large proportion on heating or cooling, respectively.
vi) Diffusion: When two solids are kept in contact with one another they do not mix with each other, i.e., they do not diffuse.
Some examples of solids: All metals, wood and wood products; rocks of various kinds, ice, etc.
II. Liquids
A liquid is a fluid in which the particles are loosely arranged and can freely form a distinct surface at the boundaries of its bulk material. At the microscopic scale, liquids exhibit the following properties.
Properties
i) Shape and volume: Liquids have definite mass and volume. They do not have definite shape, but take the shape of the container in which they are present.
Reason: We know that the kinetic energy of the molecules of a liquid is very large, and so is the distance between the molecules. Thus, the attractive forces between the molecules of a liquid are small as compared to the solids. Therefore, the molecules of a liquid are free to move about within the liquid and hence, the liquid can easily take the shape of the container in which it is present.
However, the volume of the liquid does not change, because, the molecules do not leave the liquid.
ii) Intermolecular spaces and forces: In liquids, the distance between the molecules is large compared to that of a solid. Thus, they have greater intermolecular spaces than in solids. Due to this they have less intermolecular forces of attraction than in solids.
iii) Fluidity: The force of attraction between the molecules of liquids is less than solids. Thus, liquids can flow from one place to another.
iv) Rigidity: Liquids are not as rigid as solids. They can be slightly compressed.
Reason: The intermolecular space in liquids is larger than in solids.
v) Free surfaces: Liquids have only one free surface.
vi) Density: The density of the liquids is generally less than that of solids.
vii) Effect of heating: Liquids expands on heating. They expand far more than solids on heating and contract for more than solids on cooling.
viii) Diffusion: The particles of two different liquids can diffuse in one another, depending upon the nature of molecules of liquids. For example, milk and water particles diffuse in one another, but the particles of oil and water do not.
ix) Some examples of liquids: Water, alcohol, benzene, milk, mercury, kerosene oil, etc,.
III. Gases
Gas is the most energetic phase of matter commonly found on earth. The particles of gas, either atoms or molecules, have too much energy to settle down attached to each other or to come close to other particles to be attracted by them.
At the microscopic scale, gases have the following properties.
Properties
i) Shape and volume: Gases have neither definite shape nor definite volume. They occupy the entire space of a given vessel in which they are enclosed.
Reason: The intermolecular distances between the molecules of a gas are very large with the result that the force of attraction between the molecules is negligible. Morever, the molecules have a very large kinetic energy. Thus, the molecules are practically free to move in any direction and hence, can fill any space. Thus, the gases have neither definite shape nor definite volume.
ii) Definite mass: A gas contained in a vessel has a definite mass.
iii) Intermolecular spaces and forces: In gases, the distance between the molecules is very large compared to that of solids and liquids. Thus, they have the greatest intermolecular spaces compared to solids and liquids. Due to this they have least intermolecular forces of attraction than in solids and liquids.
iv) Compressibility: Gases are highly compressible. The high compressibility of gases is due to the fact that they have large intermolecular spaces. On applying pressure, these molecules simply come close to each other, thereby decreasing the volume of a gas.
v) Expansibility: The volume of a given mass of a gas can be increased either by decreasing pressure or by increasing temperature. When the pressure on an enclosed gas is reduced, its molecules simply move apart, thereby increasing intermolecular spaces and hence, the volume increases.
When an enclosed gas is heated, the kinetic energy of its molecules increases. Thus, the molecules move faster and farther from each other. This in turn results in the increase in volume.
vi) Free surfaces: Gases have no free surfaces.
vii) Density: The gases occupy an extremely large volume as compared to those solids of and liquids. As inter molecular spaces between the gas molecules is large, they occupy greater volume compared to solids and liquids of same mass. Thus, mass per unit volume of a gas is very small as compared to the liquids and solids. This accounts for the low density of the gases.
viii) Diffusion: Gases have a very high rate of intermixing and diffusion. The intermolecular spaces in a gas are very large. Thus, when two gases are brought in to contact with each other, their molecules just move into one another’s intermolecular space, thereby forming a homogeneous mixture.
ix) Exertion of pressure: The molecules of a gas, constantly bombard the sides of the containing vessel and hence, exert some force per unit area on the sides of the container, which is commonly called pressure. It has been seen that at a given temperature, the number of molecules striking the walls of containing vessel per unit time, per unit area is same. Thus, we can say that gases exert same pressure in all directions.
6. CHANGE OF STATE OF MATTER
A change of state means an interconversion between two states of matter namely
Solid Liquid Gas ; without changing the composition of a substance. A substance may convert from solid state to liquid state and liquid state to gaseous state.
By heating ice (solid), ice turns into water (liquid) on further heating, it turns into steam(gas). When the steam is cooled down it turns into water and on further cooling it turns into ice.
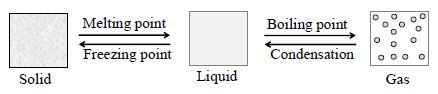
I. Solid-Liquid-Interconversion
Solid to Liquid :
When we heat a solid substance, kinetic energy of the particles increases with the increase in temperature.
They vibrate vigorously at a particular temperature and gets a fixed position, this temperature is called melting point of solid. Thus solid becomes a liquid.
Liquid to Solid:
When a liquid is condensed or cooled, kinetic energy of particles decreases and the particles moves closer. At a particular temperature, particles attains their mean positions and gets rigid. This temperature is called freezing point. At this point, liquid converts into solid.
II. Liquid -Gas-Interconversion
Liquid to Gas
Particles in a liquid collide due to their free movement. When the temperature increases in the liquid, their particles collide more vigorously (they attain more kinetic energy) leading to evaporation at a particular temperature. This temperature is called boiling point.
Gas to Liquid
Particles in a gas have more kinetic energy. When temperature in a gas is decreased, the kinetic energy of the particles also decreases. On further decrease in temperature, the gas condenses into liquid state. A substance exist in solid state only when it is below its melting point and in liquid state below its boiling
point or above its freezing point.
III. Solid – Gas Interconversion
Some solids, on heating, directly change into gaseous state, without changing into the liquid state.
Conversely, the gaseous state, on cooling, changes back into solid state, without changing into the liquid state. Such a process is called sublimation. The gaseous form of solid is called sublime. The solid state, formed from the gaseous state on cooling, is called sublimate.
Example of subliming solids: Ammonium chloride, iodine, solid carbon dioxide (dry ice), naphthalene and camphor, the moth balls become smaller in size. With the passage of time. It is because, the naphthalene changes into its vapours, at room temperature.
|
Summary |
|
|
Phase changes |
Methods |
|
Solid to liquid |
Melting |
|
Liquid to gas |
Evaporation |
|
Gas to solid |
Sublimation |
|
Liquid to sol id |
Freezing |
|
Solid to gas |
Sublimation |
|
Gas to liquid |
Condensation |
7. COMPOUNDS AND MIXTURES
A substance composed of two or more different elements is called a Compound. It is chemically combined in fixed proportion by weight. The constituents in a compound can be separated by chemical reaction only.
Eg: Water (), Ammonia (), Sodium chloride (NaCl)…etc.
Mixture consists of two or more different types of elements or compounds which are not chemically combined.
Eg: Chalk in water, sand in water.








