Plant physiology (Physis = nature of life; logos = study) is the branch of botany which deals with the study of life activities of plants. It includes the functional aspects of life processes both at cellular as well as sub-cellular level. Life process or physiological process may be defined, as any chemical or physiological change occurring within a cell or organism and any exchange of substances between the cell or organism and its environment.
1. CONCEPT OF WATER RELATION
Water is mainly absorbed by the roots of the plants from the soil, then it moves upward to different parts and is lost from the aerial parts, especially through the leaves. Before taking up the absorption and movement of water in plants, it is worthwhile to understand the phenomenon of imbibition, diffusion and osmosis involved in the water uptake and its movement in the plants.
Imbibition (L. imbibere – to drink)
The process of adsorption of water by hydrophilic surfaces of a substance without forming a solution is called ‘imbibition’. It is a type of diffusion by which movement of water takes place along a diffusion gradient. The solid particles which adsorb water or any other liquid are called imbibants. The liquid which is imbibed is known as imbibate. Agar, cellulose, pectic substances, protoplasmic protein and other organic compound in plant cells show great power of imbibition.
Characteristics of imbibition
The phenomenon of imbibition has three important characteristics:
Volume change
During the process of imbibition, imbibants increase in volume. It has been observed that there is an actual compression of water. This is due to arrangement of water molecules on surface of imbibant and occupy less volume than the same molecules do when are in free stage in the normal liquid. e.g., If a dry piece of wood is placed in water, they swell and increases in its volume.
Production of heat
As the water molecules are adsorbed on the surface of the imbibant, their kinetic energy is released in the form of heat which increases the temperature of the medium. It is called heat of wetting (or heat of hydration). e. g., during kneading, the flour of wheat gives a warm feeling due to imbibition of water and consequent release of heat.
Development of imbibitional pressure
Imbibition pressure can be defined as the maximum pressure that an imbibant will develop when it is completely soaked in pure water. Imbibition pressure is also called as the matric potential because it exists due to the presence of hydrophilic substances in the cell which include organic colloids and cell wall.
Factors influencing the rate of imbibition
Nature of imbibant: Proteins are the strongest imbibants of water, starch less strong, cellulose being the weakest.
Surface area of imbibant: If more surface area of the imbibant is exposed and is in contact with liquid, the imbibition will be more.
Temperature: Increase in temperature causes an increase in the rate of imbibition.
Degree of dryness of imbibant: If the imbibant is dry it will imbibe more water than a relatively wet imbibant.
Concentration of solutes: Increase in the concentration of solutes in the medium decreases imbibition.
pH of imbibant: Proteins, being amphoteric in nature, imbibe least in neutral medium. Towards highly acidic or highly alkaline pH, the imbibition increases till a maximum is reached, there after it starts slowing down.
Significance of imbibition
- The water is first imbibed by walls of root hairs and then absorbed.
- Water is absorbed by germinating seeds through the process of imbibition and helps in rupturing of seed coat (made up of cellulose).
- The water moves into ovules which are ripening into seeds by the process of imbibition.
Diffusion
The movement of the molecules of gases, liquids and solids from the region of higher concentration to the region of lower concentration is known as diffusion. It may occur between gas and gas (e.g., diffusion of ammonia into air), liquid and liquid (e.g., diffusion of alcohol into water), or solid and liquid (e.g., diffusion of sugar into water).
Diffusion pressure
It is a hypothetical term coined by Meyer (1938) to denote the potential ability of the molecules or ions of any substance to diffuse from an area of their higher concentration to that of their lower concentration.
Diffusion pressure deficit (DPD) or Suction pressure (SP)
The term diffusion pressure (DP) and diffusion pressure deficit (DPD) were put forth by B.S. Meyer in 1938. Now a days, the term water potential (ψ) is used which is equal to DPD, but negative in value. The term suction pressure put forth by Renner (1915). The amount by which the diffusion pressure of water or solvent in a solution is lower than that of pure water or solvent is known as diffusion pressure deficit (DPD)’. Diffusion pressure deficit is the water absorbing capacity of a solution. Therefore, DPD can also be called suction pressure (SP).
Factors influencing rate of diffusion Temperature:
Increase in temperature leads to increase in the rate of diffusion.
Pressure: The rate of diffusion of gases is directly proportional to the pressure. So the rate of diffusion increases with increase in pressure. Rate of diffusion ∝ pressure.
Size and mass of diffusing substance: Diffusion of solid is inversely proportional to the size and mass of molecules and ions.
Rate of diffusion ∝
Density of diffusing substance: The rate of diffusion is inversely proportional to the square root of density of the diffusing substance. Larger the molecules, slower will be the rate of diffusion. This is also called Graham’s law of diffusion.
D ∝ (D = Diffusion and d = Density of diffusing substance).
According to the density the diffusion of substances takes place in following manner:
Gas > Liquid > Solid
The vapours of volatile liquids (scent or petrol) and solids (camphor) also diffuse like gases.
Density of the medium: The rate of diffusion is slower, if the medium is concentrated. Thus, a gas would diffuse more rapidly in vacuum than in air. Substances in solution also diffuse but at a much slower rate than gases.
Diffusion pressure gradient (DPG): The rate of diffusion is directly proportional to the difference of diffusion pressure at the two ends of a system and inversely proportional to the distance between the two.
Significance of diffusion
- Gaseous exchange during the processes of photosynthesis and respiration takes place with the help of diffusion.
- The process of diffusion is involved in the transpiration of water vapours.
- Aroma of flowers is due to diffusion of volatile aromatic compounds to attract pollinating animals.
- During passive salt uptake, the ions are absorbed by process of diffusion.
- Diffusion helps in translocation of food materials.
- Gaseous exchange in submerged hydrophytes takes place by general surface of the cells through diffusion.
Permeability
Permeability is the degree of diffusion of gases, liquids and dissolved substances through a membrane. Different types of membranes may be differentially permeable to different substances.
Types of membranes
On the basis of permeability.
Permeable membrane: These membranes allow free passage of solvent (water) and most of the dissolved substances. e.g., cell wall in plant cells. Filter paper is made up of pure cellulose it also functions as permeable membrane.
Impermeable membrane: This type of membranes with deposits of waxy substances like cutin and suberin, do not allow the entry of water, dissolved substances and gases. e.g., suberized walls of cork cells, cuticle layer of leaf.
Semi-permeable membrane: These membranes permit the movement of solvent molecules only through them, but prevent the movement of solute particles. e.g., egg membrane, animal bladder, parchment paper, copper ferrocyanide membrane, membranes of collodion.
Selectively or Differentially permeable membrane: This type of membranes allow selective passage of solutes along with solvent, through them.
Many biological membranes such as cell membrane (plasmalemma), tonoplast (vacuolar membrane) and the membranes surrounding the sub-cellular organelles are selectively permeable. A non-living selectively permeable membrane is cellophane.
Osmosis
Osmosis (Gr. Osmos = a pushing or impulse) was discovered by Abbe Nollet in 1748 and also coined the term ‘osmosis’. First of all Traube (1867) used copper ferrocyanide and developed semipermeable membrane to show its utility in the osmosis of plant physiology. First time Pfeffer in (1887) developed osmoscope by using semipermeable membrane.
Osmosis is special type of diffusion of a liquid, when solvent moves through a semipermeable membrane from a place of higher diffusion pressure to a place of lower diffusion pressure.
or
It is the migration of solvent from a hypotonic solution (of lower concentration) to hypertonic solution (of higher concentration) through a semi-permeable membrane to keep the concentration equal. In for malin preserved spirogyra filament, selective permeability of plasma membrane is lost and hence no effect on placing in hypertonic solution.
Reverse Osmosis
It is the reverse movement of water through a semipermeable membrane from a more concentrated solution to a more dilute solution by applying external pressure on the more concentrated solution. It is used in removing salts from saline water as well as extra – purification of water.
Osmotic pressure (OP)
Pfeffer coined the term osmotic pressure.
Osmotic pressure is that equivalent of maximum hydrostatic pressure which is produced in the solution, when this solution is separated from its pure solvent by a semipermeable membrane.
Types of osmosis
Depending upon the movement of water into or outside of the cell, osmosis is of two types.
Endosmosis: The osmotic flow of water into a cell, when it is placed in a solution, whose solute concentration is less than that of the cell sap, is called endosmosis e.g., swelling of raisins, when they are placed in water. When a fish of marine water kept in fresh water then it will die due to endosmosis.
An animal cell placed in pure water will swell up and bursts.
Pollen grains of some of plants germinate on stigma soon but they burst in water or dilute sugar solution.
Exosmosis: The osmotic outflow of water from a cell, when it is placed in a solution, whose solute concentration is more than that of the cell sap, is called exosmosis. e.g., shrinkage of grapes, when they are placed in strong sugar solution.
Osmotic concentrations (Types of solutions)
Hypotonic solution (hypo = less than). A solution, whose osmotic concentration (solute potential) is less than that of another solution or cell sap is called hypotonic solution. If a cell is placed in such a solution, water starts moving into the cell by the process of endosmosis, and cell becomes turgid.
Hypertonic solution (hyper = more than). A solution, whose osmotic concentration (solute potential) is more than that of another solution or cell sap is called hypertonic solution. If a cell is placed in such a solution, water comes out of the cell by the process of exosmosis and cell becomes flaccid. If potato tuber is placed in concentrated salt solution it would becomes shrink due to loss of water from its cell.
Isotonic solution (iso = the same). A solution, whose osmotic concentration (solute potential) is equal to that of another solution or cell sap, is called isotonic solution. If a cell is placed in isotonic solution, there is no net changes of water between the cell and the solution and the shape of cell remain unchanged.
In xerophytes, the osmotic concentration of cell sap is more than the normal. The osmotic pressure of given solution can be calculated by following formula.
Osmotic pressure = CST
Where, C = Molar concentration of solution, S = Solution constant, which is 0.082 and T = Absolute temperature i.e., 273°K.
Significance of osmosis in plants
- The phenomenon of osmosis is important in the absorption of water by plants.
- Cell to cell movement of water occurs throughout the plant body due to osmosis.
- The rigidity of plant organs (i.e., shape and form of organism) is maintained through osmosis.
- Leaves become turgid and expand due to their OP.
- Growing points of root remain turgid because of osmosis and are thus, able to penetrate the soil particles.
- Opening and closing of stomata is affected by osmosis.
- Movement of plants and plant parts, e.g., movement of leaflet of Indian telegraph plant.
Turgor pressure (TP)
The plant cell, when placed in pure water, swells but does not burst. Because of negative osmotic potential of the vacuolar solution (cell sap), water will move into the cell and will cause the plasmalemma be pressed against the cell wall. The actual pressure that develops that is the pressure responsible for pushing the membrane against cell wall is termed turgor pressure.
Significance of turgidity in plants
- It provides stability to a cell.
- Turgidity keeps the cell and their organelles (mitochondria, plastids and microbodies) fully distended. This is essential for plants to live and grow normally.
- Turgor pressure helps in cell enlargement, consequently in stretching of the stems and in keeping leaves erect and fully expanded.
- The turgid cells provide mechanical support necessary for the non woody tissues (maize, sugarcane, banana etc.).
- Loss of turgidity leads to wilting of leaves and drooping of shoots.
- The opening and closing of stomata are regulated by the turgidity of the guard cells.
- Leaf movements (seismonastic movement) of many plants (such as bean, sensitive plant Mimosa pudica) are controlled by loss and gain of cell turgor.
- Due to turgor pressure plumule and radicles force out from seeds at the time of seed germination.
Wall pressure (WP)
Wall pressure (WP) may, therefore, be defined as ‘the pressure exerted by the cell wall over the protoplast to counter the turgor pressure. Normally wall pressure is equal and opposite to turgor pressure (WP =TP) except when the cell become flaccid. The value of the two forces continue to rise with the continued entry of water, till the cell becomes fully turgid.
Interrelationship of DPD (S.P.), OP and TP (WP)
DPD indicates the sucking power of suction pressure. As water enters into the cell the TP of the cell is increased. Cell wall exerts equal and opposite WP against TP. The actual force responsible for entry of water will be therefore OP–TP
i.e., DPD = OP – WP (As WP = TP)
DPD = OP – TP
Consider that a plant cell with OP = 10 atm. is immersed in pure water. In the beginning TP inside the cell is zero
i.e., DPD = OP = 10 atm.
When water enters into the cell, TP increases. Turgidity increases and cell wall develops equal and opposite WP. At the stage of equilibrium TP = 10 atm. and DPD will become zero. It is important to note that OP was same when cell was flaccid and turgid.
DPD = OP – TP = 10 – 0 = 10 (when flaccid) = 10 – 10 =0 (when turgid)
The entry of water in cell to cell depends upon the DPD and not on OP and TP. This can be examplified as follows:
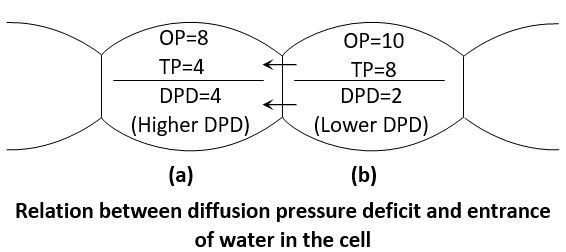
Since the DPD of cell A is more, it has less water and, therefore water would diffuse from cell B into the cell A (because that DPD of cell B is less than that of A or it has more water than cell A has). The entry of water into the cell A would stop when DPD of both the cells become equal. In this way water moves from a cell with less DPD into the cell with more DPD. Thus, DPD is the osmotic parameter, which determines the flow of water from one cell to another.
Under given suitable conditions, the DPD is more than OP when TP is negative.
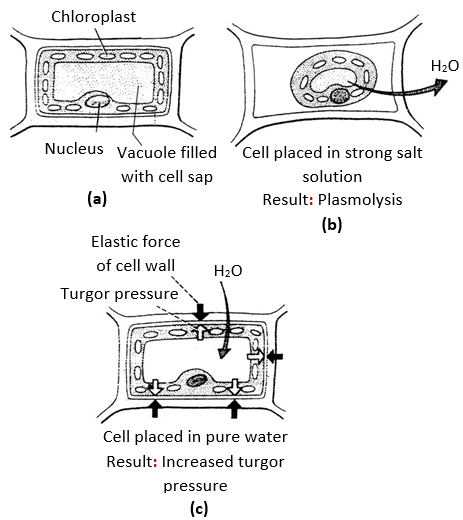
Plasmolysis (Gr. Plasma = something formed; lysis = loosing)
“The shrinkage of the protoplast of a living cell from its cell wall due to exosmosis under the influence of a hypertonic solution is called plasmolysis”. The stage of plasmolysis, when the protoplast just begins to contract away from the cell wall is called incipient plasmolysis. The stage when the cell wall has reached its limit of contraction and the protoplast has detached from cell wall attaining spherical shape is called evident plasmolysis. If a cell with incipient plasmolysis is placed in a hypertonic solution it will show more plasmolysis.
Deplasmolysis
“The swelling up of a plasmolysed protoplast due to endosmosis under the influence of a hypotonic solution or water is called deplasmolysis’. Deplasmolysis is possible only immediately after plasmolysis otherwise the cell protoplast becomes permanently damaged. The value of TP becomes zero at the time of limiting plasmolysis and below zero during incipient and evident plasmolysis. Leaf of Tradescantia is used for demonstration of plasmolysis in laboratory.

Significance of plasmolysis
- The OP of a cell can be measured by plasmolysis. The OP of a cell is roughly equal to the OP of a solution that causes incipient plasmolysis in the cell.
- Salting of pickles, meat, fishes etc. and addition of sugar to jams, jellies, cut fruits etc., prevent their decay by microbes, as the latter get killed due to plasmolysis or due to high concentration of salt or sugar.
- By salting, the weeds can be killed from tennis courts and the growth of plants can be prevented in the cracks of walls.
- Plasmolysis is helpful in determining whether a particular cell is living or dead as plasmolysis does not occur in a dead or non living cell.
Water potential (Ψ)
The term water potential was coined by slatyer and Taylor (1960). It is a modern term which is used in placed of D.P.D. The movement of water in plants cannot be accurately explained in terms of difference in concentration or in any other linear expression. The best way to express spontaneous movement of water from one region to another is in terms of the difference of free energy of water between two regions. Free energy is the thermodynamic parameter, that determine the direction in which physical and chemical changes must occur. The potential energy of water is called water potential. e.g., water is stored behind a dam. When the water runs downhill, its potential energy can be converted to electrical energy. This conversion of energy of water is due to gravity. The other source that provides energy to water is pressure. The increasing pressure increases the free energy thereby increasing water potential.
Water running downhill due to gravity can be made to run uphill by overcoming the water potential (energy) by applying pressure. This means that water moves from the point, where water potential is greater to the other, where water potential is less. The difference in water potential between two points is a measure of the amount of work or energy needed to move water from one point to the other. Thus, based on the concept of water potential, the direction of water movement can be predicted. Water potential is measured in terms of pressure.
Measurement unit of water potential is pascal, Pa (1 mega pascal, Mpa = 10 bars). It is represented by Greek letter, Psi (Ψ). Water potential (Ψw) is the difference between chemical potential of water at any point in a system (μω) and that of pure water under standard conditions (μω°). The value of water potential can be calculated by formula: Ψw = (μω) – (μω°) = RT 1 n e/e°
where Ψw = water potential, R is gas constant, T is absolute temperature (K), e is the vapour pressure of the solution in the system at temperature T, and e° the vapour pressure of pure water at the same temperature.
The direction in which water will move from one cell to another cell depends on water potential in two regions. Water potential is measured in bars. A bar is a pressure unit which equals 14.5 lb/in2, 750 mm Hg or 0.987 atm. Water potential of pure water at normal temperature and pressure is zero. This value is considered to be the highest. The presence of solute particles reduces the free energy of water and thus decreases the water potential. Therefore, water potential of a solution is always less than zero or has negative value.
Component of water potential
The water potential (Ψ) in a plant cell or tissue can be written as the sum of the matric potential (Ψm) due to binding of water to cell walls and cytoplasm, the solute potential (Ψs) due to concentration of dissolved solutes, which by its effect on the entropy components reduces the water potential and the pressure potential (Ψp) due to hydrostatic pressure, which by its effect on the energy components increases the water potential:
Ψ = Ψm +Ψs + Ψp …… [1]
Matric potential (Ψm)
Matric is the term used for the surface (such as, soil particles, cell walls, protoplasm’s, etc.) to which water molecules are adsorbed. The matric potential (Ψm) is the component of water potential influenced by the presence of a matrix. It has got a negative value. In case of plant cells and tissues, the matric potential is often disregarded because it is not significant in osmosis. Thus, the above equation [1] may be simplified as follows:
Ψ = Ψs + Ψp …… [2]
In normal cells of mesophytes and hydrophytes it is almost negligible.
Solute potential (Ψs)
Solute potential is also known as Osmotic potential. It is defined as the amount by which the water potential is reduced as a result of the presence of solute. Solute potentials or osmotic potentials (Ψs) are always in negative values (number). The term solute potential takes the place of osmotic pressure (π; Pi) expressed in bars with a negative sign. Ψs = –π
Pressure potential (Ψp) Plant cell wall is elastic and it exerts a pressure on the cellular contents. As a result of inward wall pressure, hydrostatic pressure is developed in the vacuole termed as turgor pressure. The pressure potential is usually positive and operates in plant cells as wall pressure and turgor pressure. Its magnitude varies between +5 bars (during day) and +15 bars (during night).
Physical states of cell
Three physical states of cell, according to their water potential, are as follows:
In case of fully turgid cell: In case of fully turgid cell, the net movement of water into the cell is stopped. The cell is in equilibrium with the water outside. The water potential in such a case will be zero (0).
Water potential = Osmotic potential + Pressure potential
Ψ= Ψs + Ψp
A cell at full turgor has its osmotic potential and pressure potential equal but opposite in sign. Therefore, its water potential will be zero. For example, supposing a cell has its Ψs of –10 bars and Ψp of 10 bars the resultant water potential will be zero as follows:
Ψ= Ψs + Ψp
Ψ= –10 bars + 10 bars
Ψ= 0 bars
In case of flaccid cell: When a plant cell is flaccid, its turgor becomes zero (corresponding to a turgor pressure of a 0 bars). Zero turgor is approached under natural conditions when a tissue is severely wilted. A cell at zero turgor has an osmotic potential (Ψs) equal to its water potential (Ψ). For example, supposing a flaccid cell has an osmotic potential of –10 bars and pressure potential (Ψp) of 0 bars.
Water potential = Osmotic potential + Pressure potential
Ψ= Ψs + Ψp
Ψ= –10 bars + 0 bars
Ψ= –10 bars
The water potential of the cell will be –10 bars, which is less as compared to the water potential of pure water (0 bars).
In case of plasmolysed cell: When the vacuolated parenchymatous cells are placed in solutions of sufficient strength the protoplast decreases in volume to such an extent that they shrink away from the cell wall. The cells are plasmolysed. Such cells have negative value of pressure potential (negative turgor pressure). The resultant water potential will be more negative, as for example, a plasmolysed cell has osmotic potential of –10 bars and pressure potential of –2 bars the water potential of the cell will be –12 bars.
Water potential = Osmotic potential + Pressure potential
Ψ= Ψs + Ψ p
Ψ= –10 + (–2)
Ψ= –12 bars
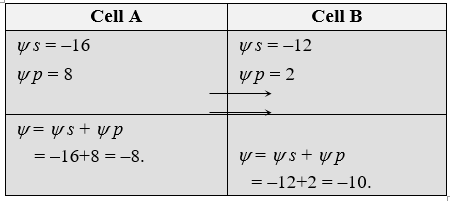
Movement of water between two adjacent cells
Suppose A and B are two adjacent plant cells where osmotic movement of water can occur. Cell A has osmotic potential (Ψs) of –16 bars and pressure potential of 8 bars. The cell B has osmotic potential of –12 bars and pressure potential of 2 bars. The movement of water will be as follows:
Wilting
A plant usually fails to survive if it is conditioned to water deficiency. The symptoms appear in the plant, plant parts or in the cells due to scarcity of water are termed as wilting. It is loss of turgidity causing folding and drooping of leaves and other soft aerial parts of the plant. It is of three types:
- Incipient wilting: There is no external symptoms but the mesophyll cells lose a part of their water content during midday due to transpiration.
- Temporary wilting: It occurs during midday and is visible externally due to drooping of leaves and young shoots. At noon the rate of transpiration is quite high as compared to water absorption, which decreases further due to depletion of water around rootlets. It is corrected in the afternoon when transpiration decreases.
- Permanent wilting: It is the last stage in wilting when the aerial parts do not regain turgidity even if placed in water saturated atmosphere. It is caused by decrease in water content of the soil which increases TSMS (Total soil moisture stress) or resistance to absorption to such an extent that plant roots are unable to absorb water. Permanent Wilting Percentage (PWP) is the percentage of water on the dry weight basis of the soil that is present in the soil when the plants growing in it first touch the condition of permanent wilting. This value varies between 1–15% and depends upon the texture of the soil e.g., clay has higher PWP than sand.
2. ABSORPTION OF WATER
Water is absorbed from soil by root system and specially by younger parts (i.e., root tips). In higher plants water is absorbed through root hairs.
Soil water
The chief source of soil water is rain. In soil water is found in different forms. The total amount of water present in the soil is called holard, of this the available to the plant is called chresard and the water which cannot be absorbed by the plants is called echard.
Water occurs freely deep in the soil and above the parent rock, it is called ground water. These are briefly described below
Gravitational water
When the water enters the soil and passes the spaces between the soil particles and reaches the water table, the type of soil water is called gravitational water.
Capillary water
It is the water which is held around soil particles in the capillary space present around them due to force like cohesion and surface tension. This is the water which can be utilised by the plants. It is also called growth water. It occurs in the form of films coating smaller soil particles.
The availability of capillary water to the plant depends upon its diffusion pressure deficit which is termed as the soil moisture stress. The plant cells have a DPD much more than the soil moisture stress for proper absorption of water.
Hygroscopic water
This is the form of water which is held by soil particles of soil surfaces. The water is held tightly around the soil particles due to cohesive and adhesive forces. Cohesive and adhesive forces greatly reduce the water protential (Ψω) and thus this type of water in soil is not available to plants.
Run-away water
After the rain, water does not enter the soil at all, but drained of along the slopes. It is called run-away water. Plants fail to avail this water.
Chemically combined water
Some of the water molecules are chemically combined with soil minerals (e.g., silicon, iron, aluminium, etc.). This water is not available to the plants.
Water vapour
That portion of the pore space in a soil which is not occupied by liquid water contain a soil atmosphere that always includes water vapour.
Water holding capacity
The amount of water actually retained by the soil is called field capacity or water holding capacity of the soil. It is about 25–35% in common loam soil. The excess amount of water beyond the field capacity produces water logging.
Soil atmosphere
In moderately coarse soils as well as in heavy soils (fine textured soil) that are with aggregated particles; there exists large interstitial spaces which facilitate the diffusion of gases. As a result the CO2 produced in a soil by respiration of soil organisms and roots is able to escape rather easily and oxygen used up in this process diffuses into the soil with corresponding case.
Soil organisms
The soil fauna includes protozoans, nematodes, mites, insects, earthworms, rats. Protozoans alone are approximately 1 million per gram of soil. Blue green algae and bacteria increase nitrogen content by nitrogen fixation in soil.
Water absorbing organs
Plants absorb water mostly from the soil by their roots, but in some plants even aerial parts like stem and leaves also do the absorption of atmospheric water or moisture. Some important examples of such plants are Vitis, Solanum, Lycopersicon, Phaseolus, Kochia baosia and Beta.
However, maximum absorption of water is done by the roots.
This area is usually characterized by the presence of root hairs which serve to increase the area of contact between the root surface and soil.
The root hairs develop mainly at the tip just above the zone of elongation (cell maturation). A root hair is the unicellular tubular prolongation of the outer wall of the epiblema.
During water absorption the plasma membrane of root hair, the cytoplasm and the vacuole membrane (tonoplast) behave together as a single differentially permeable membrane. Root hairs are at the most 1.25 cm in length and never more than 10 mm in diameter.
The root-hairs of plants increase the absorption surface of a root system about 5 to 20 times and because they extend so widely through the soil they make available a supply of water that the plant could not otherwise obtain. Water potential of root hair cells is generally –1 to –4 atm.
Pathway of water movement in root
Water in the root moves through three pathways. Munch coined the term apoplast and symplast.
Apoplast pathway: The apoplastic movement of water occurs exclusively through the cell wall without crossing any membrane.
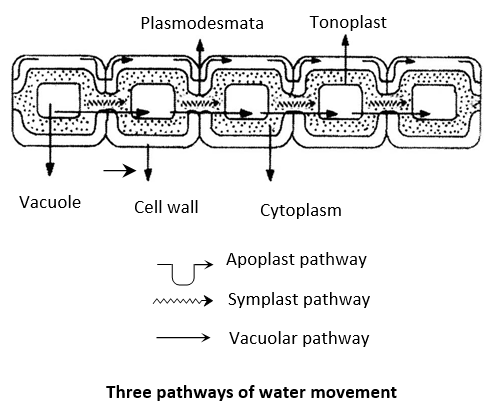
Symplast pathway: The symplastic movement of water occurs from cell to cell through the plasmodesmata.
Transmembrane pathway: Water after passing through cortex is blocked by casparian strips present on endodermis. The casparian strips are formed due to deposition of wax like substance, suberin. In this pathway, water crosses at least two membranes from each cell in its path. These two plasma membranes are found on entering and exiting of water. Here, water may also enter through tonoplast surrounding the vacuole i.e., also called as vacuolar pathway.
Mechanism of water absorption
Two distinct mechanisms which are independently operated in the absorption of water in plants. These mechanisms are:
1. Active absorption 2. Passive absorption
Renner (1912, 1915) coined the term active and passive water absorption.
1. Active absorption: Active absorption takes place by the activity of root itself, particularly root hairs. The factor responsible for water absorption is present within the roots. It utilizes metabolic energy. There are two theories of active absorption:
Osmotic theory: It was proposed by Atkins (1916) and Priestley (1922). It is purely a physical process, which does not directly require expenditure of energy.
A root hair cell functions as an osmotic system. Water is absorbed by the root hair due to osmotic differences between soil water and cells sap. The osmotic pressure of soil water remains below 1 atm, but that of cell sap is usually 2–8 atms. Thus, there exists a great difference in the osmotic pressures of the two sides or in other words there exists, water potential gradient between the soil solution and cell sap. The soil solution having less OP, has higher water potential than the cell sap with more OP (i.e., the cell sap has more negative water potential). Thus, water moves from the region of higher water potential towards the region of lower water potential.
Non-osmotic theory: It was proposed by Thimann (1951) and Kramer (1959). It has been observed that absorption of water still occurs, if the concentration of cell sap in the root hair is lower than that of the soil water, or water is absorbed against concentration gradient (i.e., from higher DPD to lower DPD). Such type of water absorption occurs on the expense of energy obtained from respiration.
Following evidences support the view that energy is utilized during active absorption of water:
(i) Rate of water absorption is directly proportional to the rate of respiration.
(ii) Respiratory inhibitors such as KCN, which inhibit the absorption of water.
(iii) Auxins (growth hormones), which increase respiration also promote water absorption.
(iv) Wilting of plants occur in non-aerated soils such as water logged soils, as roots fail to absorb water in absence of respiration.
2. Passive absorption: It is the most common and rapid method of water absorption. The factor responsible for water absorption is present somewhere else than roots. It accounts for about 98% of the total water uptake by plant.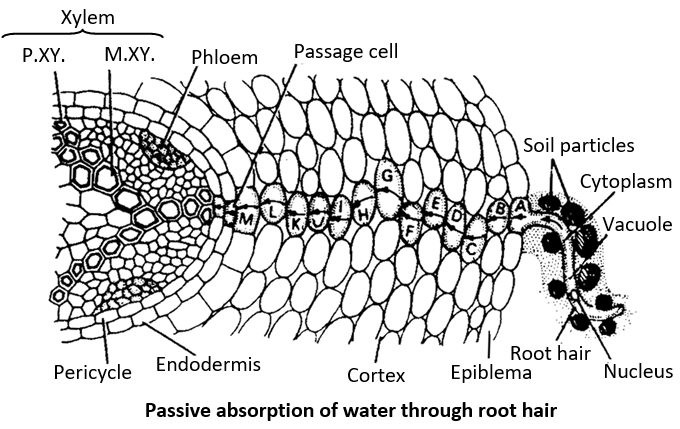
According to this theory, the forces responsible for absorption of water originate not in the cells of roots but in the cells of transpiring shoots. The root cells remain passive.
Due to transpiration, the DPD of mesophyll cells in the leaves increases which causes absorption of water by these cells from the xylem vessels of leaves. As the water column is continuous from leaves to roots, this deficit is transmitted to the xylem elements of roots and finally to root hairs through pericycle, endodermis and cortex. In this way water is continuously absorbed due to transpiration pull created in the leaves. This type of water transport occurs mainly through the apoplast in cortex but through the symplast in endodermis and pericycle.
The path of water from soil up to secondary xylem is:
Soil →Root hair cell wall →Cortex → Endodermis →Pericycle →Protoxylem →Metaxylem.
Factors affecting rate of water absorption
The different factors which influence the rate of water absorption by a plant can be divided into external or environmental and the internal factors.
External or Environmental factors
The amount of soil water: It is optimum at field capacity. Water absorption decreases above it. It begins to decline and stops at PWP.
Concentration of the soil solution: If the concentration of solutes increases in the soil water, its OP also increases which slows down or even inhibits the absorption of water. It happens due to addition of enough fertilizers in the soil increasing its salinity. This is popularly called as physiological dryness.
Soil aeration: Water absorption is done more efficiently in well aerated soil. Any deficiency of oxygen stops the respiration of roots and causes accumulation of CO2 thus the protoplasm becomes viscous and the permeability of plasma membrane decreases. Due to all these factors the rate of water absorption is reduced. This is the reason for death of plants in flooded areas.
Soil temperature: The optimum temperature for maximum rate of water absorption ranges between 20°C and 30°C. Too high temperature kills the cells. At very low temperatures (4°C) water absorption is reduced or stopped and about OºC it is almost checked.
Transpiration: The rate of absorption of water is almost directly proportional to the rate of transpiration. A higher rate of transpiration increases the rate of water absorption.
Internal factors
Efficiency of the root system: A plant with deep and elaborate root system can absorb more water. The number of root hairs will be more in a highly branched and elaborate root system, thus its more surface area will be in contact with water.
In gymnosperms, the root hairs are absent, even then they are able to absorb water due to presence of mycorrhizal hyphae.
In epiphytes (orchid), the roots develop a special type of hygroscopic tissue called as velamen which can absorb atmospheric moisture.
Metabolic activity of roots: The poor aeration or use of metabolic inhibitors (e.g., KCN) inhibits the rate of water absorption. The metabolic activities help in proper growth of root system and generation of energy for absorption of certain vital minerals.
Absorption of water through leaves: Many species of plants can absorb at least limited amounts of water through the leaves. Temporary immersion of aerial organs in flood waters takes place in some cases. Also the aerial organs of plants frequently become wet as a result of fog, dew or rain. Most of the water enters through the epidermal cells, although in some species hairs and specialized epidermal cells provide regions of high permeability. In general water absorption is more rapid in young leaves than in old leaves of the same plant.
3. ASCENT OF SAP
‘The upward transport of water along with dissolved minerals from roots to the aerial parts of the plant is called Ascent of sap’. It is also called translocation of water. The water with dissolved minerals is called sap.
Path of ascent of sap
It is now well established that the ascent of sap takes place through xylem. In herbaceous plants almost all the tracheary elements participate in the process, but in large woody trees the tracheary elements of only sap wood are functional. Further, it has been proved experimentally that sap moves up the stem through the lumen of xylem vessels and tracheids and not through their walls.
Theories of ascent of sap
The various theories put forward to explain the mechanism of ascent of sap in plants can be placed in following three categories:
- Vital force theories
- Root pressure theory
- Physical force theories
1. Vital force theories
According to these theories the forces required for ascent of sap are generated in living cells of the plant. These theories are not supported by experimental evidences hence they have been discarded. Some of the important vital force theories are mentioned below:
According to Westermaier (1883), ascent of sap occurs through xylem parenchyma; tracheids, and vessels only act as water reservoirs.
Relay pump theory (Clambering theory): According to Godlewski (1884) ascent of sap takes place due to rhythmatic change in the osmotic pressure of living cells of xylem parenchyma and medullary rays and are responsible for bringing about a pumping action of water in upward direction. Janse (1887) supported the theory and showed that if lower part of the shoot is killed upper leaves were affected.
Criticism
(i) Strasburger (1891) and Overton (1911) used poisons (like picric acid) and excessive heat to kill the living cells of the plant. When such twigs were dipped in water, ascent of sap could still occur uninterrupted. This definitely proved that no vital force is involved in ascent of sap.
(ii) Xylem structure does not support the Godlewski’s theory. For pumping action living cells should be in between two xylem elements and not on lateral sides as found.
Pulsation theory: Sir J.C. Bose (1923) said that living cells of innermost layer of cortex, just outside the endodermis are in rhythmatic pulsations. Such pulsations are responsible for pumping the water in upward direction. According to Bose, the pulsatory cells pump the water into vessels.
Criticism: Dixon failed to verify the results of Bose. It has been estimated that sap should flow through 230–240 pulsating cells per second to account for normal rate of pulsations. This rate is several times higher as would be possible to the Bose theory (Shull, MacDougal, Benedict).
2. Root pressure theory
It was proposed by Priestley (1916). According to this theory the water, which is absorbed by the root-hairs from the soil collects in the cells of the cortex. The cortical cells become fully turgid. In such circumstances the elastic walls of the cortical cells, exert pressure on their fluid-contents and force them towards the xylem vessels. Due to this loss of water these cortical cells become flaccid, again absorb water, become turgid and thus again force out their fluid contents. Thus the cortical cells of the root carry on intermittent pumping action, as a result of which considerable pressure is set up in the root. This pressure forces water up the xylem vessels. Thus the pressure, which is set up in the cortical cells of the roots due to osmotic action, is known as the root pressure. This term was used by Stephan Hales. According to Style, root pressure may be defined as “the pressure under which water passes from the living cells of the root in the xylem”.
Objections
(i) Taller plants like Eucalyptus need higher pressure to raise the water up. While the value of root pressure ranges from 2-5 atmospheres, a pressure of about 20 atm. is required to raise the water to tops of tall trees.
(ii) The absence of root pressure, ascent of sap continues.
(iii) Plants growing in cold, drought or less aerated soil, root pressure fails to appear and transport of water is normal.
In gymnosperms root pressure has rarely been observed.
3. Physical force theories
According to these theories the ascent of sap is purely a physical process. Some of the physical force theories are mentioned below:
Capillary force theory: It was proposed by Boehm (1809). According to him, in the fine tubes, the water rises as a result of surface tension to different heights depending on the capillarity of the tube. The finer the tube, the greater will be the rise of water in it. But the xylem vessels are sometimes broader than the capillarity range, and hence the rise due to surface tension will be negligible.
Objections
- For capillarity a free surface is required.
- Atmospheric pressure can support a column of water only up to the height of 34 feet.
- Water can rise only up to the height of one meter in xylem vessels having diameter of 0.03mm.
- In gymnosperms usually the vessels are absent.
Imbibitional theory: It was proposed by Unger (1868) and supported by Sachs (1879). According to them, water moves upward in the stem through the walls of the xylem vessels. This theory is not accepted now because it is proved that water moves through the lumen of the xylem vessels and tracheids.
Atmospheric pressure theory: Due to the loss of water by transpiration, the leaves draw water from the xylem vessels through osmotic pressure. The atmospheric pressure acting on the water in the soil forces the water to rise up in the xylem vessels to fill the vacuum. But the atmospheric pressure can force the water to a height of only 10 meters. So it is evident that atmospheric pressure alone cannot force water to a height of 100 metres or more.
Jamin’s chain theory: In xylem water and air bubbles are found alternately. Thus upward movement occurs.
Cohesion of water and transpiration pull theory: This is the most widely accepted theory put forth by Dixon and Joly in 1894, and further supported by Renner (1911, 1915), Curtis and Clark (1951), Bonner and Golston (1952), Kramer and Kozlowski (1960).
It is also known as Dixon’s cohesion theory, or cohesion-tension theory.
This theory depends on the following assumptions:
- The xylem vessels are connected with each other, thus the water in them is in a continuous column from the root hairs to the mesophyll cells.
Walls of tracheids and vessels of xylem are made up of lignin and cellulose and have strong affinity for water (adhesion). The cell wall of adjacent cells, and those between the cells and xylem vessels all through the plant do not affect the continuity of the water column.
- Due to the transpiration from leaves, a great water deficit takes place in its cells. As a result of this deficit the water is drawn osmotically from the xylem cells in leaf veins, and by the cells surrounding the veins. Thus a sort of pull is produced in the uppermost xylem cells in the leaves. It is called as the transpiration pull.
- The water molecules have a great mutual attraction with each other or in other words we can say that they have tremendous cohesive power which is sometimes as much as 350 atmospheres. Thus the transpiration pull develops a negative pressure in the uppermost xylem cells. It is transmitted from there into the xylem of stems, and from there to the roots.
In this way the water rises due to the transpiration pull and the cohesive power of water molecules from the lowest parts of the roots to the highest peaks of the trees. The osmotic pressure in the transpiring leaf cells often reaches to 30 atmospheres whereas only 20 atmospheres are needed to raise the water to the tops of highest known trees.
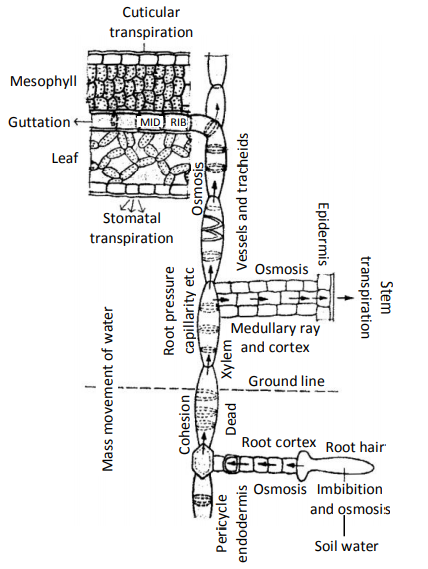
path of ascent os sap showing transpiration pull
Objections: This is the most generally accepted theory, yet there are some objections against it which it fails to explain.
The most important objection is that leaving smaller plants, the water column has been found to contain air bubbles, and so their continuity breaks at such places. This phenomenon is known as cavitation and has been demonstrated by Milburn and Johnson (1966). However, Scholander overruled this problem by suggesting that continuity of water column is maintained due to presence of pits in the lateral walls of xylem vessels.
Velocity of ascent of sap: Huber and Schmidt (1936) calculated the velocity of ascent of sap using radioactive 32P, specific dyes and also by heat-pulse transport between two specific points of stem. It varies between 1 and 6 meters per hour but under high transpirational conditions, it may be as high as 45 meters per hour. It is more in ring porous woods having large vessels. It is slowest in gymnosperms.
4. TRANSPIRATION
“The loss of water in the form of vapours from the aerial parts of a plant is called transpiration”. Maximum transpiration occurs in mesophytic plants.
About 98 percent of the water absorbed by land plants evaporates from the aerial parts and diffuse in to the atmosphere.
Differences between transpiration and evaporation
|
S.No. |
Transpiration |
Evaporation |
|
1. |
It is a physiological process and occurs in plants. |
It is a physical process and occurs on any free surface. |
|
2. |
The water moves through the epidermis with its cuticle or through the stomata. |
Any liquid can evaporate. The living epidermis and stomata are not involved. |
|
3. |
Living cells are involved. |
It can occur from both living and non-living surfaces. |
|
4. |
Various forces (such as vapour pressure, diffusion pressure, osmotic pressure, etc) are involved. |
Not much forces are involved. |
|
5. |
It keeps the surface of leaf and young stem wet and protects from sun burning. |
It causes dryness of the free surface. |
1. Magnitude of transpiration
A tropical palm under well watered conditions may lose as much as 500 litres of water per day. Daily loss of water by an apple tree may be 10-20 litres. A maize plant may lose 3-4 litres of water per day.
2. Types of transpiration
Most of the transpiration takes place through the leaves. It is called foliar transpiration. Stems transpire very little. Transpiration from stem is called cauline transpiration. Transpiration is of four types –
(i) Cuticular transpiration: Cuticle is a layer of wax like covering on the epidermis of leaves. If it is thin, upto 20 percent of the total transpiration may take place through it, but with the increase in its thickness (e.g., in xerophytes), the water vapour loss is reduced.
(ii) Lenticular transpiration: Loss of water vapours through lenticels is called lenticular transpiration. It amounts to about 0.1 percent of the total water loss through transpiration.
(iii) Stomatal transpiration: The loss of water vapour, which occurs through specialized pores on leaf surface (stomata) is called stomatal transpiration. It amounts 80-90 percent of the total water vapour loss from the plants. It is the most common type of transpiration.
(iv) Bark transpiration: This type of transpiration occurs through corky covering of the stems. Bark transpiration is very little but its measured rate is often more than lenticular transpiration due to larger area. The amount is 0.5% of the total transpiration.
3. Structure of stomata
Stomata are the microscopic openings most commonly found in the leaves. These may be present in young stems and sometimes even in fruits (e.g., citrus, banana, cucumber, etc.). Each stomatal opening is surrounded by two specialized epidermal cells, called as the guard cells.
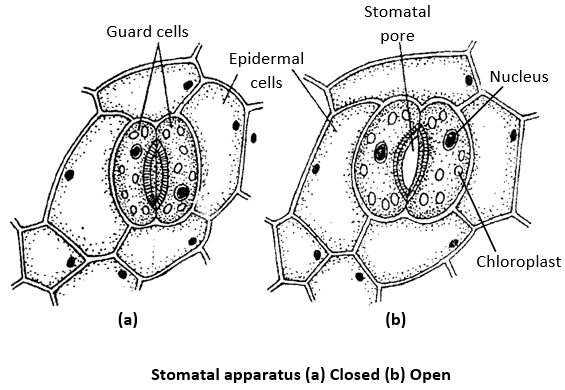
Because of their small size, the guard cells are rapidly influenced by turgor change and thus regulate the opening and closing of stomata. The guard cells of dicot leaves are kidney-shaped or raniform whereas those of monocots (family Gramineae) are dumbel-shaped or elliptical. The guard cells are surrounded by epidermal cells called as the accessory cells or subsidiary cells. These are different from the normal cells of epidermis having chloroplasts. These are also different from other cell as these develop from same cell from which guard cells develop. The stoma with subsidiary cells is called stomatal apparatus. Each stoma leads into a air space called sub stomatal cavity. Each guard cell has a thin layer of cytoplasm along the cell wall and a large vacuole. Its cytoplasm contains a distinct nucleus and several chloroplasts. The cell wall of guard cells around the stomatal pores are thickened and elastic due to presence of a secondary layer of cellulose.
The average length of stomata is 20–28mm and breadth 3–10mm.
4. Number of stomata on leaves
The number of stomata is not equal on both surface of leaves in different plants. Number of stomata per square cm is 1,000–60,000 or 10–600/mm2 in different plants sps.
5. Types of stomata
On the basis of orientation of subsidiary cells around the guard cells, Metcalfe and Chalk classified stomata into following types:
Anomocytic: The guard cells are surrounded by a limited number of unspecialized subsidiary cells which appear similar to other epidermal cells. e.g., in Ranunculaceae family.
Anisocytic: The guard cells are surrounded by three subsidiary cells, two of which are large and one is very small. e.g., in Solanaceae and Cruciferae families.
Paracytic: The guard cells are surrounded by only two subsidiary cells lying parallel to the guard cells e.g., Magnoliaceae family.
Diacytic: The guard cells are surrounded by only two subsidiary cells lying at right angles to the longitudinal axis of the guard cells. e.g., Acanthaceae and Labiatae families.
Actinocytic: The guard cells are surrounded by four or more subsidiary cells and which are elongated radially to stomata.
6. Distribution of stomata
The stomata differ in their distribution on the two surfaces of the leaf. The leaves are classified into following types on the basis of stomatal distribution on them:
Epistomatic (Water Lily type): Stomata are present only on the upper epidermis of leaves. These are found in water Lily, Nymphaea and many other floating hydrophytes.
Hypostomatic (Apple or Mulberry type): Stomata are present only on the lower surface of leaves. e.g., Apple, mulberry, peach and walnut.
Amphistomatic: Stomata are present on both the surfaces of leaves. It can further be subdivided into two types:
(i) Anisostomatic (Potato type): The number of stomata is more on the lower surface and less on the upper surface. In other words, the lower surface is multistomatic and the upper surface is paucistomatic. Such leaves are also called as dorsiventral leaves. e.g., Potato, tomato, bean, pea, and cabbage.
(ii) Isostomatic (Oat type): The stomata are equally distributed on both the surfaces of leaves. These leaves are also called as isobilateral leaves. These are found in monocots e.g., Oat, maize, grasses, etc.
Astomatic (Potamogeton type): Stomata are either absent altogether or vestigial. e.g., Potamogeton and submerged hydrophytes.
7. Daily periodicity of stomatal movement Loftfield (1921) classified the stomata into four types, depending upon the periods of opening and closing.
Alfalfa type (Leucerne type): The stomata remain open throughout the day but close during night, e.g., Pea, bean, mustard, cucumber, sunflower, radish, turnip, apple, grape.
Potato type: The stomata close only for a few hours in the evening, otherwise they remain open throughout the day and night e.g., Cucurbita, Allium, Cabbage, Tulip, Banana etc.
Barley type: These stomata open only for a few hours in the day time, otherwise they remain closed throughout the day and night, e.g., Cereals.
Equisetum type: The stomata remain always open throughout the day and night. e.g., Amphibious plants or emergent hydrophytes.
8. Mechanism of opening and closing of stomata (Stomatal movement)
Opening and closing of stomata occurs due to turgor pressure changes in guard cells. The transpiration is regulated by the movement of guard cells of stomata.
Several theories have been put forth to explain the opening and closure of stomata. Which have been discussed below:
Photosynthetic theory: According to Von Mohl (1856) the chloroplasts present in guard cells prepare osmotically active substances by photosynthesis. As a result, their osmotic pressure increases and their turgor pressure increases due to endosmosis. This results in opening of stomata.
Objection: In many cases, chloroplasts of guard cells are poorly developed and incapable of performing photosynthesis.
Starch ⇄ sugar interconversion theory: According to Lloyd (1908), turgidity of guard cells depends upon interconversion of starch and sugar. This fact was supported by Loftfield (1921) who found that guard cells contain sugar during day time when they are open and starch during night when they are closed. Later Sayre (1926) observed that stomata open in neutral or alkaline pH which prevails during day time due to constant removal of CO2 by photosynthesis. They remain closed during night when there is no photosynthesis and due to accumulation of CO2, carbonic acid is formed which causes the pH to be acidic, Sayre thus proposed that interconversion of starch and sugar is regulated by the pH. Sayre’s hypothesis was supported by Scarth (1932) and Small et al (1942). This hypothesis was further supported by detection of the enzyme phosphorylase in guard cells by Yin and Tung (1948). This enzyme is responsible for starch-glucose interconversion.
Criticism
- Starch ⇄ Sugar interconversion is a slow process which cannot account for rapid stomatal movement.
- Starch or other polymerised polysaccharide do not occur in onion plant where stomatal movement occurs.
- Glucose is not detectable in the guard cells when stomatal opening occurs.
- The theory could not explain the extra-effectiveness of blue light at the time of stomatal opening.
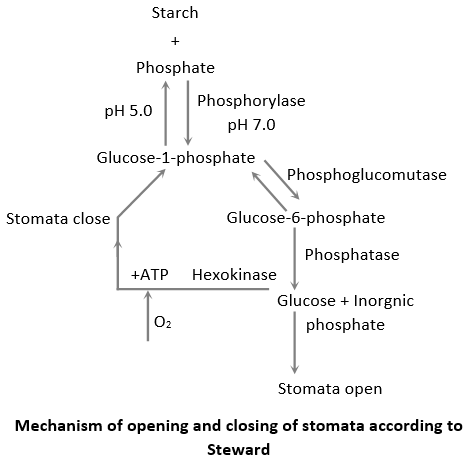
Stewards modification: Steward (1964) said that glucose-I-phosphate should be further converted into glucose as glucose-I-phosphate is not capable of changing osmotic pressure. In this process of stomatal opening and closing, enzymes like phosphorylase, phosphoglucomutase, phosphatase and hexokinase are present in guard cells.
Glycolate theory: Zelitch (1963) proposed that stomata open due to production of glycolic acid by photorespiration in guard cells under low concentration of CO2. The glycolic acid thus produced is converted into soluble carbohydrates which increase the O.P. of guard cells.
Objections
- It fails to explain the opening of stomata in dark (e.g., in succulents).
- In some plants stomata have been found to remain closed even during day times.
- It fails to explain the effect of blue light on stomatal opening.
Active K+ ion transport theory: Imamura (1943) and many other scientists found accumulation of K+ in the guard cells when they are exposed to light. Fujino (1967) suggested that stomatal opening and closing occurs due to an active transport of K+ into or out of the guard cells.
Proton transport theory: It was proposed by Levitt (1974). According to this theory stomatal opening and closing can be explained in the following manner:
Mechanism of stomatal opening
- During day time due to rapid rate of photosynthesis, the concentration of CO2 decreases in the guard cells. As a result their pH is increased. At higher pH, starch in the guard cells is converted into organic acid by the enzyme phosphoenolpyruvate carboxylase (PEPC). This enzyme was discovered by Willmer etal. (1973). It can convert several other carbohydrates into organic acids.
- The organic acid (e.g., malic acid) dissociates into H+-ions (protons) and malate ions.
- The protons (H+) are actively transported into subsidiary cells in exchange for K+ with the help of an energy (ATP) driven H+–K+-pump. The uptake of K+-ions is balanced by uptake of Cl and the negative charge on malate-ions.
- Increased concentration of K+ and malate ions in the guard cells increases the O.P. of guard cells.
- Water enters from adjoining subsidiary cells by endosmosis.
- Turgor pressure of guard cells increases. Turgidity of guard cell is controlled by potassium, chloride and malate.
- Stomata open.
Mechanism of stomatal closure
According to Cowan et.al. (1982) closure of stomata depends upon abscisic acid (ABA) which is in fact an inhibitor of K+-uptake. It becomes functional in presence of CO2 or in acidic conditions (low pH).
- During night photosynthesis stops which results in increased concentration of CO2 which causes lowering of pH.
- At lower pH, ABA inhibits K+-uptake by changing the permeability of guard cells.
- The K+-ions now start moving out of the guard cells which results in lowering of the pH.
- At low pH, organic acids are converted back into starch by PEPC.
- The O.P. of guard cells decreases and water moves out of them into subsidiary cells by the process of exosmosis, thus decreasing their turgor pressure.
- The guard cells become flaccid and the stomata close.
Stomatal opening in succulent plants (Scotoactive stomata)
The stomata in succulent plant or CAM plants (like Opuntia, Bryophyllum etc.) open during night (darkness) and remain closed during the day time and found in lower surface. This type of stomatal opening is called ‘Scotoactive type’ and the stomata which open during day are called as photoactive. Stomata closed and open due to the activity of water. This types of stomata is known as hydroactive stomata. The opening and closing mechanism of scotoactive stomata was explained by Nishida (1963). In succulent plants, during night, there is incomplete oxidation of carbohydrates and accumulation of organic acids (e.g., malic acid) without release of CO2. During day time the accumulated organic acids breakdown rapidly releasing excess amount of CO2 for photosynthesis as well as to keep the stomata closed.
During night:
During day:
Factors affecting rate of transpiration
External factors
Atmospheric humidity: If the atmosphere is humid, it reduces the rate of transpiration. When the air is dry, the rate of transpiration increases.
Temperature: It affects the rate of transpiration only indirectly. Increase in the temperature of the air decreases the humidity of the air and therefore more water is vapourised and lost from the transpiring surface.
Light: Light affects the rate of transpiration due to its effect on temperature and photosynthesis. During daytime stomata open wide but during night they close. Thus increased temperature and presence of wide open stomata increase the rate of transpiration. Light is the most important factor in the regulation of transpiration.
Maximum opening of stomata occurs in red light (660 nm), and no opening occurs in green light, UV light and far red light.
Atmospheric pressure: The rate of transpiration is inversely proportional to the atmospheric pressure.
Available soil water: If the available water in the soil is not sufficient the rate of transpiration is decreased. Under internal water deficiency the stomata are partially or completely closed.
Wind velocity: A transpiring surface of leaf continuously adds water vapours to the atmospheric air. Once the immediate area becomes saturated, it reduces the rate of transpiration. Wind velocity removes the air of that area, which is replaced by fresh air and result in an increase in the rate of transpiration. Wind velocity is measured by anemometer.
CO2 concentration: Reduced CO2 conc. favours opening of stomata while an increase in CO2 conc. promotes stomatal closing.
Internal factors/Plant factors
Leaf area: If leaf area is more, transpiration is faster. However, the rate of transpiration per unit area is more in smaller leaves than in larger leaves due to high number of stomata in a small leaf. Number of stomata per unit area of leaf is called stomatal frequency.
I = here, I = Stomatal index
S = No. of stomata per unit area
E = No. of epidermal cells in unit area.
Leaf structure: The anatomical features of leaves like sunken or vestigial stomata; presence of hair, cuticle or waxy layer on the epidermis; presence of hydrophilic substances such as gums, mucilage etc. in the cells; compactly arranged mesophyll cells etc. help in reducing the rate of transpiration.
Root shoot ratio: According to Parker (1949) the rate of transpiration is directly proportional to the root-shoot ratio.
Age of plants: Germinating seeds show a slow rate of transpiration. It becomes maximum at maturity. However, it decreases at senescence stage.
Orientation of leaves: If the leaves are arranged transversely on the shoot they lose more water because they are exposed to direct sunlight. If placed perpendicularly they transpire at slower rate.
Significance of transpiration
The advantages and disadvantages of transpiration are discussed below:
Advantages
- Transpiration is important for plants because it directly influences the absorption of water from the soil.
- Transpiration exerts a tension or pull on water column in xylem which is responsible for the ascent of sap.
- Transpiration helps in the movement of water and minerals absorbed by the roots to the other parts of the plant.
- The evaporation of water during transpiration contributes to the cooling of leaves (and also the surrounding air) and protects leaves from heat injury particularly under conditions of high temperature and intense sunlight.
Disadvantages
- Transpiration often results in water deficit which causes injury to the plants by desiccation.
- Rapid transpiration causes mid-day leaf water deficit (temporary wilting). If such condition continues for some time, permanent water deficit (permanent wilting) may develop, which causes injury to plants.
- Many xerophytes have to develop structural modifications to reduce transpiration. These modifications are extra burden on the plants.
- Excessive rate of transpiration leads to stunted growth of plants.
- Since approximately 90 percent of absorbed water is lost through transpiration, the energy used in absorption and conduction of water goes waste.
Curtis (1926) truly called ‘transpiration as a necessary evil’.
Anti-transpirants
‘The chemical substances which reduce transpiration (by increasing leaf resistance to water vapour diffusion) without affecting gaseous exchange, are called anti-transpirants’. Anti-transpirants are of two types metabolic inhibitors and film forming anti-transpirants.
Metabolic inhibitors
They reduce transpiration by causing partial closure of stomata without influencing other metabolite processes, the most important of these inhibitors are phenyl mercuric acetate (PMA), and abscissic acid (ABA).
Film forming anti-transpirants
They check transpiration by forming a thin transparent film on the transpiring surface. They are sufficiently permeable to carbon dioxide and oxygen to allow photosynthesis and respiration, but prevent movement of water vapour through them. The important chemicals of this group are silicon emulsion, colourless plastic resins and low viscosity waxes.
Guttation
The process of exudation of liquid drops from the edges of leaves is called guttation or the process of the escape of liquid from the tip of uninjured leaf is called guttation. It was first studied by Bergerstein in 1887. Usually it occurs through stomata like pores called hydathodes. Exudation may sometime occur from stem through the scars of leaves and lenticles. Guttation usually occurs when the plant is put in more saturated atmosphere.
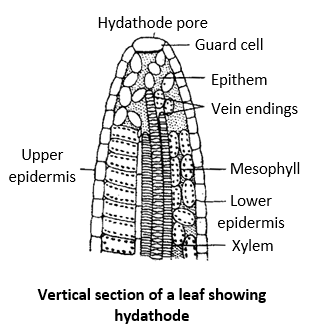
Hydathodes are generally present at the tip or margin of leaves. These pores are present over a mass of loosely arranged cells with large intercellular spaces called epithem. This mass of tissue lies above a vein ending. The xylem of a small vein usually terminates among the thin walled parenchymatous cells of epithem. Guttation is caused due to root pressure. It is found in 115 families and 333 genera of woody and herbaceous plants. e.g., Garden nasturtium (Tropeolum), Oat (Avena), Colocasia etc. growing in moist, warm soil and under humid conditions. When the absorption of water exceeds that of the transpiration, hydrostatic pressure is built up in xylem ducts. As a result, water is pushed in the xylem ducts and comes out through the hydathodes. The water of guttation contains several dissolved inorganic and organic substance.
5. TRANSLOCATION OF ORGANIC SOLUTES
“The movement of organic food or solute in soluble form, from one organ to another organ is called translocation of organic solutes.”
The process of translocation requires expenditure of metabolic energy and the solute moves at the rate of 100 cm/hr.
Directions of translocation
Downward translocation: It is of most important type, i.e., from leaves to stem and roots.
Upward translocation: From leaves to developing flowers, buds, fruits and also during germination of seeds and tubers, etc.
Radial translocation: From pith to cortex and epidermis.
Path of translocation
- Downward translocation of organic solutes: Phloem is the path for downward translocation of organic food. Following evidences are in support of it:
(i) Elimination of other tissues: Xylem is responsible for upward movement of water and minerals, so it cannot account for downward translocation of solute at the same time. Thus only phloem is left (where there is end to end arrangement of sieve tubes united by sieve pores). Which is responsible for translocation of solutes in downward direction.
(ii) Chemical analysis of phloem sap and xylem sap: Chemical analysis of sieve tube sap proves that concentrated solution of sucrose is translocated from the place of synthesis to other parts of the plant body. Glucose and fructose are sometimes found in traces only. The amount of sucrose is more in phloem sap during tshe day and less in night. In xylem the amount of sucrose is in traces and also there is no diurnal fluctuation.
(iii) Blocking of phloem: Blocking of sieve pores by ‘callose’ during winter blocks translocation of solutes.
(iv) Ringing or Girdling experiment: It was first performed by Hartig (1837). On removing the ring of bark (phloem + cambium) above the root at the base of stem, accumulation of food occurs in the form of swelling just above the ring, which suggests that in absence of phloem, downward translocation of food is stopped.
(v) Structure of phloem: The structure of phloem tissue is well modified for conduction of solutes. Phloem tissue of an angiosperm consists of sieve tubes, companion cells several kinds of parenchyma cells, fibres and scleroids. Of these sieve tubes are involved in sugar translocation. - Upward translocation of organic solutes: According to Curtis upward conduction of foods also takes place through phloem.
Mechanism of translocation
Diffusion hypothesis: Mason and Maskel (1928) working on cotton plant demonstrated that the translocation of foods occurs from the place of high concentration (place of manufacture or storage) to the place of lower concentration (place of consumption) but it is very slow process so Mason and Phillis (1936) modified this concept and proposed activated diffusion hypothesis. According to this concept the food particles are first energy activated then translocated. This hypothesis is not accepted due to lack of experimental evidence.
Protoplasmic streaming hypothesis: This concept was proposed by de Vries (1885). According to him the food is transported across by streaming current of protoplasm. The cell protoplasm shows a special locomotion movement called cyclosis. It is of two types, rotation and circulation. While rotation is circular movement of protoplasm, circulation is radial movement forming eddies around the vacuoles. The hypothesis involves two phenomenon, such as streaming of sieve protoplasm and diffusion of metabolites through sieve pores.
This hypothesis not only explains faster rate of translocation but also the bidirectional movement of metabolites across a single sieve element. This hypothesis was supported by Curtis (1950).
Transcellular streaming: Thaine (1964) suggested modification to cytoplasmic streaming theory. He defined transcellular streaming as “the movement of the particulate and fluid constituents of cytoplasm through linear files of longitudinally oriented plant cells. “He further proposed that transcellular strands are proteinaceous and characteristic microtubules to afford rhythmic contraction. Thus, transcellular streaming is an attractive mechanism as it would explain the phenomenon of bidirectional translocation.
Electro-osmotic hypothesis: A mechanism involving electro-osmosis was proposed independently by Fensom (1957) and Spanner (1958). According to this hypothesis the solute moves in the positive direction of the electrical gradient along with K+ ions.
Munch’s mass flow or pressure flow hypothesis: The mass flow or pressure flow mechanism was first proposed by Hartig (1860). It was later modified by Munch (1930). Crafts elaborated it further in (1938). Munch assumed that the protoplasm of sieve tube is connected through plasmodesmata and forms a continuous system, called as the symplast. The translocation of solutes occurs in a mass along swith cell sap through the sieve tubes form a region of higher turgor pressure to low turgor pressure (i.e., along a turgor pressure gradient).
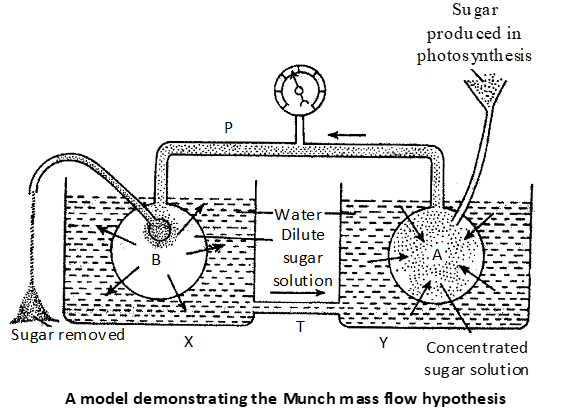
Munch’s hypothesis has been supported further by the following:
When a woody or herbaceous plant is girdled, the sap containing high sugar content exudates from the cut end. Positive concentration gradient disappears when the plants are defoliated. Movement of viruses and growth hormones is fast in illuminated leaves as compared to shaded leaves.
Objections:
The hypothesis fails to explain bidirectional movement of metabolites which is common in plants. Osmotic pressure of mesophyll cells and that of root hair do not confirm the requirements. Munch’s hypothesis gives a passive role to the sieve tube elements and the protoplasm.
Factors affecting translocation
Temperature: Optimum temperature for translocation ranges between 20-30°C. The rate of translocation increases with the increase of temperature up to an upper limit and then starts declining. At low temperature, the rate of translocation decreases.
Light: Hartt and his coworkers (1964) proposed that the movement of assimilates of a leaf can depend upon radiant energy. The increase in light intensity more food starts being translocated to roots than to shoots. At lower intensity the growth of root and shoot is inhibited thereby the rate of translocation also decreases.
Hormones: Cytokinins have a pronounced effect on the translocation of water soluble nitrogen compounds.
Oxygen: Oxygen is necessary during transfer of food from mesophyll cells into phloem which is called as phloem loading.
Minerals: Boron is highly essential for translocation of sugar. Phosphorus also helps in translocation of solutes.
Water: Translocation of photosynthates out of the leaves is highly sensitive to the amount of water in the plant cells.
Metabolic inhibitors: The metabolic inhibitors which inhibit the process of respiration (e.g., iodoacetate, HCN, carbon monoxide etc.) adversely affect the process of translocation because phloem loading and unloading require ATP.








