INTEXT QUESTIONS
Q1. Which of the following is matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Ans.
Anything that occupies space and has mass is called matter. Matter can exist in three physical states—solid, liquid, and gaseous.
Chair and almond are forms of matter in the solid state.
Cold drink is a liquid state of matter.
Air is a gaseous state of matter.
Note: The sense of smell is not matter. However, the smell or odour of a substance is classified as matter. The smell of any substance (say, perfume) can be classified as matter. This is because; perfume is in liquid state inside the bottle. It comes out in the form of tiny droplets. These droplets of perfume are matter and spread out in the atmosphere to create a sense of smell. This smell can be detected by our olfactory system.
Q2. Give reasons for the following observation: The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Ans.
Solids diffuse at a very slow rate. But, if the temperature of the solid is increased, then the rate of diffusion of the solid particles into air increases. This is due to an increase in the kinetic energy of solid particles. Hence, the smell of hot sizzling food reaches us even at a distance, but to get the smell from cold food we have to go close.
Q3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans.
The ability of a diver to cut through water in a swimming pool shows that the particles of matter have inter- -molecular spaces. The intermolecular spaces in liquids are fair enough to let the diver pass through it.
Q4. What are the characteristics of particles of matter?
Ans.
The characteristics of particles of matter are:
(i) Particles of matter have spaces between them.
(ii) Particles of matter are continuously moving.
(iii) Particles of mater attract each other.
INTEXT QUESTIONS
Q1. The mass per unit volume of a substance is called density (density = mass/volume). Arrange the following in order of increasing density − air, exhaust from chimney, honey, water, chalk, cotton, and iron.
Ans.
The given substances in the increasing order of their densities can be represented as:
Air < Exhaust from chimney < Cotton < Water < Honey < Chalk < Iron.
Q2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy, and density.
Ans.
(a) The differences in the characteristics of states of matter are given in the following table.
| Solid state | Liquid state | Gaseous state | |
| 1. | Definite shape and volume. | No definite shape. Liquids attain the shape of the vessel in which they are kept. | Gases have neither a definite shape nor a definite volume. |
| 2. | Incompressible | Compressible to a small extent. | Highly compressible |
| 3. | There is little space between the particles of a solid. | These particles have a greater space between them. | The space between gas particles is the greatest. |
| 4. | These particles attract each other very strongly. | The force of attraction between liquid particles is less than solid particles. | The force of attraction is least between gaseous particles. |
| 5. | Particles of solid cannot move freely. | These particles move freely. | Gaseous particles are in a continuous, random motion. |
(b) Rigidity can be expressed as the tendency of matter to resist a change in shape.
Compressibility is the ability to be reduced to a lower volume when force is applied.
Fluidity is the ability to flow.
By filling a gas container we mean the attainment of shape of the container by gas.
Shape defines a definite boundary.
Kinetic energy is the energy possessed by a particle due to its motion.
Density is mass per unit volume.
Q3. Give reasons:
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air, but to do the same through a solid block of wood, we need a karate expert.
Ans.
(a) There is little attraction between particles of gas. Thus, gas particles move freely in all directions. Therefore, gas completely fills the vessel in which it is kept.
(b) Particles of gas move randomly in all directions at high speed. As a result, the particles hit each other and also hit the walls of the container with a force. Therefore, gas exerts pressure on the walls of the container.
(c) A wooden table has a definite shape and volume. It is very rigid and cannot be compressed i.e., it has the characteristics of a solid. Hence, a wooden table should be called a solid.
(d) Particles of air have large spaces between them. On the other hand, wood has little space between its particles. Also, it is rigid. For this reason, we can easily move our hands in air, but to do the same through a solid block of wood, we need a karate expert.
Q4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans.
The mass per unit volume of a substance is called density (density = mass/volume). As the volume of a substance increases, its density decreases.
Though ice is a solid, it has large number of empty spaces between its particles. These spaces are larger as compared to the spaces present between the particles of water. Thus, the volume of ice is greater than that of water. Hence, the density of ice is less than that of water. A substance with lower density than water can float on water. Therefore, ice floats on water.
INTEXT QUESTIONS
Q1. Convert the following temperature to Celsius scale:(a) 300 K (b) 573 K
Ans.
(a) 300 K = (300 − 273)°C = 27°C
(b)573 K = (573 − 273)°C = 300°C
Q2. What is the physical state of water at:
(a) 250°C
(b) 100°C
Ans.
(a) Water at 250°C exists in gaseous state.
(b) At 100°C, water can exist in both liquid and gaseous form. At this temperature, after getting the heat equal to the latent heat of vaporization, water starts changing from liquid state to gaseous state.
Q3. For any substance, why does the temperature remain constant during the change of state?
Ans.
During a change of state, the temperature remains constant. This is because all the heat supplied to increase the temperature is utilised in changing the state by overcoming the forces of attraction between the particles. Therefore, this heat does not contribute in increasing the temperature of the substance.
Q4. Suggest a method to liquefy atmospheric gases.
Ans.
By applying pressure and reducing the temperature, atmospheric gases can be liquefied.
INTEXT QUESTIONS
Q1. Why does a desert cooler cool better on a hot dry day?
Ans.
When a liquid evaporates, the particles of the liquid absorb energy from the surroundings to compensate the loss of energy during evaporation. This makes the surroundings cool.
In a desert cooler, the water inside it is made to evaporate. This leads to absorption of energy from the surroundings, thereby cooling the surroundings. Again, we know that evaporation depends on the amount of water vapour present in air (humidity). If the amount of water vapour present in air is less, then evaporation is more. On a hot dry day, the amount of water vapour present in air is less. Thus, water present inside the desert cooler evaporates more, thereby cooling the surroundings more. That is why a desert cooler cools better on a hot dry day.
Q2. How does water kept in an earthen pot (matka) become cool during summers?
Ans.
There are some pores in an earthen pot through which the liquid inside the pot evaporates. This evaporation makes the water inside the pot cool. In this way, water kept in an earthen pot becomes cool during summers.
Q3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Ans.
When we put some acetone or petrol or perfume on our palm, it evaporates. During evaporation, particles of the liquid absorb energy from the surrounding or the surface of the palm to compensate for the loss of energy, making the surroundings cool. Hence, our palm feels cold when we put some acetone or petrol or perfume on it.
Q4. Why are we able to sip hot tea or milk faster from a saucer than a cup?
Ans.
A liquid has a larger surface area in a saucer than in a cup. Thus, it evaporates faster and cools faster in a saucer than in a cup. For this reason, we are able to sip hot tea or milk faster from a saucer than a cup.
Q5. What type of clothes should we wear in summers?
Ans.
We should wear cotton clothes in summers. During summers, we sweat more. On the other hand, cotton is a good absorber of water. Thus, it absorbs sweat from our body and exposes the liquid to the atmosphere, making evaporation faster. During this evaporation, particles on the surface of the liquid gain energy from our body surface, making the body cool.
NCERT TEXT BOOK EXERCISES
Q1. Convert the following temperatures to Celsius scale ?
(a) 300 K
(b) 573 K.
Ans.
(a) (300 – 273) = 27°C
(b) (573 – 273) = 300°C
Q2. Convert the following temperatures to Kelvin scale
(a) 25°C
(b) 373°C.
Ans.
(a) (25 + 273) = 298 K
(b) (373 + 273) = 646 K.
Q3. Give reasons for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid
(b) We can get the smell of perfume sitting several metres away.
Ans.
(a) Naphthalene has a tendency to sublimate i.e. it changes directly to the gaseous state. Therefore, the size of the naphthalene balls slowly decreases and ultimately they disappear. No solid residue is left.
(b) A perfume is actually a mixture of number of pleasant smelling vapours. They diffuse quite fast and can reach a person who may be even at several metres away from a person who has used perfume.
Q4. Arrange the following substances in increasing order of attraction between the particles— water, sugar, oxygen.
Ans.
Oxygen, water, sugar.
Q5. What is the physical state of water at ;
(a) 25°C
(b) 0°C
(c) 100°C ?
Ans.
(a) liquid state
(b) solid state (ice)
(c) gaseous state (water vapours).
Q6. Give two reasons to justify that:
(a) Water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Ans.
a) Water is a liquid at room temperature (25°C) due to the following reasons :
i) When placed in a beaker, its level cannot be changed on pressing.
ii) It can take the shape of any container in which it is placed.
b) An iron almirah is a solid due to following reasons :
i) Its shape does not change when pressed. This means that it is hard and rigid.
ii) It is very heavy. This means that the density is very high.
Q7. Ice at 273 K causes more cooling than water at the same temperature. Explain.
Ans.
When ice (solid state) is to melt at 273 K, it takes up certain energy from the surrounding to overcome the attractive forces in the solid particles. As a result, the temperature of the surroundings gets lowered and cooling is noticed. However, water is already in the liquid state and has no tendency to change to the vapour state at this temperature. It will hardly take up any energy from the surroundings in order to change into the vapour state. Therefore, it will cause comparative less cooling.
Q8. Why does steam produce more severe burns on the skin as compared to boiling water?
Ans.
The latent heat of vapourisation of steam is more than that of boiling water. Therefore, it produces more severe burns.
Q9. Name A, B, C, D, E and F in the following diagram showing change in its state.
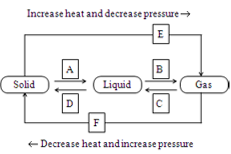
Ans.
[A] = Fusion (melting)
[B] = Vapourisation
[C] = Condensation (Liquefaction)
[D] = Solidification (freezing)
[E] = Sublimation
[F] = Solidification of gaseous state.








