1.1 INTRODUCTION
There are a large number of things around us which we see and feel. For example, we can see a book in front of us. A book occupies some space. The space occupied by the book in called its volume. If we pick up the book, we can also feel its weight. So, we conclude that the book has some mass. We cannot see the air around us, yet if we fill a balloon with air and then weigh it carefully, we will find that not only does air occupy space (bounded
by the balloon), but it also has mass.
Things like a book and air are examples of matter. Other examples of matter are wood, cloth, paper, ice, steel, water, oil etc. Further, that matter offers resistance is borne out by the fact that we cannot displace an object from one place to another without applying some force. We have to apply force to pick up a stone from the ground. Thus, matter can be defined as follows.
DEFINITION
Anything that occupies space and has mass is called Matter.
Air and water, gold and silver, table and chair, milk and oil etc., are all different kinds of matter, because all of them occupy space and have mass.
Characteristics of Matter
i) All matter is composed of particles. These particles have intermolecular spaces between them and attract each other with a force and are in continuous random motion.
ii) All material bodies have weight and hence have mass.
iii) All material bodies occupy space.
1.2 EVIDENCES OF PHYSICAL NATURE OF MATTER
I. Particle Nature of Matter
Most of the evidences for the existence of particles in matter and their motion come from the experiments of diffusion and Brownian motion.
EVIDENCE-1
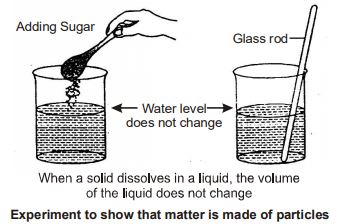
Dissolving a solid in a liquid: Take a beaker. Fill half of it with water. Mark the level of water in the beaker. Add some sugar to the water and dissolve it with the help of a glass rod. You will see that the sugar has disappeared, but there is no change in the level of water.
Conclusion: This can be explained by assuming that matter is not continuous, rather it is made up of particles. Sugar contains a large number of separate particles. These particles when dissolved in water occupy the vacant spaces between the particles of water. Therefore,
the water level in the beaker did not rise. Had sugar been continuous, like a block of wood, the water level in the beaker would have risen.
EVIDENCE-2
Movement of pollen grain in water: The best evidence for the existence and movement of particles in liquids was given by Robert Brown in 1827. Robert Brown suspended extremely small pollen grains in water. On looking through the microscope, it was found that the pollen grains were moving rapidly throughout water in a very irregular way (of zig-zag way).
Conclusion: Water is made up of tiny particles which are moving very fast (The water molecules themselves are invisible under the microscope because they are very, very small). The pollen grains move on the surface of water because they are constantly being hit by the fast moving particles of water. So, though the water particles (or water molecules) are too small to be seen, but their effect on the pollen grains can be seen clearly. The random motion of visible particles (pollen grains) caused by the much smaller invisible particles of water is an example of Brownian motion (after the name of the scientist Robert Brown who first observed this phenomenon)
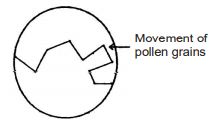
Brownian motion: Zig-zag motion (in a very irregular way) of particles is known as Brownian motion. Brownian motion can also be observed in gases. Sometimes, when a beam of light enters in a room, we can see tiny dust particles suspended in air which are
moving rapidly in a very random way. This is an example of Brownian motion is gases. The tiny dust particles move here and there because they are constantly hit by the fast moving particles of air.
The existence of Brownian motion gives two conclusions.
• Matter is made up of tiny particles.
• Particles of matter are constantly moving.
Note: Brownian motion increases on increasing the temperature.
II. Characteristics of Particles of Matter:
The important characteristics of particles of matter are the following:
(i) The particles of matter are very, very small:
Experiment: Potassium permanganate is a purple coloured solid substance and water is a liquid. We will take 2-3 crystals of potassium permanganate and dissolve them in 100 ml of water. Now we will take out 10 ml of this solution and put into another 90 ml of clear water. We will keep diluting the solution like this 5 to 8 times.
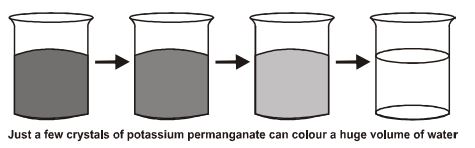
Conclusion: This experiment shows that just a few crystals of potassium permanganate can colour a large volume of water. It means that the crystal of is made of millions of tiny particles. They keep dividing themselves into smaller and smaller particles.
(ii) The particles of matter have spaces between them:
Experiment: We take about 100 ml of water in a beaker and mark the level of water. We will also take 20g of sugar. Now we will dissolve the sugar by stirring and we get a sugar solution.
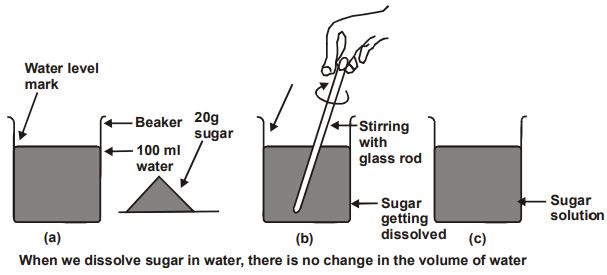
Conclusion: The level of sugar solution in the beaker is at the same mark where water level was initially in the beaker.
It shows that particles of sugar go into the spaces between various molecules of water due to which there is no change in the volume. Thus, from this experiment it can be concluded that, the molecules in water are not tightly paced they have spaces between them.
(iii) The particles of matter are constantly moving:
This property can be explained by diffusion.
Diffusion: Intermixing of particles of two different types of matter on their own is called diffusion. It is the phenomenon in which the movement of molecules or particles occur form their higher concentration towards their lower concentration.
For example, when a perfume bottle is opened in one corner of a room, its fragrance spreads in the whole room quickly. This happens because the particles of perfume move rapidly in all directions and mix with the moving particles of air in the room.
Experiment: We take a gas jar full of bromine vapours and invert another gas jar containing air over it, then after some time, the red-brown vapours of bromine spread out into the upper gas jar containing air.
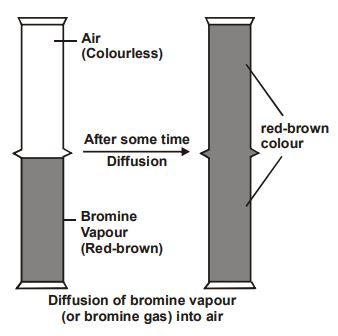
Conclusion: In this way, the upper gas jar which contains colourless air in it, also turns red-brown. The mixing is due to the diffusion of bromine vapours (or bromine gas) into air.
Note: The particles of matter possess kinetic energy and so are constantly moving. As the temperature rises, particles move faster.
(iii) Particles of matter attract each other: There are some forces of attraction between the particles of matter which bind them together.
Cohesive Forces: The forces of attraction between the particles of same substances are known as cohesive forces.
Adhesive Forces: The forces of attraction between the particles of different substances are known as called adhesive forces.
Example: Take a piece of chalk, a cube of ice and an iron nail and beat them with a hammer. Chalk will easily break into smaller pieces, but more force will be required to break a cube of ice and iron nail.
Reasons: The inter particle force of attraction in weak in chalk, a bit stronger in ice cube, very strong in iron.
1.3 KINETIC THEORY OF MATTER
The Kinetic Theory is a good way to relate the ‘micro world’ with the ‘macro world.’ Following are the postulates of Kinetic Theory of matter.
i) Composition of matter: All matter is made of atoms, the smallest bit of each element or molecules.
ii) Particles in motion: The particles of matter are in a state of unending motion. The motion of atoms or molecules can be in the form of linear or of translational, the motion vibration of atoms or molecules against one another or pulling against a bond, and the rotation of individual atoms or groups of atoms. The motion is responsible for the particles
to have kinetic energy. (Kinetic energy is the energy possessed by particles in motion)
iii) Variation in kinetic energy: With the supply of heat energy (thermal energy) to matter, the kinetic energy of particles increases, i.e., they start moving more vigorously. Reverse happens if the matter is cooled i.e., heat energy is taken.
iv) Adhesive and cohesive forces: The particles of matter attract each other with a force. This force is called cohesive force; if the particles are of same kind and adhesive force, if the particles are of different kinds.
v) Inter-particular force of attraction: There exists a force between these particles known as inter-particular force of attraction. This force decreases if the distance between them increases and vice versa.
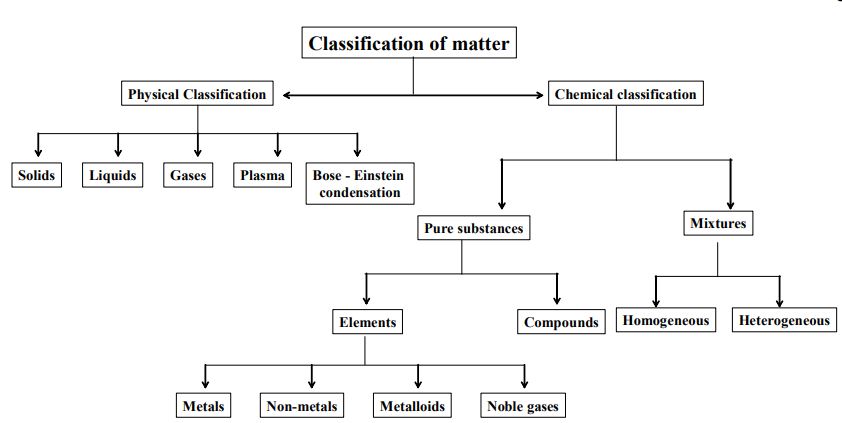
1.4 CLASSIFICATION OF MATTER
Physical Classification Of Matter
We know that matter is composed of extremely small particles. Based on the arrangement of these particles, matter is mainly divided into three types. They are solids, liquids and gases. These are also called physical sates of matter. This classification is also based on
differences of certain physical properties, namely, mass, volume, shape, rigidity, density and arrangement of particles. To understand the properties of solids, liquids and gases, we need to know the kinetic theory of matter.
1.5 SOLIDS
A solid object characterized by resistance to deformation and changes of volume.
At the microscopic scale, solids have the following properties.
Properties
i) Shape and volume: Solids have definite shape and volume.
Reason: The definite shape and volume of solids can be explained on the basis of kinetic theory of matter. In the cases of solids, the kinetic energy of molecules is least and the force of attraction between the molecules is highest. The molecules of the solids can just vibrate about their mean position, but cannot migrate from one position to another. Thus, the solids have definite shape and volume.
ii) Rigidity: Rigid means ‘unbending’ of inflexible solid is a rigid form of matter so that it maintains its shape when subjected to outside force. Solids are generally rigid.
Reason: The constituent molecules i.e., in solids, they cannot be deformed on application of force, have fixed positions in space relative to each other. This accounts for the solid’s rigidity.
(In some solids like rubber, the shape changes on the application of external force. It regains its shape, on removal of the external force.)
iii) Free surfaces: Solids have several free surfaces.
iv) Intermolecular spaces and forces: In solids, the molecules are very close to each other. Thus, they have minimum intermolecular spaces. Due to this, they have large intermolecular forces of attraction.
iv) Density: The density of the solids is generally high. This is due to the compact arrangement of the molecules.
v) Effect of heating: Solids expand on heating. But the dimensions of solids do not increase or decrease in large proportion on heating or cooling, respectively.
vi) Diffusion: When two solids are kept in contact with one another they do not mix with each other, i.e., they do not diffuse.
Some examples of solids: All metals, wood and wood products; rocks of various kinds, ice, etc.
1.6 LIQUIDS
A liquid is a fluid in which the particles are loosely arranged and can freely form a distinct surface at the boundaries of its bulk material. At the microscopic scale, liquids exhibit the following properties.
Properties
i) Shape and volume: Liquids have definite mass and volume. They do not have definite shape, but take the shape of the container in which they are present.
Reason: We know that the kinetic energy of the molecules of a liquid is very large, and so is the distance between the molecules. Thus, the attractive forces between the molecules of a liquid are small as compared to the solids. Therefore, the molecules of a liquid are free to move about within the liquid and hence, the liquid can easily take the shape of the container in which it is present.
However, the volume of the liquid does not change, because, the molecules do not leave the liquid.
ii) Intermolecular spaces and forces: In liquids, the distance between the molecules is large compared to that of a solid. Thus, they have greater intermolecular spaces than in solids. Due to this they have less intermolecular forces of attraction than in solids.
iii) Fluidity: The force of attraction between the molecules of liquids is less than solids. Thus, liquids can flow from one place to another.
iv) Rigidity: Liquids are not as rigid as solids. They can be slightly compressed.
Reason: The intermolecular space in liquids is larger than in solids.
v) Free surfaces: Liquids have only one free surface.
vi) Density: The density of the liquids is generally less than that of solids.
vii) Effect of heating: Liquids expands on heating. They expand far more than solids on heating and contract for more than solids on cooling.
viii) Diffusion: The particles of two different liquids can diffuse in one another, depending upon the nature of molecules of liquids. For example, milk and water particles diffuse in one another, but the particles of oil and water do not.
ix) Some examples of liquids: Water, alcohol, benzene, milk, mercury, kerosene oil, etc,.
1.7 GASES
Gas is the most energetic phase of matter commonly found on earth. The particles of gas, either atoms or molecules, have too much energy to settle down attached to each other or to come close to other particles to be attracted by them.
At the microscopic scale, gases have the following properties.
Properties
i) Shape and volume: Gases have neither definite shape nor definite volume. They occupy the entire space of a given vessel in which they are enclosed.
Reason: The intermolecular distances between the molecules of a gas are very large with the result that the force of attraction between the molecules is negligible. Moreover, the molecules have a very large kinetic energy. Thus, the molecules are practically free to move in any direction and hence, can fill any space. Thus, the gases have neither definite shape nor definite volume.
ii) Definite mass: A gas contained in a vessel has a definite mass.
iii) Intermolecular spaces and forces: In gases, the distance between the molecules is very large compared to that of solids and liquids. Thus, they have the greatest intermolecular spaces compared to solids and liquids. Due to this they have least intermolecular forces of attraction than in solids and liquids.
iv) Compressibility: Gases are highly compressible. The high compressibility of gases is due to the fact that they have large intermolecular spaces. On applying pressure, these molecules simply come close to each other, thereby decreasing the volume of a gas.
v) Expansibility: The volume of a given mass of a gas can be increased either by decreasing pressure or by increasing temperature. When the pressure on an enclosed gas is reduced, its molecules simply move apart, thereby increasing intermolecular
spaces and hence, the volume increases. When gas enclosed in a container is heated, the
kinetic energy of its molecules increases. Thus, the molecules move faster and farther from each other. This in turn results in the increase in volume.
vi) Free surfaces: Gases have no free surfaces.
vii) Density: The gases occupy an extremely large volume as compared to those solids of and liquids. As the inter molecular spaces between the gas molecules is large, they occupy greater volume compared to solids and liquids of same mass. Thus, mass per unit volume of a gas is very small as compared to the liquids and solids. This accounts for the low density of the gases.
viii) Diffusion: Gases have a very high rate of intermixing and diffusion. The intermolecular spaces in a gas are very large. Thus, when two gases are brought in to contact with each other, their molecules just move into one another’s intermolecular space, thereby forming a homogeneous mixture.
ix) Exertion of pressure: The molecules of a gas, constantly bombard the sides of the containing vessel and hence, exert some force per unit area on the sides of the container, which is commonly called pressure. It has been seen that at a given temperature, the number of molecules striking the walls of containing vessel per unit time, per unit
area is same. Thus, we can say that gases exert same pressure in all directions.
1.8 FOURTH AND FIFTH STATES OF MATTER
Fourth State of Matter
The fourth state of matter is plasma. Plasma is an ionized gas, into which sufficient energy is provided to free electrons from atoms or molecules and to allow species, ions and electrons, to coexist.
In effect, plasma is a cloud of protons, neutrons and electrons where all the electrons have come from their respective molecules and atoms, giving the plasma the ability to act as a whole rather than as a bunch of atoms. (Electron, proton and neutron are the subatomic
particles) Plasma is the most common state of matter in the universe comprising more than 99% of our visible universe and most of which is not visible.
Plasma occurs naturally and makes up the stuff of our sun, the core of stars and occurs in quasars, x-ray beam emitting pulsars, and supernovas. On earth, plasma is naturally occurring in flames, lightning and the auroras.
Fifth State of Matter
The collapse of the atoms into a single quantum state is known as Bose condensation or Bose-Einstein condensate is now considered as fifth state of matter.
It occurs at ultra-low temperature, close to the point that the atoms are not moving at all.
A Bose-Einstein condensate is a gaseous superfluid
phase formed by atoms cooled to temperatures very near
to absolute zero. (Zero kelvin (or) – 273 °C)
The first such condensate was produced by Eric Cornell and Carl Wieman in 1995 at the University of Colorado at Boulder, using a gas of rubidium atoms cooled to 170 nano kelvins (1 nano = ). Under such conditions, a large fraction of the atoms collapse into the lowest quantum state, producing a superfluid.
This phenomenon was predicted in the 1920s by Satyendra Nath Bose and Albert Einstein, based on Bose’s work on the statistical mechanics of photons, which was then formalized and generalized by Einstein.
Comparison of the characteristics of three states of matter
|
Property |
Solid state |
Liquid |
Gaseous state |
|
Interparticle spaces |
Very small spaces |
Comparatively large spaces than solids |
Very large spaces |
|
Interparticle forces |
Very strong |
Weak |
Very Weak |
|
Nature |
Very hard and rigid |
Fluid |
Highly fluid |
|
Compressibility |
Negligible |
Negligible |
Highly compressible |
|
Shape and volume |
Definite shape and volume |
Indefinite shape, but definite volume |
Indefinite shape as well as volume |
|
Density |
Low |
Comparatively high than solids |
Very high |
|
Diffusion |
Negligible |
Slow |
Very fast |
A gas can fill a vessel completely. This is because
1.9 INTER CONVERSION OF MATTER
We have seen that, matter exists in three different states. For a given substance, its state of matter is not permanent
i.e., a given state of matter, can always be changed to other states of matter, by altering conditions of temperature and pressure.
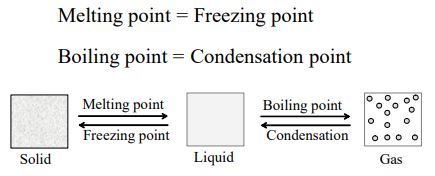
This phenomenon of change of matter, from one state to another and back to original state, by altering the conditions of temperature and pressure, is called change of state.
1.10 INTERCONVESTION OF MATTER BY CHANGE IN TEMPERATURE
a) Interconversion of matter, on heating
Consider a block of ice at 0°C, placed in a beaker and heated. It changes to liquid water. Heat the water till it boils. It slowly gets converted to vapour (gas). From this observation, it is clear that, the solids convert into liquids and liquids in turn convert to gas, when they are heated.
Here, the process by which a solid changes to liquid by absorbing heat, is called melting. And the process by which a liquid changes to gas (vapour) by absorbing Heat, is called boiling or vapourisation.
When observed carefully, it is found that a given solid changes to a liquid, at a constant temperature. This constant temperature is called melting point.
For example, ice changes to water at 0°C. Hence, its melting point is 0°C. Similarly, the constant temperature, at which a liquid changes into a gas, by absorbing heat, is called boiling point. For example, liquid water changes to water vapour (gas), at 100°C. Hence, the boiling point of water is 100°C.
(i) Melting or Fusion: The process due to which a solid changes into liquid state by absorbing heat energy is called melting or fusion.
(ii) Freezing or Solidification: The process due to which liquid changes into solid state by giving out heat energy is called freezing or solidification.
(iii) Melting Point: The constant temperature at which a solid changes into liquid state by absorbing heat energy at 1 atm pressure is called its melting point.
(iv) Freezing Point: The constant temperature at which a liquid changes into solid state by giving out heat energy at 1 atm pressure is called freezing point.
Note: The numerical value of freezing point and melting point is same.
Melting point of ice = Freezing point of water = 0°C (273.16 K)
Explanation: On increasing the temperatures of solids, the kinetic energy (K.E.) of particles increases. Due to increase in K.E, the particles start vibrating with greater speed. The energy supplied by heat overcomes the force of attraction between the particles. Then, the particles leave their fixed positions and start moving freely and thus solid
melts.
Latent Heat of Fusion: The amount of heat energy that is required in change 1 kg of solid into liquid at atmospheric pressure and its melting point is known as the latent heat of fusion. (In Greek Latent means Hidden). Latent heat of fusion of ice = J/kg.
Note: Particles of water of 0°C (273 K) have more energy as compared to particles is ice at the same temperature.
Interconversion of liquid into gaseous state and vice versa:
Liquids can be converted into gases by heating them. Similarly, gases can be converted into liquids by cooling them.
e.g.: Water at 1 atm pressure changes into gas (steam) at 100°C changes into water by giving out energy.
Melting points of common solids
|
Ice |
|
|
Sodium |
|
|
Sulphur |
|
|
Lead |
|
|
Zinc |
|
|
Iron |
Boiling points of common liquids
|
Ice |
|
|
Sodium |
|
|
Sulphur |
|
|
Lead |
b) Interconversion of matter by Cooling
We have seen the changes that take place on heating. Then, have you ever wondered, what
happens if a given state of matter is cooled?
Collect some water vapour (gas) and cool it. We will notice that, it becomes liquid water. On cooling further, the liquid water gets converted to ice(solid). It is interesting to see that a reverse process of heating is taking place on cooling. That is, a gas is converted to liquid and liquid is converted to solid, by cooling. Here, the process by which a gas gets converted to a liquid, by giving out heat is called liquefaction or condensation. The process by which a liquid gets converted to solid, is known as solidification or freezing.
Further, the above changes take place at constant temperature. The constant temperature, at which a gas is converted to a liquid, is known as condensation point. For example, vapour changes to liquid water at 100°C. Hence, its condensation point is 100°C.
The constant temperature at which a liquid changes to a solid, is known as freezing point. For example, liquid water changes to solid at 0°C. Hence, the freezing point of water is 0°C.
(i) Boiling or Vapourisation: The process due to which a liquid changes into gaseous state by absorbing heat energy is called boiling.
(ii) Condensation or Liquefaction: The process due to which a gas changes into liquid state by giving out heat energy is called condensation.
(iii) Boiling Point: The constant temperature at which a liquid rapidly changes into gaseous state by absorbing heat energy at atmospheric pressure is called boiling point.
(iv) Condensation Point: The constant temperature at which a gas changes into liquid state by giving out heat energy at atmospheric pressure is called condensation point.
Note: The numerical value of condensation point and boiling point is same.
Condensation point of vapour (water) = Boiling point of water = 100°C (373.16 K)
Explanation: When heat is supplied to water, particles starts moving faster. At a certain
temperature, a point is reached when the particles have enough energy to break the forces of attraction between the particles. At this temperature the liquid starts changing into gas.
Latent heat of vapourisation: The amount of heat which is required to convert 1 kg of the liquid (at its boiling point) to vapour of gas without any change in temperature. Latent heat of vaporisation of water = J/kg.
Note: Particles in steam (water vapour) at 373 K have more energy than water at the same
temperature. This is because steam has absorbed extra energy in the form of latent heat of
vapourisation.
Freezing points of common solids
|
Ice |
|
|
Sodium |
|
|
Sulphur |
|
|
Lead |
|
|
Zinc |
|
|
Iron |
Condensation points of common liquids
|
Water |
|
|
Ethyl alcohol |
|
|
Benzene |
|
|
Mercury |
The condensation and freezing points of some substances is as follows:
If we observe the table shown, we see the numerical values of melting point and freezing point, boiling point and condensation point, are equal. Thus, for a given substance
Melting point = Freezing point
Boiling point = Condensation point
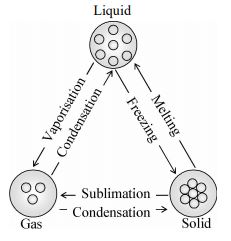
Direct Interconversion of Solid to Gaseous State And Vice Versa
Some solids, on heating, directly change into gaseous state, without changing into the liquid state. Conversely, the gaseous state, on cooling, changes back into solid state, without changing into the liquid state. Such a process is called sublimation.
The gaseous form of solid is called sublime.
The solid state, formed from the gaseous state on cooling, is called sublimate.
Example of subliming solids: Ammonium chloride, iodine, solid carbon dioxide (dry ice), naphthalene and camphor, the moth balls become smaller in size with the passage of time. This is because, they change into its gaseous state at room temperature itself.
1.11 CHANGE OF STATE BY ALTERING THE PRESSURE OF MATTER
Pressure of atmosphere helps in altering the state of matter. When pressure is lowered, boiling point of a liquid is lowered. This helps in rapid change of liquid, into gaseous state.
Examples:
i) Water boils at 100° C and rapidly changes into steam. However, if atmospheric pressure is lowered, it boils at a temperature below 100°C and changes into vapour state.
ii) Carbon dioxide is a gas, under normal conditions of temperature and pressure. It can be liquified, by compressing it, to a pressure 70 times more than atmospheric pressure.
If the pressure from liquid carbon dioxide is suddenly released, some amount of it changes into solid carbon dioxide.
1.12 CURVE (TEMPERATURE TIME GRAPH)
We can show the change of temperature with time in the form of a temperature-time graph drawn by using the readings obtained in the above experiment. Such a time-temperature graph is shown in figure.
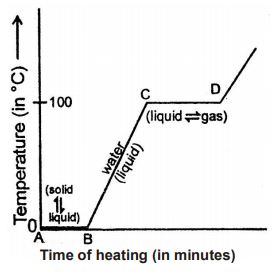
In this graph of point A, we have all ice. As we heat it, the ice starts melting to form water but the temperature of ice and water mixture does not rise. It remains constant at 0°C during the melting of ice. At point B, all the ice has melted to form water. Thus, we have only water at point B. Now, on heating beyond point B, the temperature of water (formed from ice) starts rising as shown by the sloping line BC in the graph.
1.13 EVAPORATION
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
Water changes into vapours below 100°C. The particles of matter are always moving and are never at rest. At a given temperature in any gas, liquid or solid, there are
particles with different K.E.
In case of liquids, a small fraction of particles at the surface, having higher K.E., is able to break the forces of attraction of other particles and gets converted into vapour.
Note: The atmospheric pressure of sea level is 1 atm.
Factors Affecting Evaporation
(i) Temperature: With the increase in temperature the rate of evaporation increases.
Rate of evaporation T
Reasons: On increasing temperature more number of particles get enough K.E. to go into the vapour state.
(ii) Surface Area: Rate of evaporation Surface area.
Evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation also increases. So, while putting clothes for drying up we spread them out.
(iii) Humidity of Air:
Humidity is the amount of water vapour present in air. When humidity of air is low, the rate of evaporation is high and water evaporates more readily. When humidity of air is high, the rate of evaporation is low and water evaporates very slowly.
Rate of evaporation
(iv) Wind speed: With the increases in wind speed, the particles of water vapour move away with the wind. So the amount of water vapour decreases in the surroundings.
Rate of evaporation wind speed.
(v) Nature of substance: Substances with high boiling points will evaporate slowly, while substances with low boiling points will evaporate quickly.
Difference between evaporation and boiling
|
Evaporation |
Boiling |
|
It is a surface phenomenon. |
It is a bulk phenomenon. |
|
It occurs at all temperature below B.P. |
It occurs at B.P. only |
|
The rate of evaporation depends upon the surface area of the liquid, humidity temperature & wind speed. |
The rate of boiling does not depend upon the surface area, wind speed and humidity. |
Cooling Caused by Evaporation
The cooling caused by evaporation is based on the fact that when a liquid evaporates, it draws (or takes) the latent heat of vapourisation from ‘anything’ which it touches.
For example:
• If we put a little of spirit, ether or petrol on the plain of our hand then our hand feels very cold.
• Perspiration (or sweating) is our body’s method of maintaining a constant temperature.
We wear Cotton Clothes is Summer
During summer, we perspire more because of the mechanism of our body which keeps us cool. During evaporation, the particles at the surface of liquid gain energy from the surroundings or body surface. The heat energy equal to latent heat of vapourisation, is absorbed from the body, leaving the body cool. Cotton, being a good absorber of water helps
in absorbing the sweat.
Water droplet on the outer surface of a glass containing ice cold water
If we take some ice cold in a glass then we will observe water droplets on the outer surface of glass.
Reason: The water vapour present in air on coming in contact with glass of cold water, loses energy. So water vapour gets converted to liquid state, which we see as water droplets.








