1.1 INTRODUCTION
Man’s friendship with metals dates from prehistoric times. Initially, man became familiar with those metals which were occurring free in nature, like gold and copper. Gold was found at the burial sites. Then man came across copper and this was the first metal man started using extensively. This is the reason why this period was called the Copper Age.
This was followed by the Bronze Age and the Iron Age.
There are more than 100 elements known today. On the basis of their general characteristics, they are broadly divided into two major groups :
(i) Metals and (ii) Non-metals.
There is yet another group of elements which shows characteristics of both metals and non-metals. Such elements are called metalloids. Among the total elements discovered, approximately 80% of them are metals and the remaining are either non-metals or metalloids.
Position in the periodic table : In the periodic table, metals are placed on the left hand side and in the centre, whereas non-metals are placed on the right hand side with the exception of hydrogen, which is placed with metals on the extreme left. The metalloids form a border diagonal line, separating metals from non-metals, as shown in the diagram below :
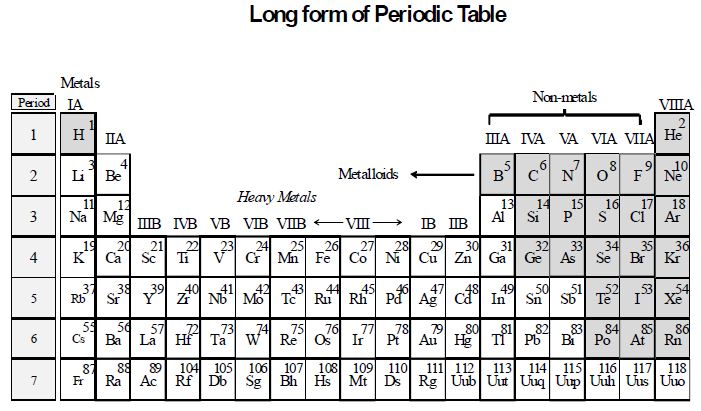
1.2 METALS
The solid state of matter, in which the atoms are very closely packed together and have a special type of bond known as metallic bond is called a metal. Because of very tight or close packing, the metals are quite hard. Out of 115 elements, nearly 70 elements are found to be metals.
LIST OF COMMON METALS
|
English Name |
Symbol |
|
English Name |
Symbol |
|
1. Lithium |
Li |
|
14. Zinc |
Zn |
|
2. Sodium |
Na |
15. Gallium |
Ga |
|
|
3. Magnesium |
Mg |
16. Silver |
Ag |
|
|
4. Aluminium |
Al |
17. Tin |
Sn |
|
|
5. Potassium |
K |
18. Barium |
Ba |
|
|
6. Calcium |
Ca |
19. Platinum |
Pt |
|
|
7. Vanadium |
V |
20. Gold |
Au |
|
|
8. Chromium |
Cr |
21. Mercury |
Hg |
|
|
9. Manganese |
Mn |
22. Lead |
Pb |
|
|
10. Iron |
Fe |
23. Radium |
Ra |
|
|
11. Cobalt |
Co |
24. Uranium |
U |
|
|
12. Nickel |
Ni |
25. Tungsten |
W |
|
|
13. Copper |
Cu |
26. Thorium |
Th |
1.3 PHYSICAL PROPERTIES OF METALS
1. Appearance
Metals usually have a silver or grey colour (except copper and gold).

Copper has a reddish-brown colour whereas gold has a yellow colour. Metals are widely used in our daily life for a large number of purposes. The cooking utensils, electric fans, sewing machines, cars, buses, trucks, trains, ships and aeroplanes, are all made of metals or mixtures of metals called alloys. In fact, the list of articles made of metals which we use in our daily life is unending.
2. Physical state
Metals are solids at the room temperature. Generally, metals are very hard solids. All the metals like iron, copper, aluminium, silver and gold, etc., are solids at the room temperature.

Exceptions: Only one metal, mercury, is in liquid state at the room temperature. Whereas gallium is a liquid at 30°C.
3. Melting and Boiling points
Metals generally have high melting points and boiling points. This means that most of the metals melt and vapourise at high temperatures. Iron is a very important metal. We use about nine times more iron than all the other metals put together. Iron is made into steel and used for making large things like bridges , as well as small things like needles. For example, iron is a metal having a high melting point of 1535°C. This means that solid iron melts and turns into liquid iron (or molten iron) on heating to a high temperature of 1535°C. Copper metal has also a high melting point of 1083°C.

Exceptions: Metals like sodium and potassium have low melting points (of less than 100°C). Another metal gallium has such a low melting point that it starts melting in hand (by the heat of our body).
4. Hardness
Metals are generally hard. Most of the metals are hard. But all the metals are not equally hard. The hardness varies from metal to metal. Most of the metals like iron, copper, aluminium, etc., are very hard. They cannot be cut with a knife.

Exceptions: Sodium and potassium are soft metals which can be easily cut with a knife.
5. Tensile strength
The ability to hold large weights without breaking is called tensile strength. Metals are hard and have high tensile strength. For example, iron metal (in the form of steel) is very strong having a high tensile strength. Due to this, iron metal is used in the construction of bridges, buildings, railway lines, gliders, machines, vehicles and chains, etc. Though most of the metals are strong but some of the metals are not strong. For example, sodium and potassium metals are not strong. They have low tensile strength.

6. Density
Metals have high densities. This means that metals are heavy substances. For example, the density of iron metal is 7.8 g/ which is quite high. There are, however, some exceptions. Sodium and potassium metals have low densities. They are very light metals.
7. Malleability
Metals can be beaten into thin sheets with a hammer (without breaking). This property of metals is called Malleability.
Gold and silver metals are some of the best malleable metals. Aluminium and copper metals are also highly malleable metals. All these metals can be beaten with a hammer to form very thin sheets called foils. For example, silver metal can be hammered into thin silver foils because of its high malleability. The silver foils are used for decorating sweets. Similarly, aluminium metal is quite malleable and can be converted into thin sheets called aluminium foils.

Aluminium foils are used for packing food items like biscuits, chocolates, medicines, cigarettes, etc. Milk bottle caps are also made of aluminium foil. Aluminium sheets are used for making cooking utensils. Copper metal is also highly malleable. So, copper sheets are used to make utensils and other containers. Thus, malleability is an important characteristic property of metals.
8. Ductility
The property of metals by which they can be drawn (or stretched) into thin wires is called ductility. All the metals are not equally ductile. Some are more ductile than the others. Gold and silver are among the best ductile metals. For example, just 100 milligrams of a highly ductile metal like silver can be drawn into a thin wire about 200 metres long. Copper and aluminium metals are also very ductile and can be drawn into thin wires which are used in electrical wiring. So, we can say that metals are malleable and ductile. It is due to the properties of malleability and ductility that metals can be given different shapes to make various articles.
9. Lustrousness
Metals are lustrous (or shiny), and can be polished. Gold, silver and copper are shiny metals and they can be polished. The property of a metal having a shining surface is called metallic lustre (chamak). The shiny appearance of metals makes them useful in making jewellery and decoration pieces. For example, gold and silver are used for making jewellery because they are bright and shiny. The shiny surface of metals makes them good reflectors of light. Silver metal is an excellent reflector of light.
10. Heat and electrical conductivity
Conductivity is the ability of a substance allows heat and electricity to pass through them easily.
Metals are generally good conductors of heat (The conduction of heat is also called thermal conductivity). Silver metal is the best conductor of heat. It has the highest thermal conductivity. Copper and aluminium metals are also very good conductors of heat. The cooking utensils and water boilers, etc., are usually made of copper or aluminium metals because they are very good conductors of heat. The poorest conductor of heat among the metals is lead. Mercury metal is also a poor conductor of heat.

Metals are good conductors of electricity. The metals offer very little resistance to the flow of electric current and hence show high electrical conductivity. Silver metal is the best conductor of electricity. Copper metal is the next best conductor of electricity followed by gold, aluminium and tungsten. The electric wires are made of copper and aluminium metals because they are very good conductors of electricity. The metals like iron and mercury offer comparatively greater resistance to the flow of current, so they have lower electrical conductivity.
11. Sonority
Metals are sonorous. This means that metals make a ringing sound when we strike them. It is due to the property of sonorousness of metals that they are used for making bells, plate type musical instruments like cymbals (manjira), and wires (or strings) for stringed musical instruments such as violin, guitar, sitar and tanpoora, etc.

Metals differ significantly from non-metals in many of the physical and chemical properties. Let us study them in detail.
1.4 NON-METALS
As the name suggests, non-metals are opposite to metals, which means that their properties are quite different, from the metals. They are comparatively less in number. Out of 118 elements, only about 14 to 15 elements are found to be non-metals. A non-metal is an element that is neither malleable nor ductile, and does not conduct electricity.
LIST OF COMMON NON-METALS
|
Non-metal |
State |
Colour |
|
Hydrogen |
Gas |
Colourless |
|
Nitrogen |
Gas |
Colourless |
|
Oxygen |
Gas |
Colourless |
|
Fluorine |
Gas |
Colourless |
|
Chlorine |
Gas |
Greenish yellow |
|
Bromine |
Liquid |
Reddish brown |
|
Iodine |
Solid |
Greyish brown |
|
Carbon |
Solid |
Grey |
|
Phosphorus |
Solid |
Waxy yellow |
|
Sulphur |
Solid |
Yellow |
1.5 PHYSICAL PROPERTIES OF NON-METALS
1. Appearance
Non-metals are dull in appearance and are present in different colours. For example, sulphur is yellow, phosphorus is white or red, graphite is black, chlorine is yellowish-green, bromine is red- brown whereas hydrogen and oxygen are colourless.

2. Physical State
Non-metals can exist in all the three physical states: solid, liquid and gaseous. For example, carbon, sulphur and phosphorus are solid non-metals; bromine is a liquid non- metal; whereas hydrogen, oxygen, nitrogen and chlorine are gaseous non-metals. Diamond ( a non-metal) is the hardest substance known.
3. Melting and boiling points
Non-metals have comparatively low melting points and boiling points This means that non- metals melt and vapourise at comparatively low temperatures.
For example, sulphur is a non-metal having a low melting point of 119°C. The majority of non- metals have very low boiling points due to which they exist as gases at room temperature.

Exception: Only one non-metal graphite has a very high melting point (of 3700°C).
4. Hardness
Non-metals are generally soft . Most of the solid non-metals are quite soft. They can be easily cut with a knife. For example, sulphur and phosphorus are solid non-metals which are quite soft and can be easily cut with a knife.

Exception: Only one non-metal carbon (in the form of diamond) is very hard. In fact, diamond (which is an allotropic form of carbon) is the hardest natural substance known.
5. Tensile strength
Non-metals are not strong. They have low tensile strength. This means that non-metals cannot hold large weights (without breaking). For example, graphite is a non-metal which is not strong. It has a low tensile strength. When a large weight is placed on a graphite sheet, it breaks.
6. Densities
Non-metals have low densities. This means that non-metals are light substances. For example, sulphur is a solid non-metal having a low density of 2 g/cm3, which is quite low. The density of gaseous non-metals is very low.

Exception: One non-metal iodine has higher density compared to other non-metals.
7. Malleability
Non-metals are not malleable and are brittle. This means that non- metals cannot be beaten into thin sheets with a hammer. Non-metals break into small pieces when hammered. For example, sulphur and phosphorus are solid non-metals which are not malleable, they cannot be beaten into thin sheets with a hammer. Thus, we cannot get thin sheets from non-metals. Sulphur and phosphorus non-metals are brittle. When beaten with a hammer, they break into small pieces. Brittleness is a characteristic property of solid non-metals.
8. Ductility
Non-metals are not ductile. This means that non-metals cannot be drawn into wires. They are easily snapped on stretching. For example, sulphur and phosphorus are non-metals and they are not ductile. When stretched, sulphur and phosphorus break into pieces and do not form wires. Thus, we cannot get wires from non-metals.
Note: Non-metals are neither malleable nor ductile. Non- metals are brittle.
9. Lustrousness
Non-metals are not lustrous (not shiny). They are dull in appearance. Non-metals do not have lustre which means that non-metals do not have a shining surface. The solid non-metals have a dull appearance.
For example, sulphur and phosphorus are non-metals which have no lustre, that is, they do not have a shining surface. They appear to be dull.
Exception: Iodine is a non-metal having lustrous appearance. It has a shining surface (like that of metals).
10. Heat and electrical conductivity
Non-metals are bad conductors of heat and electricity. This means that non-metals do not allow heat and electricity to pass through them. For example, sulphur and phosphorus are non-metals which do not conduct heat or electricity. Many of the non-metals are, in fact, insulators.
Exception: A form of the carbon element, diamond is a non-metal which is a good conductor of heat. And another form of carbon element, graphite is a non-metal which is a good conductor of electricity. Being a good conductor of electricity, graphite is used for making electrodes (as that in dry cells).
11. Sonority
Non-metals are not sonorous. This means that solid non-metals do not make a ringing sound when we strike them.
1.6 METALLOIDS AND NOBLE GASES
Metalloids
Elements that exhibit some properties of metals and some properties of non-metals are called metalloids.
Examples: Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te) and Polonium(Po) Noble Gases
These elements are found in air in the form of gas in very small amounts. Therefore, they are sometimes called rare gases.
They are also called noble gases, because they do not react chemically with any known element.
Examples: Helium, Neon, Argon, Krypton, Xenon and Radon.
Note: Helium is the second lightest element after hydrogen.
Radon is given out by the radioactive emission from earth.
1.7 ABUNDANCE OF ELEMENTS IN EARTH’S CRUST
|
Element |
Percentage by weight |
Element |
Percentage by weight |
|
Oxygen |
49.85 |
Potassium |
2.33 |
|
Silicon |
26.03 |
Magnesium |
2.11 |
|
Aluminium |
7.28 |
Hydrogen |
0.97 |
|
Iron |
4.12 |
Titanium |
0.41 |
|
Calcium |
3.18 |
Other elements |
1.39 |
|
Sodium |
2.33 |
|
|
1.8 SUMMARY OF PHYSICAL AND CHEMICAL PROPERTIES OF METALS AND NON-METALS
|
Physical property |
Metals |
Non-metals |
|
1. State |
All metals are solids at room temperature. Eg : Iron |
Generally gases at ordinary temperature. Eg: chlorine. Exception : Bromine is a liquid. Iodine is a solid. |
|
2. Lustre (ability to reflect light) |
Lustrous and can be polished. |
Possess no metalli cluster and cannot be polished. Exception: Iodine and diamond |
|
3. Hardness |
Generally hard Exception: Sodium, potassium, mercury and lead |
Generally soft Exception: Diamond (hardest substance known) |
|
4. Density |
Generally have high density Exception: Sodium, potassium |
Generally have low density. |
|
5. Melting and boiling point |
Generally have high M.P. and B.P. Exception: Sodium, potassium, gallium, mercury |
Generally have low M.P. and B.P. Exception: Carbon, boron and silicon. |
|
6. Sonority (ability to produce sound) |
They are sonorous (make sound when struck). |
Non-sonorous. |
|
7. Malleability (Ability to mould into shapes and sheets without breaking) |
Generally malleable. Eg: Silver and gold-highly malleable. |
Non-malleable. |
|
8. Ductility (ability to be drawn into a thin wire) |
Generally ductile. |
Non-ductile. |
|
9. Conductivity |
Good conductors of heat and electricity. Exception: Tungsten |
Poor conductors of heat and electricity. |
|
10. Solubility |
Usually metals are insoluble in water or other solvents. But if a metal dissolves, it does by reacting chemically with the solution. Eg: Na with water |
Non-metals are soluble even without chemical reaction and reobtained by evaporation. Eg: Sulphurin carbon disulphide |
|
11. Alloy formation (A mixture made by combining two or more metals) |
Forms alloys. Eg: Brass, bronze etc. |
|
1.9 CHEMICAL PROPERTIES OF METALS AND NON-METALS
|
Chemical property |
Metals |
Non-metals |
|
1. Electro chemical nature |
Electropositive in nature. |
Electronegative in nature. |
|
2. Action with oxygen |
Exception : acidic |
Exception : CO, NO, Neutral |
|
3. Action with water |
Very reactive metals like potassium, calcium, sodium react with water and displace hydrogen by forming metal hydroxide. |
Non – metals do not react with wa ter. In fact, they are kept in water to prevent the action of air on them. |
|
4. Action with acids |
Metals such as sodium, magnesium, zinc and iron react with dilute hydrochloric acid and sulphuric acid to for m salt and hydrogen. |
Majority of non metals do not react with acids. But sulphur reacts with concentrated nitric acid to form sulphur dioxide, nitrogen dioxide and water |
|
5. Hydrides |
Generally do not form hydrides. Exception : Sodium, potassium |
Generally for m hydrides with hydrogen. |
|
6. Action on litmus paper |
Magnesium ribbon is burnt and t he residue is collected in glass tumbler. A little quantity of water is added to it and is tested with both red and blue litmus papers. The blue litmus paper remains unaffected. Whereas the red litmus paper changes to blue indicating it is a basic oxide. |
Sulphur is heated in a spoon and transferred to glass tumbler. Water is added to it and it is tested with both red and blue litmus papers. The red litmus paper remains unaffected. Whereas blue litmus paper changes to red indicating it is acidic oxide. |
1.10 METAL ACTIVITY SERIES
Some metals are very reactive and others are less reactive. They can be arranged in a series according to their reactivity. This series is called metal activity series.
|
Reaction |
Magnesium |
Zinc |
Iron |
Copper |
|
On heating |
Burns very easily with a brilliant white flame. |
Does not burn as easily as magnesium. |
Iron filings burn much less easily than zinc. They glow red hot and produce a few sparks. |
With copper powder there is very little reaction. It just glows a little. |
|
With acid |
Very fast reaction. |
Fast reaction |
Slow reaction |
No reaction |
From the above observations we conclude that the metals differ in their reactivity. Some are very reactive, some are less reactive and few are unreactive.
When we arrange the above metals in their order of reactivity, we have the following series.
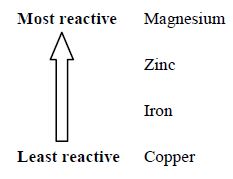
Let us extend the above analogy to a bigger group of metals and the resulting series in their decreasing order of reactivity is called reactivity series or activity series of metals.
The series and its members are as shown below :
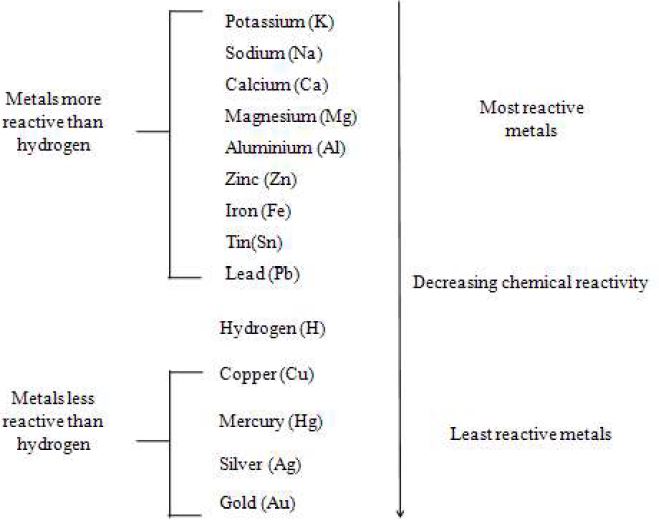
Salient features of Reactivity Series
1. The metals present higher position in the series are more reactive.
2. The metals present at the bottom are less reactive.
3. The metal present at the top position can replace the metals at the lower position from their salts.
4. Although hydrogen is not a metal, it is placed in the reactivity series of metals as hydrogen has the ability to lose electrons to form positive ions like metals.
1.11 DISPLACEMENT OF METALS
(i) Decolourisation of solution
When a strip of Zn metal is placed in a blue coloured solution of , the colour of the solution fades away gradually and the solution becomes colourless due to the formation of . The copper gets deposited on Zn strip. Here copper of is displaced by Zn, as Zn is more reactive than copper.
(ii) Colour change of solution
Similarly, when a strip of iron is placed in a blue coloured solution of , the colour of the solution becomes green due to the formation of . Here copper of is displaced by iron, since iron is more reactive than copper.
1.12 USES OF METALS AND NON-METALS
|
Metals |
Uses |
Non-metals |
Uses |
|
Copper Aluminium and Iron |
• Making house hold • Copper and aluminium are used in making wires that conduct electricity. |
Silicon |
• In making silicon steel alloy |
|
Zinc |
• It is used for galvanizing iron to protect it from rusting. It is also a constituent of enzymes which a ct as biological catalysts. |
Phosphorus |
• In manufacturing phosphoric acid and super phosphate fertilizer. |
|
Chromium and nickel |
• They are used for |
Sulphur |
• In the manufacture of |
|
Silver and gold |
• They are used to make |
Graphite |
• As a dry lubricant. |
|
Sodium titanium and zirconium |
• They are used in projects related to atomic energy and space science. |
||
1.13 NOBLE METALS
The noble metals are a group of metallic elements consisting of gold, platinum, iridium, rhodium, osmium, ruthenium, palladium and silver.
These metals are called noble because,
(i) They are non-reactive, hence, occur free in nature.
(ii) They maintain their metallic lustre for a longer time.
(iii) The availability of these metals is very scarce.
Some important Noble Metals and their Uses
|
Noble metal |
Description |
Uses |
|
Gold |
i) Gold is soft, shiny, metallic element. ii) Its chemical symbol is ‘Au’. |
i) It is used in jewellery and ornaments. ii) Gold is used as currency for trade in various countries. iii) Gold can easily be beaten into different shapes, hence, it is used in dentistry, fillings and crowns. iv) Gold can be beaten into sheets. It is used for gold lettering. |
|
Platinum |
i) Platinum is a rare silvery element. ii) Its chemical symbol is ‘Pt’. |
i) It is used to make surgical instruments and chemical equipments. ii) It is used in number of different alloys. iii) It is used to make fine jewellery. iv) It is used as catalyst in many chemical reactions. |
|
Silver |
i) Silver is scarce and a highly valued metallic element ii) The chemical symbol of silver is ‘Ag’. |
i) It is used in making jewellery, utensils and table ware. ii) It is used to make coins and medals. iii) It is used to make contacts in some kinds of electrical equipment. iv) Silver is used in electroplating. v) Silver compounds are widely used in photography. Silver iodide, chloride and bromide are all photography elements since they are sensitive to light. |
1.14 ALLOYS
I. Introduction
An alloy is a homogenous mixture of two or more metals or a metal and a non-metal. For example, iron is the most widely used metal. But it is never used in the pure form. This is because iron is very soft and stretches easily when not. But when it is mixed with a small amount of carbon (about 0.05%), it becomes hard and strong. The new form of iron is called steel.
II. Objective of Alloy Making
Alloys are generally prepared to have certain specified properties which are not possessed by the constituent metals. The main objects of alloy-making are:
(a) To increase resistance to corrosion: For example, stainless steel is prepared which has more resistant to corrosion than iron.
(b) To modify chemical reactivity: The chemical reactivity of sodium is decreased by making an alloy with mercury which is known as sodium amalgam.
(c) To increase the hardness: Steel, an alloy of iron and carbon is harder than iron.
(d) To increase tensile strength: Magnesium is an alloy or magnesium and aluminum. It has greater tensile strength as compared to magnesium and aluminum.
(e) To produce good casting: Type metal is an alloy of lead, tin and mercury.
(f) To lower the melting point: For example, solder is an alloy of lead and tin (50) Pb and 50% Sn). It has a low melting point and is used for welding electrical wires together.
III. Some Important Alloys
The approximate composition and used of some important alloys are given below:
A. Alloys of Iron
(i) Steel: Steel is an alloy of iron and carbon containing 0.1 to 1.5% carbon. Steel is very hard, tough and strong. It is used for making rails, screws, girders, bridges, railway lines etc. Steel can also be used for the contraction for building, vehicles, ships etc.
(ii) Alloy Steel: Steel obtained by the addition of other elements like chromium, vanadium, titanium, molybdenum, manganese, cobalt or nickel to carbon steel is called Alloy Steel.
B. Alloys of Aluminum
This common alloys of aluminum are:
(i) Duralumin: It is an alloy containing aluminum, copper and traces of magnesium and manganese. The composition of different elements in Duralumin : – Al – 95%, Cu = 4%, Mg = 0.5 % Mn = 0.5% It is stronger than pure aluminum, Since duralumin is light and yet strong, it is used for making bodies of aircrafts, helicopter, jets and kitchenware’s like pressure cookers etc.
(ii) Magnalium: It is an alloy of aluminum and magnesium having the composition: Al – 95%, Mg = 5% It is very light and hard. It is harder than pure aluminum. It is used for making light instruments, balance beams, pressure cookers etc.
(iii) Alnico: It is an alloy containing aluminum, iron, nickel, and cobalt. It is highly magnetic in nature and can be used for making powerful magnets.
C. Alloys of Copper
The important alloys of copper are Brass and Bronze.
(i) Brass – It is an alloy of copper and zinc having the composition: Cu = 80% Zn = 20%. Brass is more malleable and stronger than pure copper. It is used for making cooking utensils, condenser sheets, pipes, hardware, nuts, bolts, screws, springs etc.
(ii) Bronze – It is an alloy of copper and tin having the composition: Cu = 90% Sn = 10% Bronze is very though and highly resistant to corrosion. It is used for making utensils, statues, cooling pipes, coins, hardware etc.
(iii) German Silver – It is an alloy of copper, zinc and nickel having the composition: Cu = 60%, Zn = 20%, Ni = 20%. It is used for making silverware, utensils and for electroplating.
D. Alloying of Gold
Pure gold is very soft and cannot be used as such for jewellery. Therefore, it is generally alloyed with other metals commonly copper or silver to make it harder and modify its colour. The purity of gold is expressed as carats. Pure gold is of 24 carat. A 18 carat gold means that is contains 18 parts of gold is 24 parts by weight of alloy. Most of the jewellery is made of 22 carat gold.
Amalgams
Amalgams are homogenous mixtures of a metal and mercury. For example, sodium amalgam contains sodium and mercury. Different amalgams are prepared according to their used. For example,
i) Sodium amalgam is produced to decrease the chemical reactivity of sodium metal. It is also used as a good reducing agent.
ii) Tin amalgam is used for silvering cheap mirrors.
iii) The process of amalgamation is used for the extraction of metals like gold or silver from their native ores.








