1. INTRODUCTION
Water is a colourless, odourless and tasteless liquid that covers over 70% of the Earth’s surface. It is present in rain, clouds and the seas. Water is not only all around us, but it is inside us as well. On an average, the human body contains almost 65% of water. Some foods are almost all water. For example, a ripe tomato consists of nearly 95% water.
Historical Note
In 1669, Becher and Stahl put forward the theory of phlogiston, an imaginary constitute of combustible bodies, which escapes from them when they burn. Phlogiston was represented by the Greek letter .
In 1781, Henry Cavendish showed that water could be prepared by introducing an electric spark through a mixture of two volumes of hydrogen and one volume of oxygen.
Cavendish was a strong believer of phlogiston theory. He thought that, water pre-existed in the two gases as hydrogen and oxygen.
Importance of Water
Role of Water in the Human Body
1. Water is a medium of transport of chemicals to and from cells.
2. Metabolic reactions occur in water.
3. Water regulates the temperature of the body by the process of sweating and evaporation.
4. Blood is a colloidal solution of many compounds such as salts, proteins, enzymes, glucose etc, in water.
Role of Water in Plants
1. Germination of Seeds: Water helps in the germination of seeds.
2. Photosynthesis : Along with carbon dioxide, plants use water for manufacturing food.
3. Transport of Minerals : Minerals present in the soil dissolve in the water and form a solution. This solution is then absorbed by the roots and conducted upwards through the plant tissues.
Importance of Sea Water
Extraction of Common Salt from Sea Water:
1. In warm climate, seawater is evaporated in large shallow ponds called sea pans or meadows by the heat of the sun. Sea water is pumped into these pans. On evaporation, salt is left behind. This salt is called solar salt. The slower the evaporation takes place, the larger are the crystals obtained in the pan. The mother liquor obtained after the removal of common salt crystals is called bitteru and it contains magnesium salts and bromides of the sea water. 2. Sea provides the biggest habitat for living organisms. The majority of Earth’s producers and consumers live in sea water. Phytoplankton comprising sea algae and sea plants are the major producers while zooplanktons, protozoans, crabs, fishes, sea snakes, sea turtles, crocodiles and sea birds are the primary consumers in sea water.
Reasons for Life in Sea Water
1. Sunlight can easily pass through sea water, helping water plants to carry on photosynthesis.
2. Temperature variation in the sea is mild. Thus, there is no threat to the life of these life forms when the weather changes.
3. Sea water contains dissolved oxygen, which is utilized by animals and plants for respiration.
4. Sea water contains dissolved carbon dioxide, a vital substance to carry out photosynthesis to produce food for sea plants.
5. Sea plants and animals require traces of salts and minerals containing nitrogen, phosphorus, potassium etc, for their growth. These minerals are provided by sea water.
The mystery of Dead Sea
The dead sea is a terminal lake with no outlet, meaning water can only leave through evaporation. Water from its surrounding tributaries flow in to the dead sea. Bringing with them all sorts of minerals, including salts. Since, there is no outlet, the water evaporates depositing minerals and salts. This is the basic reason why dead sea has such high concentration and thus life cannot sustain in it.
2. OCCURRENCE OF WATER
Water is widely distributed in nature in all the physical states. It is found in the combined states in certain minerals and crystalline substances.
In Solid State
It occurs as ice, snow and hails stones in Polar regions and mountainous areas of the Earth.
As the temperature rises during summer, some amount of these forms melt down.
In Liquid State
Water in the liquid state covers about three-fourth of the surface of the Earth. The volume of water in ocean is estimated to be cubic metres.
In Gaseous State
A large amount of water is present in the form of vapours in the atmosphere. This water plays a vital role in sustaining life in plants and animals. A dry weather (without moisture) is injurious to plant and animal tissues.
Note: Water is the only substance that can exist in all three states (solid, liquid, gas), at ordinary temperature and pressure.
3. CLASSIFICATION OF WATER
Underground Water
Part of rain water goes into the soil. It cannot go beyond a certain level. It gets collected there. This reservoir of water over the hard rock, below the surface of Earth is called underground water.
Well Water The reservoir of water above the impervious rocks is called a well and water in this type of reservoir is called well water. The depth of well varies from place to place. Well water contains soluble impurities.
Spring Water Rain water collects over the impervious rocks under the Earth. This water exerts pressure and comes out in the form of spring from any opening in the Earth.
Surface Water Surface water is present on the surface of the Earth. It is of three types:
River and Lake Water The water in rivers and lakes comes from rain and melting of snow on the mountains.
Sea Water Rivers finally flow into the sea and therefore, seas are the largest reservoirs of natural water. The water which contains large amounts of dissolved salts is called saline water.
97.4% of the total water available on the Earth is in oceans. It is salty and hence is unfit for consumption or agriculture.
Only 0.01% of the fresh water is available in the form of lakes, rivers, underground water, etc., which is fit for human and the animals use. It is this water which is continuously recycled in nature.
4. WATER CYCLE
Water vapour is continuously added into the air by the following natural processes: Direct evaporation of water from water bodies by the heat of Sun. Release of water into the air by the leaves of plants by the process of transpiration. Breathing of plants and animals releases water vapour in air. Burning of various kinds of fuels releases water vapour in air.
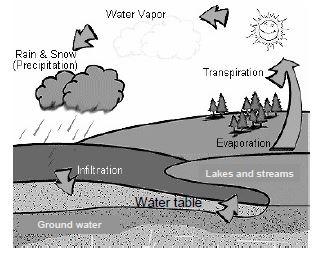
The enormous amount of water vapour released into the air, rises up in the atmosphere, as it is lighter than air. In the upper regions of atmosphere, the water vapour condenses to form tiny droplets of water, commonly called clouds. When the clouds contain too much of water, they cause rain. The rain water flows over the surface of earth in the form of streams, and rivers. Some amount of rain water collects within the earth in the form of sub-soil water. Ultimately, the water is absorbed by the soil or flowing water of surface reaches the sea through rivers. Thus, a natural balance is built, i.e., the amount of water which vaporises is equal to amount of water which returns to earth in the form of rain.
5. PHYSICAL PROPERTIES OF WATER
Colour, odour and taste
Water is a colourless, odourless and tasteless liquid. Deep water bodies like ponds, lakes, rivers and appear greenish blue due to the scattering of light.
Melting point of ice or freezing point of Water
Ice melts and water freezes at 0°C at 1 atm. The melting point decreases with increase in pressure, and rises with decrease in pressure.
Boiling point
Water boils at 100°C at 1 atm.
As is the case with other liquids, the boiling point of water rises as the pressure is raised and decreases as the pressure is lowered.
In a pressure cooker, food is cooked better and quicker because the temperature of the boiling water inside the cooker, i.e., under pressure, is higher than 100°C, say 110°C or so. Surgical instruments are sterilized in an autoclave, which works on the same principle as a pressure cooker. At temperatures higher than 100°C, the bacteria are killed.
The atmospheric pressure decreases and the boiling point of water is reduced at higher altitudes. For example, water boils at around 70°C at Mount Everest. Hence, cooking food at high altitudes becomes difficult. Using a pressure cooker, however, solves the problem.
Freezing point
Pure water freezes at 0°C at 760 mm of mercury pressure.
a. Presence of impurities lowers the freezing point, i.e., the water freezes at temperature lower than 0°C.
b. At higher pressure, the freezing point is slightly less than 0°C.
Conduction
Pure water is a bad conductor of heat and electricity.
Density the anomalous behavior of Water
A behavior that goes against a general rule is said to be anomalous.
Usually, the density of a substance in the solid state is higher than that in the liquid state. Also, the density of a liquid decreases as the temperature rises. However, water shows a peculiar behavior below 4°C.
The density of water is maximum (1 g/mL) at 4°C, and lower at greater and lower temperatures. Thus ice is lighter than water and floats on it.
Physical Constants of Water at a glance
Latent heat of vaporization: 2268 J/g
One gram of water at 100°C absorbs 2268 joules of heat and is converted to steam.
The steam releases 2268 joules of heat (per gram of water) and changes back to water.
Latent heat of fusion of ice: 336 J/g
One gram of water at 0°C loses 336 joules of heat and is converted to ice.
One gram of ice absorbs 336 joules of heat and changes back into water.
Density: 1 g/cm3 at 4°C
Specific heat: 4.2 J/g°C, which is highest among all substances.
Factors effecting the solubility of air in Water
Effect of temperature on the solubility of air in Water
The solubility of gases in water decreases with the rise in temperature. It is because of the decrease in solubility that the dissolved air is expelled out from tap water.
It is the expulsion of air from boiled water that it tastes flat. However, if same water is shaken with air, the taste returns because of the dissolution of air.
It is for the same reason that chilled soda water bottle does not produce large effervescence on opening, as compared to another soda water bottle at room temperature, because the solubility of gases increases with the fall in temperature.
Effect of pressure on the solubility of air in Water
An increase of pressure on the surface of water, increases the solubility of gases in it and vice versa.
The solubility of gases in water at a fixed temperature can be stated by ‘Henry’s law’ as under: At any given temperature, the mass of gas dissolved by a fixed volume of liquid is directly proportional to the pressure on the surface of liquid.
6. CHEMICAL PROPERTIES OF WATER
Nature : Pure water has no effect on litmus solution, i.e., it is neutral to litmus.
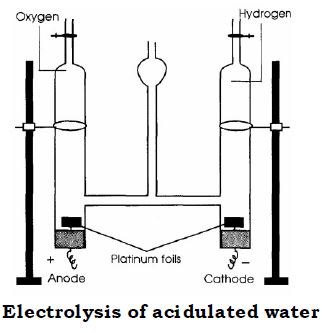
Stability : Water is a stable compound, i.e., it does not decompose on heating. At very high temperatures (between 2000°C-3500°C), it decomposes very slightly to form hydrogen and oxygen. However, the water molecule can be split by the passage of an electric current. This process is called electrolysis.
Splitting up of the Water molecule by Electrolysis
(i) Water acidified with dilute sulphuric acid is taken in a glass apparatus with three interconnected limbs. This apparatus is also called Hoffman’s Voltameter.
(ii) The lower ends of the two side limbs are fitted with platinum plates called electrodes. The plate connected to the positive end of a battery is called the anode, while the plate connected to the negative terminal of the battery is the cathode.
(iii) Electric current is passed through the electrolyte for some time.
(iv) Oxygen gas accumulates at the anode.
(v) Hydrogen gas accumulates at the cathode.
Catalytic nature of Water :
In many chemical reactions, water acts as a catalyst.
(i) Hydrogen and oxygen, when sparked together, combine only if moisture is present.
(ii) Yellow phosphorus burns in air in the presence of moisture.
Electrolysis :
It is a chemical process by which a chemical substance in its fused or aqueous solution is decomposed by the passage of an electric current, leading to the discharge of ions at the electrodes.
Reaction of Water with Metals
Since, water is a compound containing hydrogen, most metals above hydrogen in the activity series are able to displace hydrogen from cold water or steam, and form the corresponding alkali or base.
Cold Water and Potassium [K]
When a piece of potassium is added to a trough of water to which a few drops of red litmus solution has been added, the following changes are observed:
(i) Potassium being lighter than water darts around on the water surface.
(ii) The reaction with water is extremely vigorous and large amounts of heat is given off.
(iii) A colourless gas (hydrogen) is given off. It is ignited due to the heat produced. Although hydrogen burns with a pale blue flame, the flame appears lilac due to the presence of potassium vapours.
(iv) The water turns blue indicating that the solution formed (potassium hydroxide) is alkaline,
Cold Water and Sodium [Na]
When sodium is added to a trough of cold water to which the red litmus solution has been added, the following changes are observed :
(i) The reaction is less vigorous and exothermic than potassium. The sodium melts, forms a silvery globule and revolves at its position.
(ii) The hydrogen evolved along with sodium vapour, burns with a golden yellow flame.
(iii) The hydrogen gas can be collected, if the sodium is wrapped in a wire gauze.
(iv) The solution left behind is alkaline due to the formation of sodium hydroxide.
Cold Water and Calcium [Ca]
Calcium is a hard and heavy metal [density 1.55 g/cm3] with a high m.p. [810°C]. When added to a trough of cold water, the following observations are noted:
(i) The reaction is less exothermic and proceeds smoothly, forming alkaline calcium hydroxide and hydrogen gas.
(ii) The solution left behind is turbid due to an insoluble suspension of calcium hydroxide.
(iii) The hydrogen that is liberated can be collected as shown in the diagram below.
Reaction of Metals with Steam
Magnesium, aluminium, zinc and iron react with steam to form the oxide and liberate hydrogen.
(i) Magnesium
It reacts slowly with boiling water to form magnesium oxide and hydrogen. Since magnesium oxide is slightly soluble in water, some amount of magnesium hydroxide is also formed. Magnesium liberates hydrogen more rapidly with steam.
(ii) Aluminium and Steam
At ordinary temperatures, there is no reaction between aluminium and steam. This is because, a coating of aluminium oxide forms on the surface of aluminium, due to atmospheric oxidation. Reaction occurs at temperature above 800°C, after the oxide layer is destroyed by heat.
(iii) Iron and Steam
Iron reacts reversibly with steam to form triferric tetroxide and hydrogen. This reaction is also exothermic.
[A reversible reaction never reaches completion. This is because, the products formed are constantly reacting to form the original reactants.]
(iv) Copper and Lead
Lead, being only slightly above hydrogen in the activity series, and copper being below hydrogen in the activity series, do not react with water at any temperature. Hence these find use in making water pipes and boilers.
Reaction of Water with Oxides
(i) Metallic oxides
Water reacts with oxides of sodium, potassium and calcium to form the corresponding hydroxides. Magnesium oxide is very slightly soluble in water while iron oxide is insoluble in water.
(ii) Non-metallic oxides
Water reacts with oxides of non-metals to form the respective acids. Carbon dioxide forms carbonic acid
Sulphur dioxide forms sulphurous acid
Sulphur trioxide forms sulphuric acid
Phosphorus pentoxide forms phosphoric acid
Activity Series of Metals
K > Na > Ca > Mg > Al > Zn > Fe > Pb > > Cu > Ag.
Metals more reactive than hydrogen, can displace hydrogen from compounds containing hydrogen.
Example:
but
Action of water with non-metals
Formation of water gas
Action of water with chlorine gas
.
7. TESTS FOR WATER
1. Water turns white anhydrous copper [II] sulphate blue.
2. Water turns blue cobalt chloride paper pink.
|
The list given below gives the number of water molecules associated with one molecule of |
||
|
Name of the compound |
Formula |
Colour of the compound |
|
Copper (II) sulphate Iron (II) sulphate Sodium carbonate dihydrate Sodium carbonate decahydrate Sodium sulphate Bauxite Borax Potash alum Chrome alum |
CuSO4.5H2O FeSO4.7H2O Na2CO3.2H2O Na2CO3.10H2O Na2SO4.10H2O Al2O3.2H2O Na2B4O7.10H2O K2SO4.Al2(SO4)3.24H2O K2SO4.Cr2(SO4)3.24H2O |
Blue Green White White White White White White Green |
8. USES & IMPORTANCE OF WATER
General uses of Water
1. Domestic purposes: Water is used for drinking (potable water), cooking and washing purposes and for preparing soluble injectables with distilled water.
2. Agriculture: In agriculture, water is used for irrigation purposes through rivers and canals.
Fertilizers and pesticides dissolve in water.
3. Cooling Agent: Due to its ability to absorb large quantities of heat, water is used as a coolant (cooling agent), in car radiators.
4. Electricity generation: Water is used to produce hydro-electricity in dams and electricity in the rural power plants and nuclear plants.
5. Solvent and Reagent: Water is used as a solvent for carrying out chemical reactions. Water is also used as a reagent to prepare many chemicals.
6. Manufacture of Water Gas: Water is used in the manufacture of Water gas. When steam is passed over white hot coke, water gas is formed. Water gas is an important fuel used to produce heat in furnaces, in welding and in the manufacture of hydrogen.
7. Water is a very good solvent known as universal solvent because it can dissolve many substances in it.
Uses in Life Processes
i) Water is used for drinking purpose.
ii) It helps in dissolving of food materials in our digestive system, which are then assimilated by our body cells.
iii) It also helps in the excretion of waste materials from our body.
iv) It regulates the body temperature by the processes of sweating and evaporation.
Uses in Industry
Water is used in a large number of industries, such as dyeing industry, tanning industry, steel industry, alcohol industry, etc.
Uses in Manufacturing Chemicals
Water can be broken into hydrogen and oxygen by chemical processes. Hydrogen and oxygen are raw materials for the manufacture of chemicals, such as ammonia, nitric acid, hydrochloric acid, etc.
Uses in Medicine
Distilled water is used in injections as well as a number of medical formations, which are solutions of medicines in distilled water.
Uses in Laboratory
In laboratory distilled water is used for :
i) Purification of water soluble compounds, by crystallisation.
ii) For analytic purpose.
|
IMPORTANCE OF WATER A LIFE PROCESS |
|
|
Activity |
Amount of water used |
|
1. To satisfy the biological needs of an adult human being. 2. To maintain personal and environmental 3. To produce one liter of milk. 4. To produce one liter of milk. 5. To produce 1 Kw of electricity. 6. To produce 1 Kg of steel. 7. In agriculture. |
About 2.5 liters of water per day is needed.
More than 100 liters of water is used. Nearly 5 liters of water is used. 10 liters of water is used. 360 liters of water is required. 300 liters of water is used. Maximum water is used. |
9. TYPES OF CHEMICAL SUBSTANCES IN RELATION WITH WATER
Efflorescent Substances
Crystalline hydrated salts on exposure to the atmosphere lose their water of crystallization partly or completely to the atmosphere. Such substances are called efflorescent substances.
Examples:
Efflorescent crystals
i) Copper sulphate
ii) Glauber’s salt
iii) Washing soda
Residue after efflorescence
i) Anhydrate
ii) Anhydrate
iii) Monohydrate
Hydroscopic Substances
Some substances absorb moisture but do not undergo change after absorption. Such substances are called hygroscopic substances.
Examples:
Solid
i) Quick lime
ii) Anhydrous calcium chloride
Liquid
i) Concentrated sulphuric acid
Deliquescent substances
Some substances absorb moisture some dissolve in it. Such substances are called deliquescent substances.
Examples:
i) Ferric chloride
ii) Calcium chloride
iii) Potassium hydroxide [KOH]
iv) Sodium hydroxide [NaOH]
v) Magnesium chloride
10. DISSOLVED SOLIDS IN WATER
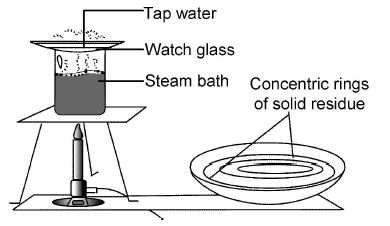
Experiment showing dissolved solids in tap water
Tap water is placed in a watch glass and placed over a beaker containing water as shown below.
When the water in the beaker is boiled, the heat evolved causes the water in the watch glass to evaporate slowly.
After all the water has evaporated in the watch glass, hold the watch glass against the light.
A number of concentric rings of solid matter are observed.
The concentric rings are deposits of the dissolved solids left behind after evaporation.
Importance of dissolved salts in water
Dissolved salts provide a specific taste to water. It is for the same reason that water at different places tastes differently.
Dissolved salts act as micronutrients for plants and help in their growth and development.
11. DISSOLVED GASES IN WATER
Large water surfaces are in direct contact with atmospheric air. Since, the forces of attraction between gas molecules are small, the gases present in air dissolve to some extent in the water.
Air is a mixture. Hence, the solubility of air in water is actually the solubility of each of the constituents of air i.e., oxygen, nitrogen and carbon dioxide. Oxygen being more soluble in water than nitrogen, air dissolved in water contains a higher percentage of oxygen (30-35%) than ordinary air (21%).
Experiment showing dissolved Air in tap Water
One litre round bottom flask and delivery tube are completely filled with tap water.
A graduated tube filled with water, is inverted and clamped over the end of the delivery tube.
Gas bubbles evolved on heating the flask, travel through the delivery tube and collect in the inverted graduated tube. The heating is stopped, when no more of the gas is given off.
The volume of the gas collected is a measure of the volume of air dissolved in one litre of tap water.
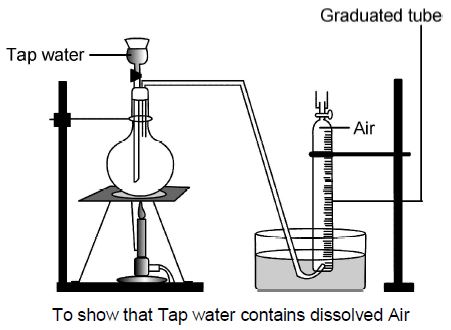
The solubility of gases in water decreases with the increase in temperature. Hence, dissolved gases can be removed by boiling.
Importance of dissolved air in water
The survival of aquatic organisms depends on the gases dissolved in water.
Aquatic plants and animals use oxygen dissolved in water to respire.
(i) Photosynthesis by aquatic plants
The plants use carbon dioxide dissolved in water for the preparation of their food in the form of carbohydrates.
(ii) Formation of shells by marine animals:
Calcium is essential for the formation and strengthening of shells of snail and crabs. Carbon dioxide dissolved in water reacts with limestone present in rocks to form soluble calcium bicarbonate . This is absorbed by the marine animals.
12. TYPES OF WATER
Types of Water
There are two types of water: Hard water and soft water.
Water that lathers with soap easily is called soft water.
Water that does not lather with soap easily is called hard water.
What makes water hard?
The presence of soluble salts (like bicarbonates, sulphates or chlorides) of calcium and magnesium in a sample of water makes it hard.
Soap contains sodium salts of fatty acids. (Fatty acids are organic acids containing a large number of carbon atoms.) These salts produce lather with water. However, the calcium and magnesium salts of these fatty acids are insoluble. So, when a soap is treated with hard water, the calcium and magnesium salts of the fatty acids precipitate in the form of a scum. As a result, the soap is consumed but no lather is produced.
Precipitation over clothes leaves dirty stains and that over your body causes irritation of the skin.
It is obvious that hardness of water increases with the amount of dissolved calcium and magnesium salts. But remember that dissolved sodium or potassium salts (e.g., NaCl, , etc.) do not make water hard. This is because the sodium and potassium salts of fatty acids do not precipitate. And water containing sodium and potassium salts does lather with soap.
Types of Hard water
The hardness of some water samples can be removed by boiling, but not of all. On this basis, hard water is classified into two types.
When the hardness of a water sample can be removed by boiling, it is called temporarily hard water.
When the hardness of a water sample cannot be removed by boiling, it is called permanently hard water.
Temporary hardness is caused by the dissolved bicarbonates of calcium and magnesium. Permanent hardness is caused by the dissolved sulphates and chlorides of calcium and magnesium.
Softening of Water
If the hardness of water is removed, soft water is produced and the process is called softening of water.
The following methods are used to soften water.
Boiling: Temporarily hard water can be softened by boiling it. When such water is heated, the bicarbonates of calcium and magnesium are decomposed to the carbonates. Being insoluble, the carbonates precipitate out.
Treating with washing soda Permanent hardness of water is removed by treating with washing soda . A solution of washing soda is added to the water, and the carbonates of calcium and magnesium are precipitated.
Sodium chloride formed will not make the water hard.
Clark’s Method
Temporary hardness can also be removed when hard water is mixed with slaked lime in solid or liquid form. The bicarbonates present in the water are changed into carbonates. These are not soluble in water. So they form a residue at the bottom. By this method temporary hardness of water can be removed and soft water is obtained.
Calcium bicarbonate + Calcium hydroxide Calcium Carbonate + water
+ +
Magnesium bicarbonate + Calcium hydroxide Calcium Carbonate + Magnesium Carbonate + watert
+ + +
Types of Modern Processes for removal of Permanent Hardness
Hardness of water can be removed by the following two Processes.
1) Permutit Process
2) Exchange of Ions Process.
1) Permutit Process: Sodium Permutit is filled in the cylindrical tube as shown in the figure. Allow the flow of hard water from above the cylindrical tube. The chlorides and sulphides of Calcium and Magnesium are changed into permutites. These permutites remain in the cylindrical tube and soft water is obtained at the bottom.
After some time the cylindrical tube contains permutites of Calcium and Magnesium. At this stage the rate of reaction in changing the hard water into soft water is decreased. In order to avoid this, solution of Sodium chloride is sent through a tube. Thus the reaction takes place in the normal stage.
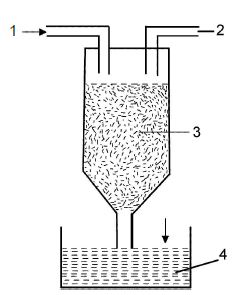
2) By Exchange of Ions: Hard water contain Ions of Calcium , magnesium (cations) Sulphate (anions) and Chloride . By displacement of Positive Ion with Negative Ions the hardness of water can be removed.
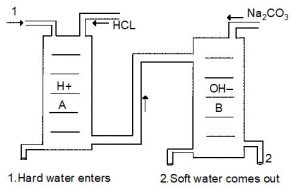
There are two cylindrical tubes A and B, The tube A contains zero cation and tube B contains diacetite. Tube A is known as cation exchanger and tube B is known as anion exchanger. When hard water is sent through tube ‘A’ the positive Ion present in the hard water are attracted by Hydrogen Ion (). When water flows through the tube ‘B’ negative Ions are attracted by Ions. These and Ions combine and form water.
After some time the capacity of and Ions decreases in the A and B tubes. At this stage Hydrochloric acid and Sodium carbonate are added in the tubes.
Importance of Soft Water
Why is it necessary to soften water?
It is necessary to soften water because hard water is unfit for most domestic and industrial purposes.
Hard water is unfit for laundries as it
(a) consumes too much soap, and
(b) leaves dirty stains of calcium and magnesium salts of fatty acids on cloth.
Hard water is not very suitable for bathing. The precipitates of calcium and magnesium salts of fatty acids, formed on reaction with soap, causes irritation of the skin.
It is not possible to properly cook hard food stuff, like pulses, in hard water.
Though not injurious to health, hard water does not have an agreeable taste.
When used for industrial purposes (mainly in boilers), hard water produces white deposits of insoluble substances, called scales. The scales consist mainly of and . They deposit on the walls of the boiler and do not allow proper conduction of heat. They also block the pipes, which may cause serious accidents.








