NOBLE GAS
• The Noble gases are the elements (gases) of group 18 of periodic table i.e., p-block elements (gases) except He.
• Group 18 consists of elements (i.e., gases) helium (He), neon (Ne), Argaon (Ar), Krypton (Kr), Xenone (Xe) and radon (Rn).
• These gases are called rare gases of atmosphere because they occurs in trace.
• These gases at ordinary temperature do not have chemical reactivity and therefore, these were called inert gases.
• However, now a days number of compound of Xe and Kr have been reported. This shows that these gases are not completely inert. Consequently these gases are called noble gases instead of inert gases.
Occurrence:
• Due to the inert nature of noble gases, they always occur in the free state.
• Except radon, all these gases are present in atmosphere in the atomic state.
• Helium is second most abundant element in the universe.
• Argon is the most abundant of all the inert gases in the atmosphere.
|
Element |
Abundance (Volume %) |
|
He |
5.24 10-4 |
|
Ne |
1.82 10-9 |
|
Ar |
0.934 |
|
Kr |
1.14 10-3 |
|
Xe |
8.7 10-6 |
General characteristics:
1. Electronic configuration: ns2 np6:[Except helium – ns2]
|
Element |
Symbol |
At. No. |
Electronic configuration |
|
Helium |
He |
2 |
1s2 |
|
Neon |
Ne |
10 |
[He]2s2 2p6 |
|
Argon |
Ar |
18 |
[Ne] 3s23p6 |
|
Krypton |
Kr |
36 |
[Ar] 3d10 4s2 4p6 |
|
Xenon |
Xe |
54 |
[Kr] 4d10 5s2 5p6 |
|
Radon |
Rn |
86 |
[x] 4f14 3d10 6s26p6 |
2. Physical state: All the noble gases are colourless and tasteless gases.
3. Abundance: In 1% air the abundance follows the order Ar > Ne > He > Kr > Xe.
4. Atomicity: All the noble gases are monoatomic.
5. Solubility: These gases are slightly soluble in water and solubility increases from Helium to Radon.
6. Melting and boiling points: Melting and boiling points of the noble gases increase from He to Rn, due to increase in magnitude of van der Waal’s forces.
7. Liquification: The liquefication of noble gases is extremely difficult, due to weak van der Waal’s force of attraction among their molecules. Hence, they posses low value of critical temperature also.
8. Critical temperature (TC) and Critical pressure (PC):
|
Element |
He |
Ne |
Ar |
Kr |
Xe |
Rn |
|
TC (K) |
5.1 |
44.3 |
150.6 |
211.0 |
256.4 |
375.5 |
|
Pc(atm) |
2.26 |
26.86 |
47.99 |
54.3 |
58.2 |
62.4 |
9. Atomic radii: The atomic radii of noble gases increase from He to Rn.
10. Ionization energies (enthalpies):
The ionization enthalpies of noble gases are very high this is attributed to the stable completely filled configurations of noble gases. However, the ionization enthalpies decrease with increase in atomic number from He to Rn due to increase in atomic size.
11. Electron affinity:
Due to presence of stable electronic configuration they have no tendency to accept additional electron. Therefore, electron affinity is almost zero.
Discovery (1795 – 1898):
i) Helium: It was observed in the spectrum of sun, hence name helium is derived , from helios means sun by P.J.C.Janssen, J.N. Lockeyer and Frankland. Ramsay was successful to obtain it, from gases occluded in uranium minerals. Its vapour density was found to be 2 and molecular weight as 4.
ii) Argon: The credit of discovery goes to Sir Henry Cavendish who performed number of experiments to study the composition of air. It was isolated by Rayleigh and confirmed by Ramsay from air. It was named as argon due to its inertness (Argon means lazy).
iii) Neon, Krypton and Xenon: These were obtained by fractional distillation of liquid air under reduced pressure. They were named as neon means ‘new’, krypton means ‘hidden’ and xenon means ‘stranger’.
iv) Radon: It was discovered by Dorn and isolated by Rutherford and Soddy by disintegration of radium.
Isolation of the noble gases from the atmosphere:
1. Physico-chemical methods:
A. Separation of Noble gas mixture from air:
i) Ramsay – Rayleigh’s first method:
• In this method pure and dry air is passed over soda – lime and potash solution to remove carbon dioxide and then passed through a long tube containing red hot copper to remove oxygen from air.
• Thereafter, the air is passed over heated magnesium ribbon, when nitrogen is removed by the following reaction.
3Mg + N2 →
• The process is repeated several times, so that, nitrogen and oxygen are completely removed, leaving behind the mixture of noble gases. Since, it takes a very long time for completion.
ii) Ramsay – Rayleigh’s second method:
• In this method nitrogen and oxygen present in the air are first converted into oxides of the nitrogen by passing an electric discharge. They are absorbed by NaOH circulating in the flask to form sodium nitrite and nitrate.
e.g.,
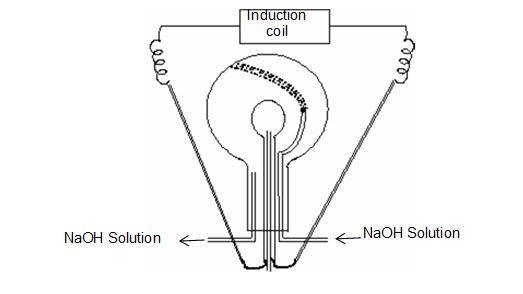
• In this method a large flask of 50 – 60 litre capacity is fitted with a five holed rubber cork.
• Two stout platinum electrods are introduced through two holes.
• Two long tubes for the circulation of NaOH solution are introduced through two other holes.
• In the fifth hole a long tube is inserted for sending a mixture of dry air and oxygen in the ratio of 9 : 11, then an electric discharged of 6000 – 8000V is passed.
• As a result, nitrogen of the air combines with oxygen to form oxides of nitrogen. CO2 and NO2 are absorbed by NaOH and trace of oxygen is absorbed or removed by alkaline pyrogallol.
iii) Fischer – Ringe’s method:
• In this method dry and pure air is passed over a mixture containing calcium carbide and 10% calcium chloride placed in an iron tube heated to 8000C Oxygen and nitrogen gases are removed by the following reactions.
C + O2 → 2 CO2
2C + O2 → 2CO
• The carbon monoxide is removed by passing over cupric oxide.
• Carbon dioxide is removed by passing through KOH solution.
• The remaining mixture of all the noble gases is dried over concentrated H2SO4 and finally over P2O5.
B. Separation of noble gases by Dewar’s method:
a) Principle:
i) Except helium, all the noble gases can be adsorbed over activated charcoal. The extent of adsorption increases down the group, due to increase van der Waal’s forces.
ii) Adsorption on the charcoal also depends on the temperature.
iii) Lower the atomic weight of the noble gas, lower is the temperature needed to adsorb it, i.e., noble gases with less atomic weights get more adsorbed at lower temperature.
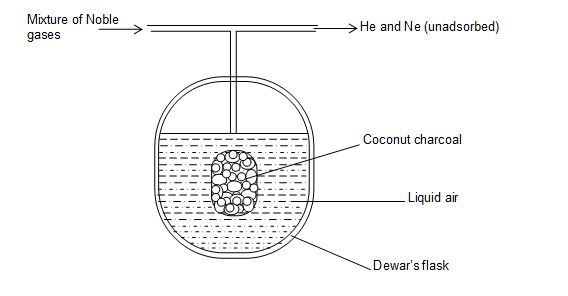
b) Procedure:
Separation of Noble gases is carried out in a flask designed by Dewar called as Dewar’s flask. It is double walled flask containing activated coconut charcoal in the middle.
A tube is arranged above the flask to introduce the gaseous mixture and to remove the unabsorbed gases. The procedure involves the following steps.
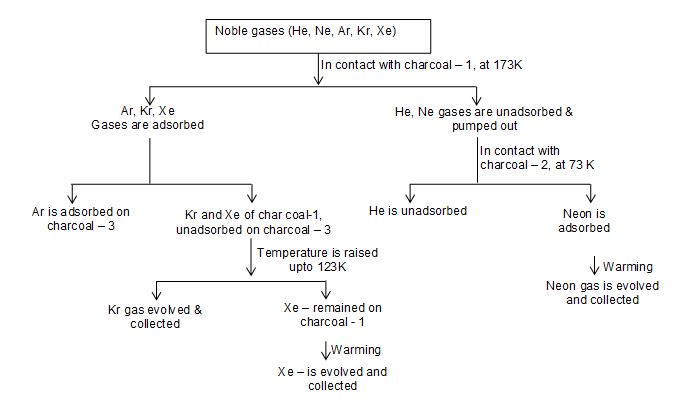
1. The mixture of noble gases is brought in contact with charcoal (charcoal – 1) kept in a Dewar’s flask maintained at a temperature of 173K upto one hour. Gases like Ar, Kr, Xe are adsorbed while He and Ne do not adsorbed, hence, they are pumped out of the bulb.
2. The mixture of helium and neon is introduced into another bulb containing coconut charcoal (charcoal – 2) mainted at a temperature of 93K. Only neon gas is adsorbed leaving behind helium which is pumped out. This charcoal is warmed to recover neon.
3. The first charcoal adsorbing Ar, Kr and Xe is brought in contact with another charcoal (charcoal – 3) at liquid air temperature of 77K. Argon being a lighter gas diffuse into charcoal – 3 at 77K and is recovered by warming separately.
4. Remaining gases (Kr, Xe) on the charcoal – 1 are separated by raising the temperature of charcoal to 183K. At this temperature krypton gas is evolved and collected, while xenone remaining on charcoal is released by warming and is collected.
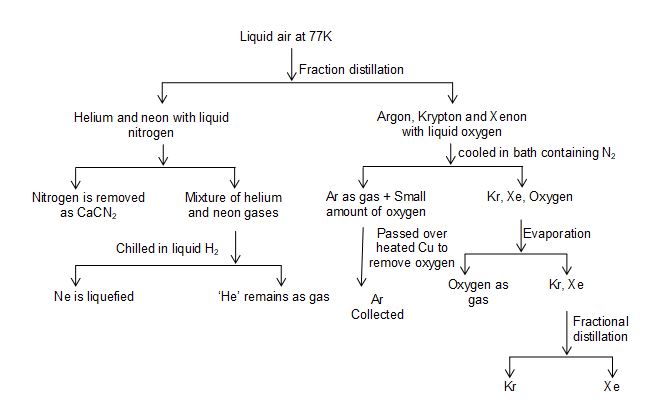
II. Physical methods based on fractional distillation:
a) Separation of noble gases from liquid air:
The difference in the boiling points of various components of liquid air make possible their separation by fractional distillation.
Table : Boiling points (in K) of noble gases
|
Gas |
He |
Ne |
N2 |
Ar |
O2 |
Kr |
Xe |
|
BP |
4 |
27 |
77 |
87.6 |
90.1 |
121.3 |
165.0 |
• Following conclusion can be drawn from this table.
1. Helium and neon being volatile, they can pass along with nitrogen.
2. As the difference in the boiling points of argon and oxygen is small the O2 is always found mixed argon.
3. Krypton and xenon are less volatile. They remain behind and can be separated by fractional distillation method.
Procedure:
• The fractionating column used for the fractional distillation of noble gases is called Claud’s apparatus.
• In this apparatus cold and compressed air is passed from the bottom and allowed to go up through the tubes and rectifiers which are surrounded by liquid oxygen.
• Both oxygen and nitrogen get condensed here and are collected in separate receivers.
• The liquid fractions are pumped up from the bottom. Due to the difference in the boiling points of the constituents, following fractions are collected.
1. Helium and neon with liquid nitrogen.
2. Argon, kryopton and xenon with a liquid oxygen.
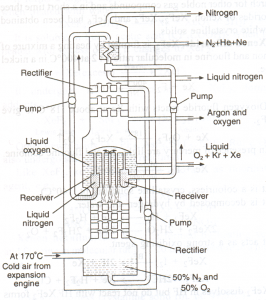
Fig: Claud’s apparatus – fractional distillation of liquid air.
Isolation of helium and neon:
• The portion containing helium, neon and liquid nitrogen is passed over heated calcium carbide at 800 – 10000
• The nitrogen gas is removed as calcium cyanamide leaving behind a mixture of helium and neon.
• The resulting mixture is than chilled in liquid hydrogen (20K), the neon gas changes to liquid neon while helium remains as a gas (it is difficult to liquefy helium gas at this temperature) and can be separated from liquid neon by pumping out (the helium gas).
Isolation of Argon, Krypton and Xenon:
• The portion containing argon, krypton and xenon with a liquid oxygen is first cooled in a bath containing liquid nitrogen then krypton, xenon and oxygen being less volatile get liquidified while argon remains as a gas.
• The argon gas containing, small amount of oxygen is passed over heated copper to remove oxygen and to obtain the argon in pure state.
• On the other hand, the liquid portion containing oxygen, krypton and xenon is subjected to fractional distillation by which these gases are separated.
Note: The mixture of noble gases can be separated either by adsorption or fractional distillation of liquid mixture.
Illustration 1: The noble gas which was discovered first in the sun and then on the earth is?
(A) Argon (B) Xenon (C) Neon (D) Helium
Solution: (D) Helium was first observed in the spectrum of sun by PJC Janssen, J.N. Lockyer and Frankland then observed on the earth by Ramsay.
Illustration 2: The correct order of solubility for He, Ne, Ar, Kr, Xe in water is
(A)
(B)
(C)
(D)
Solution: (C) Noble gas are slightly soluble in water and the solubility increases from Helium to Randon.
Illustration 3: The most abundant inert gas in the atmosphere is
Solution: (C) Argon is the most abundant inert gas in the atmosphere.
Illustration 4: The value of ionization potential for inert gases is
(A) zero (B) low (C) high (D) Negative
Solution: (C) Ionization potential of inert gases is highest in periodic table due to stable electronic configuration.
Illustration 5: Coconut charcoal at – 1000C adsorbs a mixture of
(A) Ar, Kr and Xe (B) He and Ne (C) He and Kr (D) Ne and Xe
Solution: (A) Coconut charcoal at – 1000C (i.e., 173k) adsorb a mixture of AR, Kr and Xe gases.
Chemical properties of Noble gases:
• The noble gases are generally inert and do not participate in the chemical reactions easily.
• The inertness of noble gases is due to the following reasons.
i) The noble gases have completely filled electronic configurations (i.e., ns2np6) in their valence shells (except helium – ns2).
ii) The noble gases have exceptionally high ionization energies.
iii) The noble gases have very low electron affinities i.e., zero.
• Therefore, they have neither tendency to gain nor to lose any electron and do not enter in chemical combinations.
• Before 1962, it was thought that noble gases do not combine at all with other elements and hence, no serious attempts were made to synthesise their compounds.
• In 1962, N.Bartlett noticed that O2 reacts with platinum hexafluoride, PtF6 (powerful oxidizing agent) to form ionic compound, dioxygenyl hexafluoroplatinate (v), O2+ [PtF6]–.
O2(g) + PtF6(g) → O2+ [PtF6]–
In this compound, O2 is oxidized to O2+ by PtF6.
• Moreover, since, first ionization energy of X oxygen (1166 kJ mol-1) and Xenon (1170 KJ mol–) are fairly comparable, he thought that an analogous reaction should be possible with Xenon ( Molecular diameter of oxygen and atomic radius of Xe are similar (4Å)).
• On the basis of this assumptions, Bartlett tried the reaction between Xe and PtF6 by direct interaction and synthesized the first compound of noble gases Xe+ [Pt F6]– in the form of red crystalline solid.
• The formation of this compound has shown that Xenon is not totally inert.
• Recent studies have shown that this compound is formulated as [XeF]+ [Pt2F11]–.
• At present many compounds of Xenon are known with fluorine or oxygen.
• The compound of krypton are fewer, only the difluoride, KrF2 has been studied in detail.
• The compounds of radon have not been isolated so far but have been identified by radiotracer techniques.
• In last, the compounds of He, Ne or Ar are not known.
Compounds of Xenon:
A) Fluorides of Xenon: The three common fluorides of xenon are known i.e., XeF2, XeF4, XeF6.
1) Xenon difluoride (XeF2):
Preparation:
Properties:
i) It is colourless solid.
ii) Reaction with water – XeF2 hydrolyses slowly but completely in acidic, neutral or alkaline solutions.
iii)
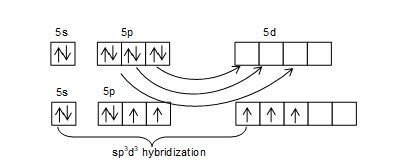
Structure of XeF2:
• XeF2 has linear structure.
• It involves sp3d hybridization of Xenon which adopts trigonal bipyramidal geometry in which three positions are occupied by lone pairs i.e., Xe – 5s2 5P6 5d0.
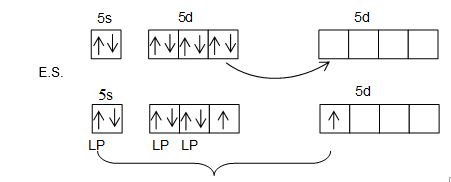
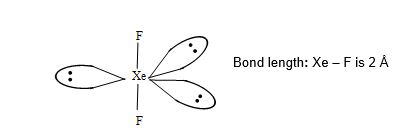
• Because of the symmetrical arrangement of three lone pairs (each at an angle of 1200) the net repulsion on the Xe-F bond pair is zero. Thus, XeF2 has linear geometry.
Xenon tetrafluoride, XeF4:
Preparation:
Properties:
i) Reaction with water:
• XeF4 undergoes slow hydrolysis with moisture and forms XeO3 which is highly explosive
• If the reaction is controlled at – 800C, it forms xenon oxyfluoride. (i.e., partial hydrolysis).
ii)
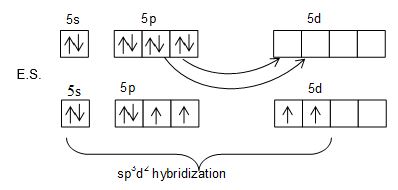
Structure of XeF4:
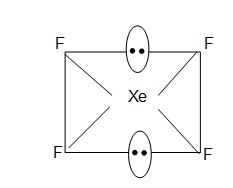
• XeF4 has square planar structure.
• It involves sp3d2 hybridization of Xenon which adopts octahedral geometry in which two positions are occupied by lone pairs.
3. Xenon hexafluoride (XeF6):
Preparation:
Properties:
i) Reaction with water:
• It undergoes slow hydrolysis with atmospheric moisture and forms XeO3 which is highly explosive.
(XeF6 can not be stored in glass vessels because it form XeO3 an explosive oxide)
• Careful hydrolysis (Partial hydrolysis) in nickel vessel gives colourless stable liquid, XeOF4.
ii)
iii) XeF6 + MF → M+ [XeF7]– (M = Na, K, Rb, Cs).
Structure of XeF6:
• XeF6 has distorted octahedral structure in which all six positions are occupied by fluorine atoms and the lone pair is present at the centre of one of the triangular faces.
• It involves sp3d3 hybridization of xenon.
E.C : Xe – 5s2 5P6 5d0
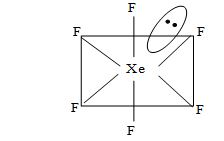
Fig. Distorted octahedral structure of XeF6.
B) Oxides of Xenon: Xenon combines with oxygen to form two types of oxides.
i) Xenon trioxide (XeO3) (ii) Xenon tetraoxide (XeO4)
i) Xenon trioxide (XeO3):
Preparation:
Xenon Trioxide is prepared by the hydrolysis of XeF4 or XeF6.
i) 6XeF4 + 12H2O → 2XeO3 + 4Xe + 6HF + 3O2
ii) XeF6 + 3H2O → XeO3 + 6HF
(explosive)
However, the second reaction proceeds through the following stages.
Great care should be taken during its preparation because it is highly explosive.
Properties:
It is a colourless solid, highly explosive and powerful oxidizing agent. XeO3 reacts with aqueous alkali to form xenate ion, XeO4– which slowly disproportionate to give Xe and per xanate ion, XeO44– in which xenon has + 8 oxidation state.
Structure of XeO3:
• It has pyramidal structure.
• It involves sp3 hybridisation of xenon. Xe – 5s2 5p6 5d0
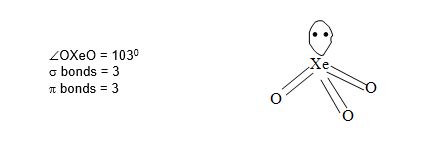
• XeO3 is the anhydride of Xenic acid, H2XeO4.
ii) Xenon tetraoxide (XeO4):
Preparation:
It is prepared by the action of conc H2SO4 on sodium or barium xenate (Na4XeO6 or Ba2XeO6) at room temperature.
Property: It is quite unstable gas and decomposes to xenon & oxygen.
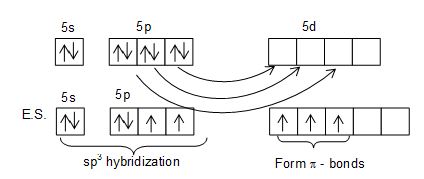
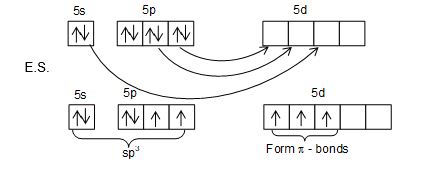
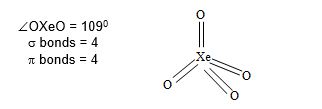
Structure of XeO4: XeO4 molecule has tetrahedral structure.
• Hybridization of Xe is sp3.
• Hybrid orbitals form sigma bond while unhybrid orbitals form Pi bonds.
C) Oxyfluorides of Xenon:
The common oxyfluorides of xenon are, XeOF2, XeOF4 and XeO2F2.
i) Xenonoxydifluoride (XeOF2):
Preparation:
It is prepared by the slow and partial hydrolysis of XeF4 at low temperature.
Structure of XeOF2:
• It has T-shaped structure.
• Xenon involves sp3d hybridization giving trigonal bipyramidal geometry in which two positions are occupied by lone pairs.
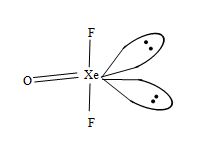
Fig. T – shaped structure of XeOF4.
ii) Xenon oxytetrafluoride (XeOF4):
Preparation:
i) It is prepared by partial hydrolysis of xenon hexafluoride.
ii) It can also be prepared by the action of XeF6 on silicon dioxide.
The content are immediately quenched with solid CO2 as soon as the yellow colour of XeF6 disappears. It is done to avoid the formation of XeO3 (explosive) as
XeOF4 + SiO2 → XeO3 + SiF4
Structure of XeOF4:
• It has square pyramidal structure.
• It involves sp3d2 hybridization of xenon and one position is occupied by a lone pair.
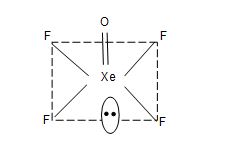
Fig. Square pyramidal structure of XeOF4.
Properties:
i) Reaction with water.
It reacts with water giving xenon dioxydifluoride (XeO2F2) which further reacts with water to give explosive compound, XeO3 as
XeOF4 + H2O → XeO2F2 + 2HF
XeO2F2 + H2O → XeO3 + 2HF
iii) Xenon dioxydifluoride (XeO2F2):
Preparation:
i) It is prepared by mixing XeO3 and XeOF4 at low temperature of – 780C
.
It is purified by fractional distillation.
ii) It is also formed when XeOF4 is hydrolysed or reacts with silica.
2XeOF4 + SiO2 → 2XeO2F2 + SiF4
XeOF4 + H2O → XeO2F2 + 2HF
Prop: Reaction with water:
It is easily hydrolysed to give xenon trioxide;
Structure of XeO2F2:
• It has distorted trigonal bipyramidal geometry.
• It involves sp3d hybridization in which one position is occupied by a lone pair.

Fig. Distorted trigonal bipyramidal structure of XeO2F2
Illustration 6: Helium and Neon are chemically inert why?
Solution: Helium and neon are chemically inert due to following reasons.
1. high ionization energies
2. Absence of d-orbitals in the valency shell of these elements.
Hence, they are chemically inert.
Illustration 7: Krypton and Xenon are not chemically inert. Why?
Solution: Krypton and xenon are chemically reactive because of following reasons.
1. Low ionization energy.
2. Presence of empty d – orbitals for he excitation of electrons from p orbitals in the valency shell.
Hence, compared to others they are chemically active.
Illustration 8: Radon, however, has low I.E. and empty d-orbitals but it does not form compounds with other elements.
Solution: However, Radon has low I.E. and empty d-orbitals but its half life is so low that, it is difficult, during this time to form compounds with other elements.
Illustration 9: Account for the following; Noble gases exhibit low chemical reactivity.
Solution: Noble gases exhibit low chemical reactivity because they have stable electronic configuration, high ionization energies and zero electron affinity values. As a result, losing or gaining an electron or sharing of electrons is difficult.
Illustration10: Why do noble gas form compounds with fluorine and oxygen only?
Solution: Fluorine and oxygen are small atoms with high values of electronegativity. Fluorine is also highly reactive in nature. It is for this reason they form compounds with noble gases.
Uses of Noble gases:
1) Uses of helium:
i) In filling of observation balloons: Because of its lightness and non-inflammability, helium is used to fill air ships and observation balloons or metero logical balloons.
ii) In artificial respiration by deep sea divers: Unlike nitrogen, helium is not soluble in blood, under pressure. Hence, a mixture of 80% helium and 20% oxygen is used, instead of ordinary air in modern diving apparatus. This prevents ‘bends’ which is the pain caused by formation of nitrogen bubbles in blood veins when a diver comes to the surface.
iii) In treatment of asthma: A mixture of helium and oxygen is used to help patients suffering from respiratory diseases like asthma, in breathing.
iv) Liquid helium is used as a cryogenic liquid to provide low temperatures for studying various phenomenona occurring near absolute zero.
v) In nuclear reactors, helium is used as heat transfer agent.
vi) Helium is used in electric transformers.
vii) Helium gas is used in gas thermo meters.
viii) Helium is used to provide inert atmosphere in a number of metallurgical operations in the preparation of reactive metals such as magnesium.
ix) Helium is also used in melting and welding of Mg, Al and stainless steel. Since, these metals are attacked by oxygen and nitrogen at high temperature (red hot), then welding is carried out in an atmosphere of helium.
Note: It helium is cooled to 2.2K at 1 – atmospheric pressure, it forms a new form of liquid called helium – II. The viscosity of this liquid is low. Unlike normal liquids, it flows upwards instead of flowing downwards.
Uses of Neon:
i) Mixed with helium it is used to protect electrical instruments from high voltages.
ii) Neon has a special property of giving an orange red colour in a discharge tube at 2mm of pressure. Hence, it is widely used in filling of glow lamps known as neon tubes for advertising purposes. It produces different colours when mixed with mercury vapour or with argon.
Table: Colours produced by noble gases
|
Gases filled in tube |
Colour of tube |
Visible colour |
|
1. Neon + argon + mercury |
Colourless |
Light blue |
|
2. Neon + argon + mercury |
Grey colour |
Dark blue |
|
3. Neon + argon + mercury |
Green colour |
Light green |
|
4. Neon + helium |
Colourless |
White |
|
5. Neon + helium |
Amber |
Golden yellow |
|
6. Neon |
Colourless |
Orange red |
|
7. Neon |
Light colour |
Dark red |
iii) Neon light is also used as signal lights and as becon lights for safe air navigation as the light possess fog, mist and storm – penetrating power.
iv) Neon lamps are also used in botanical gardens and the green houses as it stimulates growth and is effective in the formation of chlorophyll.
Uses of Argon:
i) A mixture of argon and mercury vapour is used in fluorescent tubes.
ii) A mixture of argon and nitrogen is used in filling of electric bulb because argon is more inert than nitrogen.
iii) It is also used in Geiger counter tubes and other discharge tubes.
Uses of Krypton:
i) It is used in high efficiency miners cap lamps.
ii) Kr – 85, the isotope of krypton is used in electronic tube for voltage regulations.
iii) Kr – 85 is used for the measurement of the thickness of the metal sheets and joints.
Uses of Xenon:
i) It is used in Photographic flash tubes or bulbs.
ii) It is also mixed with other inert gases and filled in lamps to get light of different colours.
iii) Liquid xenon is used for the detection of neutral mesons and gamma photons in the bubble chamber.
Some important points:
i) Lord Rayleigh and Sir William Ramsay were awarded noble prizes in 1904 for their discovery of noble gases.
ii) Noble gases neither act as reducing agent nor as oxidizing agent.
iii) In many reactions of xenon oxides, fluorides and oxifluorides can be systematized in terms of generalized acid-base theory in which any acid (here, defined as an oxide acceptor) can react with any base (oxide donor) lying below it in the sequence of descending acidity.
iv) Xenon in its compounds, exhibits even oxidation states from two to eight, i.e., + 2 in XeF2, + 4 in XeF4, + 6 in XeF6, and xenate ion XeO42-, + 8 in XeO4, and in perxenate ion, XeO64–.
v) XeF2 oxidises iodine in the presence of fluoride ion acceptor to give IF.
reacts with NO to give nitrogen fluoride.
vi) Under high energy conditions, several molecular ions such as He2+, HeH+, HeH2+ and Ar22+ are formed in discharge tubes. They only survive momentarily and are detected spectroscopically.
vii) Clatherates:
• When noble gases get entrapped in cavities of crystal lattices of certain organic or inorganic substances, clatherates or case compounds are formed.
• Clatherate = Organic or inorganic molecule (Host) + inert gas (Guest).
• Clatherales are not true compound.
• They are held by weak van der Waal’s forces (dipole induced dipole interaction) and there is no chemical bonding.
• These are non-stoichiometric compound.
• Ar, Kr and Xe form clatherates but He & Ne do not (they are too small to be entrapped in the cavities of crystal lattice of substances).
viii) Enthalpy, ease of diffusion and thermal conductivity decrease in the order.
He > Ne > Ar > Kr > Xe
ix) He, due to smaller size form interstitial compounds with Fe & Pb as FeHe, Pb He. Other noble gases do not form interstitial compounds due to their large sizes.
FORMULAE AND CONCEPTS AT A GLANCE
1. Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn) elements are called noble gases or inert gases or rare gases.
2. Noble gases are placed in ‘0 group’ of the periodic table.
3. The outer electronic configuration of inert gases is ns2np6 except He. The configuration of He is 1s2.
4. ‘0 group’ elements are known as inert gases because they are chemically inert.
5. ‘0 group’ elements are known as rare gases because they are present in extremely small concentrations in air.
6. Noble gases are naturally occur in air. So these are known as aerogens. The order of abundance of noble gases in air is Ar > Ne > Kr > Xe > He.
7. The most abundant noble gas in the atmosphere is Argon.
8. Helium was discovered by Janssen and Loekyer in the chromosphere of the sun.
9. Argon was discovered by Rayleigh and Ramsay from the atmosphere.
10. Neon, krypton and xenon were discovered by Ramsay and Travers by the fractional distillation of liquid air.
11. Radon was discovered by Dorn in the disintegration of radium. It is also known as niton.
12. Common names of noble gases are
Helium (Helios = Sun)
Neon – New gas
Argon – Lazy gas
Krypton – Hidden gas
Xenon – Stranger gas
13. All noble gases except radon occur in the universe in the free state. They are also present in stars, earth’s atmosphere, in natural gas and in minerals.
14. The most abundant source of helium is natural gas.
15. The radioactive minerals like monozite sand and clevite, on heating give helium.
SOLVED PROBLEMS
Prob 1. Which one of the following statement regarding helium is incorrect?
(A) It is used to produce and sustain powerful superconducting magnets
(B) It is used as a cryogenic agent for carrying out experiments at low temperatures
(C) It is used to fill gas balloons instead of hydrogen because it is lighter and non-inflammable.
(D) It is used in gas-cooled nuclear reactors.
Sol: (C) Helium is heavier than hydrogen although it is non inflammable.
Prob 2. Density of nitrogen gas prepared from air is slightly greater than that of nitrogen prepared by chemical reaction from a compound of nitrogen due to the presence of
(A) Argon
(B) Carbon dioxide
(C) SomeN3 molecules analogous to O3
(D) Greater amount of N2 molecules derived from N-15 isotope.
Sol: (A) Air contains 1% inert gases mainly Ar (At wt = 40). Therefore it happens.
Prob 3. The atomic weight of noble gases is obtained by using the relationship
(A) Atomic weight = equivalent weight / valency
(B) Atomic weight = equivalent weight / valency
(C) At. weight = valency / equivalent weight
(D) 2 V.D. = molecular weight = atomic weight
Sol: (D) The atomic weight of noble gases is obtained by using the relationship 2 V.D. = at.wt (since inert gases are monoatomic).
Prob 4. Which has the same electronic configuration as of inert gas
(A) Ag3+ (B) Cu2+ (C) Pb4+ (D) Ti4+
Sol: (D) Ti 4+ has the same E.C. as of inert gas: Ti4+ = 1s22s22p63s23p6.
Prob 5. In Kroll and ICl process of the production of titanium, the inert gas used to
(A) Ne (B) Ar (C) Kr (D) Xe
Sol: (B) Argon is used in kroll and ICI process for titanium to provide an inert atmosphere.
Prob 6. In order to prevent the hot metal filament from getting burnt, when the electric current is switched on, the bulb is filled with
(A) CH4 (B) An inert gas (C) CO2 (D) Cl2
Sol: (B) Inert gases do not support combustion.
Prob 7. Which one of the following is correct pair with respect to molecular formula of Xenon compound and hybridization state of Xenon in it?
(A) XeF4, sp3 (B) XeF2, sp (C) XeF2, sp3d (D) XeF4, sp2
Sol: (C) XeF2 – sp3d
Prob 8. Which of the following is the life saving mixture for an asthmas patient?
(A) Mixture of helium and oxygen
(B) Mixture of neon and oxygen
(C) Mixture of xenon and nitrogen
(D) Mixture of argon and oxygen
Sol: (A) Mixture of (He + O2) is used for asthma.
Prob 9. In the clathrates of xenon with water, the nature of bonding between xenon and water molecule is
(A) Covalent
(B) Hydrogen bonding
(C) co-ordinate
(D) Dipole-induced dipole interaction
Sol: In cathrates the forces are dipole induced dipole interaction.
Prob 10. Match List I with List II and select the answer using the codes given below.
|
Code |
List I |
Code |
List II |
|
a |
XeF4 |
1 |
Distorted octahedral |
|
b |
XeF6 |
2 |
Tetrahedra |
|
c |
XeO3 |
3 |
Square planar |
|
d |
XeO4 |
4 |
Trigonal pyramidal |
(A) a–4, b–1, c–3, d–2
(B) a–2, b–3, c–1, d–4
(C) a–1, b–4, c–2, d–3
(D) a–3, b–1, c–4, d–2
Sol: (D)
XeF4 – Square planar
XeF6 – distorted octahedral
XeO3 – trigonal pyramidal
XeO4 – tetrahedral








