GROUP 16 ELEMENTS
Introduction:
Group 16 (VIA) of the periodic table consists of the elements oxygen (O), sulphur (S), selenium (Se), tellurium (Te) and polonium (P*o). These elements are known as chalcogens i.e., ore forming elements (except Po*). These elements are p-block elements as the last differentiating electron is accommodated on np shell. These elements have six electrons in their valency shell and thus placed in the VI group.
The first four elements are non metals while last member (polonium) is a radioactive metal and its half life is 13.8 days.
Occurrence: Oxygen is the most abundant element. It constitutes 46.6% of earth’s crust by weight 21% of air by volume and 89.1% of ocean by weight. It also occurs as carbonates, sulphates, nitrates borates etc and as ozone in the upper atmosphere and is of great importance. (e.g., Pyrolusite – MnO2; Haematite – Fe2O3.
Sulphur is the 16th most abundant element and constitution 0.034% by weight of the earth’s crust. It occurs in the combined form as sulphide ores and sulphate ores. e.g., Iron pyrite – FeS2, zinc blende – ZnS. Most of the sulphur is converted to SO2, then to SO3 and finally to H2SO4.
Order of abundance: O > S > Se > Te > P0*
General characteristics of chalcogens:
A. Electronic configurations: ns2np4:
|
Element |
At. NO. |
E.C. |
|
Oxygen (O) |
8 |
[He] 2s2 2p4 |
|
Sulphur (S) |
16 |
[Ne] 3s2, 3p4 |
|
Selenium (Se) |
34 |
[Ar] 3d10 4s2 4p4 |
|
Tellurium (Te) |
54 |
[Kr] 4d10 5s2 5p4 |
|
Polonium (Po) |
84 |
[Xe] 4f14 5d106s26p4 |
B. Atomic and physical properties:
1. Atomic and ionic radii: Increase regularly from ‘O’ to ‘Po’. This is due to increase in the number of shells.
2. Ionization enthalpies: First ionization enthalpies of these elements are less than those of corresponding elements of nitrogen family. This is due relatively symmetrical (exactly half filled) and stable electronic configuration of the elements of nitrogen family as compared to the elements of oxygen family.
The second ionization enthalpies, of these elements are higher than those of the elements of nitrogen family because of smaller size of the ions formed and greater effective nuclear charge and more stable (exactly half filled) electronic configuration of ions (M++).
As we move down the group, ionization enthalpies decreases sharply from O to S, and then fall regularly from S to Te.
Reason: Atomic radii and shielding effect increase from O to Po.
|
Element |
O |
S |
Se |
Te |
Po |
|
IE1 (kJ mol–1) |
1314 |
1000 |
941 |
869 |
813 |
|
IE2 (kJ mol–1) |
3388 |
2251 |
2045 |
1790 |
– |
3. Electronegativity: These elements have higher value of electronegativity than the corresponding elements of nitrogen family.
Reason: Due to their small size and because they have short of only two electrons to attain stable electronic configuration.
Oxygen is the second most electronegative elements, the first being fluorine.
As we move down the group the electronegativity decreases gradually. eg.,
|
Element |
O |
S |
Se |
Te |
Po |
|
EN |
3.5 |
2.44 |
2.48 |
2.01 |
1.76 |
4. Melting and boiling points: The melting and boiling points increase with the increase in atomic number as we go down the group.
Reason : On going down the group, the molecular size increases. As a result the magnitude of the van der Waals forces increases with increase in atomic number and there fore melting and boiling point increases. The melting point of polonium is, however small (exception).
|
Element |
O |
S |
Se |
Te |
Po |
|
MP (0C): |
-219 |
119 |
217 |
450 |
254 |
|
BP (0C) |
–183 |
445 |
688 |
990 |
962 |
5. Electron affinity: These elements have high electron affinity, which decreases from ‘O’ to ‘Po’.
Reason: As the size of the atom increases the extra added electrons feels lesser attraction by nucleus and hence electron affinity decreases.
|
Element |
O |
S |
Se |
Te |
Po |
|
EA1 (kJ mol–1) |
–142.0 |
–200 |
–125 |
–190 |
– |
|
EA2 (kJ mol–1) |
+ 780.0 |
+ 590 |
+420 |
+295 |
– |
6. Non-metallic and metallic character:
7. Catenation: The tendency to form chains of identical atoms is known as catenation. It follows the order
|
Catenation |
S-S > |
Se – Se > |
O – O > |
Te – Te |
|
BE (kJ mol–1) |
226 |
172 |
142 |
– |
The higher the bond strength, the higher is the catenation.
Oxygen chains are limited to two atoms as in peroxides but sulphur chains contain upto four atoms as in poly sulphides.
8. Electrical conductivity:
9. Molecular structure – Atomicity:
Oxygen has strong tendency to form multiple (pp – pp) bonds and hence exist as a diatomic gaseous molecule.
Sulphur, selenium and tellurium because of bigger size have no tendency to form pp – pp multiple bonds but prefer to form single bonds. Thus these elements exist as octa-atomic (i.e., S8, Se8, Te8) solid molecules with puckered ring structure or crown structure. The puckered S8 rings differ in the overall packing in the crystal.
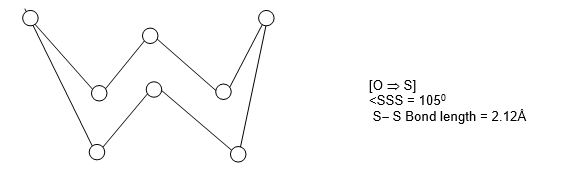
10. Tendency to form rings:
11. Density:
|
|
O |
S |
Se |
Te |
Po |
|
(g/ml) |
1.14 (liq) |
2.07 |
4.70 |
6.10 |
– |
12. Allotropy: All these elements of group 16 exhibit allotropy.
|
Element |
|
Allotropes |
|
Oxygen |
– |
Ordinary dioxygen and ozone. |
|
Sulphure |
– |
Rhombic (S8) or α S8 |
|
|
– |
Monoclinic or β (or S8) – Most stable |
|
|
– |
Plastic sulphur (or λ) open chain. |
|
|
– |
Colloidal (or δ) sulphur |
|
|
|
|
|
|
– |
Cyclo S6-20 from. In cyclo – S6 the ring adopts the chain form |
|
Selenium |
– |
Rhombic Se8 |
|
|
– |
Monoclinic Se8 |
|
|
– |
Grey – More stable form consisting of regularly arranged spirals of Se atoms. |
|
Tellurium |
– |
Non-metallic (amorphous) |
|
|
– |
Metallic (More stable) |
|
Polonium |
– |
α and β both metallic |
Illustration 1: What is transition temperature? Give an example.
Solution: The temperature at which to allotropes exists equilibrium is called transition temperature. e.g. Rhombic sulphur Monoclinic sulphur.
C. Oxidation states: The common oxidation state of these elements is – 2 because they have ns2 np4 configuration (i.e., 6 valance electrons) in their outer most orbits. They gain two electron to complete the octet in their valence shell. e.g., In sodium monoxide (Na2O) and calcium oxide, oxidation state of oxygen is – 2. Besides this (-2) oxidation state, the chalcogens exhibit other oxidation states also.
|
Elements: |
O |
S |
Se |
Te |
Po |
|
O.S : |
– 1, – 2, + 2 |
-2, + 2, + 4, + 6 |
-2, +2, + 4, + 6 |
-2, +2, + 4, + 6 |
-2, + 2, + 4, + 6 |
In O2F2 the oxidation state of oxygen is + 1, In peroxides (H2O2) the oxidation state of oxygen is -1 and in OF2 is + 2, but it does not show + 4 and + 6 O.S. due to absence of d-orbitals in its valence shell.
However, as we move down the group, the stability of + 4 oxidation state increases while that of + 6 oxidation state decreases due to inert pair effect.
Thus, in Po, + 4 oxidation state is more important than + 6 oxidation state. Hence, S, Se and Te are tetravalent in their typical compounds with oxygen and hexavalent in their compound with fluorine.
Illustration 2: Oxygen is divalent but sulphur is hexavalent. Why?
Solution: Oxygen has no vacant d-orbitals. Hence it is divalent. It cannot form 4 or 6 bonds. But sulphur has vacant d-orbitals. Hence, it possess six unpaired electrons and forms six bonds.
D. Chemical properties:
1. Formation of hydrides:
All these elements form volatile, stable, bivalent hydrides (H2M) such as H2O, H2S, H2Se, H2Te and H2Po). These are formed either by direct combination with hydrogen or by the action of acids on metal sulphides, selenides and telurides. i.e.,
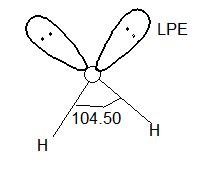
Water is a colourless, odourless liquid while other hydrides are colourless, bad smelling poisonous gases. All these hydrides have angular or V-shape structure which involves sp3 hybridization of central atom. e.g.,
Characteristics of hydrides:
(i) Melting and boiling points:
|
Hydrides: |
H2O |
H2S |
H2Se |
H2Te |
|
MP (0C) |
1000C |
– 85 |
– 65 |
– 51 |
|
BP (0C) |
1000C |
– 60 |
– 41 |
– 4 |
Melting point and Boiling point of hydrides of charcogens decrease in the following order.
ii) Volatility: All the hydrides are volatile in nature on decreasing the boiling point, volatility increases. Hence, volatility increases from H2O to H2S and then decreases. This is clear from their boiling points. Thus, the correct trend of volatility is
Thus H2O is least volatile and H2S is most volatile hydrides.
Reason: The high B.P. and therefore low volatility of water is due to the association of H2O molecule through hydrogen bonding. However hydrogen bonding is not present in other hydrides.
The inter molecular force between the hydrides are van der Waal’s forces (except H2O)
These forces increases with increase in molecular size and therefore, boiling points increase on moving from H2S to H2Te.
iii) Acidic character: These hydrides are weakly acidic in nature.
e.g., H2S is a weak diprotic acid
the acidic character increase from H2O to H2Te as
Reason: The increase in acidic character is due to the reason that as the size of the atom ‘M’ in H2M (acid) increases, the strength of H – M bond decreases.
As a result, the tendency to release hydrogen as proton increases down the group. (it is least in case of ‘O’ and maximum in case of ‘Po’).
This is evident from their dissociation constant (Kr) values in aqueous solution at 250C.
|
Hydrides |
H2O |
H2S |
H2Se |
H2Te |
|
Ka1 |
1.0 ´ 10–14 |
1.3 ´ 10–7 |
1.3 ´ 10–4 |
2.3 ´ 10–3 |
iv) Thermal stability: The thermal stability of the hydrides decreases from H2O to H2Te as
Reason: This is due to the reason that as the size of central atom in H2M increases, the H-M bond become weaker and breaks easily on heating, and therefore thermal stability decreases.
Thus water dissociate at about 2073 – 2273 K, H2S at 673 – 873 K, H2Se at about 423K. While H2Te decomposes even at ordinary temperature.
v) Reducing character: All the hydrides except H2O are reducing agent, the reducing power of these hydrides increases from H2O to H2Te, due to the decrease in thermal stability of these hydrides, the order of reducing power is
Thus greater the unstability of hydrides, the greater is its reducing character.
Illustration 3: Water is liquid but H2S is a gas at room temperature. Why?
Solution: Water is liquid due to the presence of intermolecular H-bonding in it. Whereas H2S is a gas because it has no H – bonding.
Illustration 4: What are the bond angles in H2O and H2S? Why are they different?
Solution: The bond angles in H2O and H2S are 104031¢ and 920 respectively. In H2O, central atom (O) undergoes sp3 hybridization. In H2S pure ‘p’ orbitals of ‘S’ participate in bond formation. Hence there is difference in bond angles.
2. Halides formation: All these elements form a number of halides.
a) Monohalides: Only the monochloride, monobromide (Dimeric) and monofluorides of S and Se are known. They exist as dimer i.e., S2F2, S2 Cl2, S2Br2, Se, Br2.
The electron diffraction studies have shown that the structure of S2Cl2 is similar to H2O2 as shown below:

Properties of monohalides (Physical state): The important physical properties of sulphur monohalides are given below
|
Halides |
S2F2 |
S2Cl2 |
S2Br2 |
|
Physical state |
Colourless liq |
Yellow liq |
Red liquid |
|
M.P (0C) |
-120.50c |
– 80 |
– 46 |
|
B.P (0C) |
– 390 |
138 |
90 |
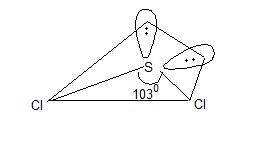
b) Dihalides: All these element form stable dichlorides and dibromides. g. OF2, SCl2, TeBr2. Only SCl2 is best known. It has tetrahedral geometry (sp3) but bent or angular shape due to presence of lone pairs of electrons on ‘S’ atom, and the bond angle is about 1030 instead of normal tetrahedral angle of 109.50.
Preparation : i)
Properties:
1. It is a red liquid having melting point – 790C and boiling point – 590
2. It is unstable and disproportionates as
3. It readily oxidize to thionyl chloride
c) Tetrahalides: With the exception of S Br4, SI4 and SeI4 all tetrahalides are known. SF4 is gaseous, SeF4 is solid, SCl4 is unstable liquid.
Preparation:
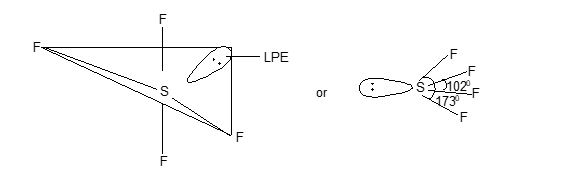
Structure: They have trigonal bipyramidal shape with sp3d hybridization.
Properties of SF4: (i) It is colourless gas, mp = – 1210C and B.P. = – 380C.
(ii) It is a water sensitive and get hydrolysed.
(iii) It slowly undergoes direct oxidation to SOF4.
Structure: It has trigonal bipyramidal structure in which one position is occupied by a lone pair of electrons. Sulphur undergoes sp3d hybridization as shown below.
Properties of SCl4: i) It is unstable liquid and its freezing point is – 310C.
ii) It forms H2SO3 with
iii) It forms adduct with Lewis acids.
Structure: Structure of Scl4 is similar to that of SF4.
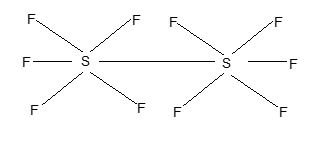
d) Pentahalides: Only sulphur forms one pentahalide, S2, F10. It has the structure as shown below, in which two SF5 units are linked through sulphur atoms having octahedral geometry.
Preparation: i) It is formed by the fluorination of sulphur as a by product along with SF6.
ii) It is best prepared by the photolytic reduction of SClF5 as
Properties:
i) It is a liquid having melting point – 920C and boiling point 290
ii) It is extremely toxic.
iii) It has intermediate reactivity between SF4 and SF6. Like SF6, it is inert and does not get hydrolysed by water or alkalies.
e) Hexahalides: All the elements except oxygen form covalent hexafluorides such as SF6, SeF6 and TeF6 (all are gases). No other halogen forms hexahalides. The haxafluorides have actahedral shape with sp3d2 hybridization of central atom.
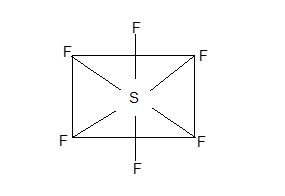
Thus the case of hydrolysis varies as SF3 < SeF6 < TeF6.
Halogen compounds of oxygen: Iodine oxides are most stable.
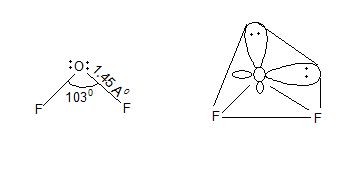
Oxygen difluoride (OF2):
Prep : i)
Prop: i) It is a pale yellow gas.
ii) It is more poisonous gas than fluorine.
iii) It dissolves in water out does not give any oxyacid solution.
Dioxygendifluoride (O2F2):
Its formula resembles those of dimeric monohalides M2X2 (e.g., S2Cl2).
Prep:
Structure: Similar to that of H2O2 i.e., open book structure.
Illustration 5: What products are formed in the hydrolysis of SCl4? Give the equation.
Solution: SCl4 on hydrolysis gives sulphurous acid
Illustration 6: SF6 is not easily hydrolysed. Explain.
Solution: SF6 is chemically inert and therefore, does not get hydrolysed. Its inert nature is due to the presence of stearically protected sulphur atom which does not allow thermodynamically favourable hydrolysis reaction.
Illustration7: SF6 is known but SCl6 is not known. Give reason.
Solution: Due to small size of ‘S’, six large atoms can not be accommodated around S atom. But small six F atoms can be easily accommodated around ‘S’ atom to form SF6.
Moreover, because of low electronegativity of Cl, it cannot easily cause promotion of electrons in S to form S (VI).
3. Formation of oxides: These elements form a variety of oxides in different oxidation states from + 2 to + 6.
|
Element |
Monoxides |
Dioxides |
Trioxides |
Heptoxides |
Other oxides |
|
S |
SO |
SO2 |
SO3 |
S2O7 |
S2O, S2O3, SO4 |
|
Se |
– |
SeO2 |
SeO3 |
– |
– |
|
Te |
TeO |
TeO2 |
TeO3 |
– |
– |
|
P0 |
P0O |
P0O2 |
|
|
|
1. Sulphur Monoxide (SO):
Preparation: i)
ii)
Properties: i) It is a colourless unstable gas. It is stable only under reduced pressure and break up into sulphur dioxide at pressure greater than 40 mm Hg.
ii) It reacts with water to give hydrogen sulphide and SO2
2. Sulphur dioxide (SO2): It contains ‘S’ in + 4 oxidation state. It is anahydride of H2SO3 and is called sulphurous anhydride.
Preparation. i) On laboratory scale.
ii) On industrial scale:
In this process a small amount of SO3 is also formed.
iii)
iv)
This method is particularly used in a place where gypsum occurs abundantly.
Properties:
i) It is a colourless, toxic gas with a pungent and suffocating odour and forms descrete molecules even in the solid state.
ii) It is acidic in nature and is also called anhydride of sulphurous acid.
iii) It can act as a reducing agent and also as anoxidising agent.
iv) It also act as a bleaching agnet in the presence of a moisture.
v) It bleaches due to reduction and bleaching action is temporary.
The colour is, however, restored when the bleached article is exposed to air since the oxygen of the air oxidizes the colourless compound back to the original coloured substance.
vi) It turns lime water milky due to formation of CaSO3. Milkiness disappears on passing excess SO2 due to the formation of Ca(HSO3)2 (soluble).
SO2 is dried by bubbling it through cone H2SO4. It is not dried over quick lime as it reacts with it to form calcium sulphide.
Structure: SO2 molecule has angular or bent shape structure. It is resonance hybrid of two structures (a) and (b) as shown below:
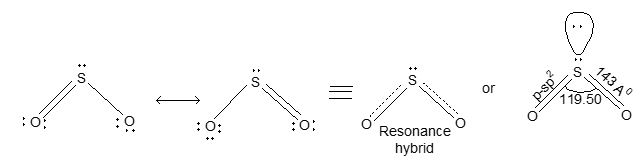
The multiple bonding in S – O bond in SO2 is due to pπ – pπ bonding. Sulphur is sp2 hybridized and the lone pair of electrons on ‘S’ reduces the angle from 1200 to 1190. In addition, it has been suggested that there is possibility of pπ- pπ bonding due to overlap of filled pp orbitals of oxygen with vacant 3d-orbitals of the ‘S’ as shown below.
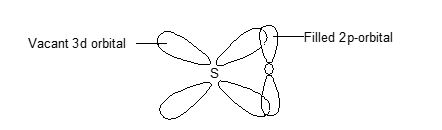
Gaseous SeO2 has the same structure but the solid form consist of infinite non-planar chains of SeO2 units with briding oxygen atoms.
3. Sulphur trioxide (SO3): It contains ‘S’ in + 6 oxidation state. It is an anhydride of H2SO4 and is called sulphuric anhydride.
Preparation:
i)
ii)
iii)
Properties:
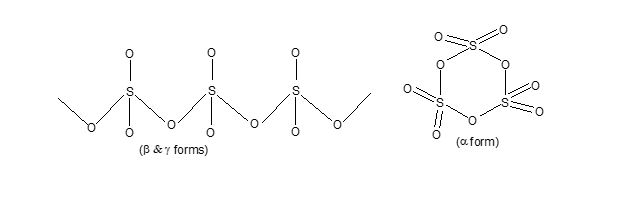
i) It is a white crystalline solid and exists in three allotropic forms, i.e., α, β and γ. Out of these a is the most common form. Chemically all the three form are identical, although α-form is comparatively more reactive. Mp = 170C, BP = 450
ii) It is an acidic oxide and is regarded as an anhydride of H2SO4. It turns blue litmus red.
iii) It acts as an oxidizing agent.
Structure: In the gaseous phase it exists as planar triangular molecular species involving sp2 hybridization of the sulphur atom. It has three S – O σ bonds formed by sp2 – p overlap and three S – O π bonds, one formed by pπ – pπ overlap. The O – S – O bond angle is of 1200 and all S – O bonds are equivalent (142 pm).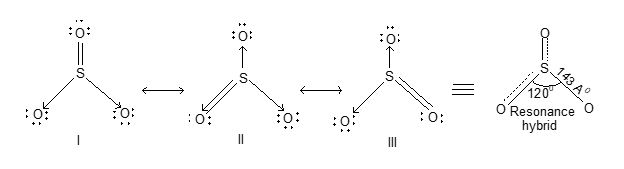
In solid state, it exist as linear chain or cyclic trimer.
Selenium trioxide (SeO3): It is a white hygroscopic solid having melting points 1180C. It exists as monomer in the vapour state (a) and as a cyclic tetramer (Se4O12) in the crystalline state (b).
General characteristics of Oxides:
ii) Acidic character: Acidic character of oxides of this group (VIA), decreases as we move from top to bottom. Thus SO2 and SeO2 are acidic while TeO2 and PoO2 are amphoteric. Thus order of acidic character is
Acidic character of oxides of a particular element increases with the increases in oxidation number of the central element. Thus, SO is less acidic than SO2 which is less acidic than SO3. The oxidation number of ‘S’ increases from + 2 to + 4 and + 6 in these oxides, respectively.
Thus, the order of acidic character varies as
ii) Thermal stability: The oxides of ‘S’ are more stable than the corresponding oxides of selenium, e.g., SO2 is more stable than SeO2.
SO3 is more stable than SeO3. SO is known while SeO is not known. The thermal stability of dioxides of these elements varies as
|
|
SO2 |
TeO2 |
SeO2 |
PoO2 |
|
Decomposition temperature |
Very high temperature |
> 1000 K |
~ 873 K |
773 K |
4. Oxoacid formation : ‘S’, ‘Se’ and ‘Te’ form a variety of oxoacids, of these, the oxoacid of ‘S’ are more numerous and more important than those of ‘Se’ and ‘Te’.
Oxoacids of sulphur: Sulphur form number of oxoacids. Only a few of these have been isolated as free acids while a number of these have been found only as their aqueous solutions or salts.
|
Name |
Formula |
Structure |
Oxidation state of ‘S’ T C Av |
Comments |
|
I. Sulphurous acid series |
|
|
|
|
|
a) Sulphurous acid (Red. In nature) |
H2SO3 |
-, + 4, + 4 |
Tautomeric structures are possible. Unstable in free state. Its salts sulphites are well known. It has pyramidal or tetrahedral structure with a lone pair. Hence sp3 hybridization undergone. (pπ-dπ) bond is formed with one oxygen atom. |
|
|
b) Thiosulphurous acid |
H2S2O2 |
|
-2, + 4, + 1 |
Sp3 hybridization is involved. pπ-dπ bond between ‘S’ atoms is formed. |
|
c) Disulphurous acid or pyrosulphurous acid |
H2S2O5 |
+3, +5, +4 |
Known only in the forms of its salts. Two (pπ – dπ) bond with S in + 5 OS and one (pπ – dπ) bond with ‘S’ in +3 OS are formed. |
|
|
d) Dithionounsacid or, hyposulphurous acid or hydrosulphurous acid |
H2S2O4 |
+3, +3, +3 |
Unstable in solution known in the form of its salts. One (pπ-dπ) bond on each sulphure. |
|
II. Sulphuric acid series:
|
a) Sulphuric acid |
H2SO4 |
-, + 6, + 6 |
Several resonance structures are present. Two (pπ-dπ) bonds are formed. |
|
|
b) Thiosulphuric acid |
H2S2O3 |
(S-S, linkage) |
-2, + 6, + 2 |
Unstable in free state. Its salts are well known. Two pπ-dπ bonds are formed one with oxygen and the other with terminal ‘S’. |
|
c) Pyrosulphuric acid or disulphuric acid (olium). |
H2S2O7 |
(S-O-S, linkage) |
+ 6, + 6,+ 6 |
It is believed to be a solution of SO3 in concentrated H2SO4. This is also known as fuming sulphuric acid. Two pπ – dπ bonds present on each of the sulphur atoms. |
III. Thionic acid Series:
|
a) Di thionic acid |
H2S2O6 |
+5, + 5, + 5 |
Known in aqueous solution and also as its salts. Two pπ – dπ bonds, on each ‘S’ are formed. |
|
|
b) Polythionic acid |
H2Sn+ 2 O6 |
+5, – , + 5 |
Free acids are unstable known in the form of salts. Two pπ – dπ bonds are present on ‘S’ atoms present in SO3H groups. |
IV. Peroxoacid series or peroxy acid series:
|
a) Peroxomono sulphuric acid (caro’s acid) |
H2SO5 |
-, + 6, + 6 |
Fairly stable acid. Two pπ – dπ bonds are formed by ‘S’. |
|
|
b) Peroxodisulphuric acid or persulphuric acid (or) (Marshall’s acid) |
H2S2O8 |
+6, + 6, + 6 |
Stable compound. Its salts are well known. Two pπ – dπ bonds are formed on each ‘S’ in SO3 H groups. |
Note: T = Terminal S; c = central S; Av = Average O.S. of S. Oxyacids of selenium: Selenous acid H2SeO3; Selenic acid H2SeO4.
Oxygen:
Chinese observed the presence of oxygen in air. Priestley and Scheeley prepared oxygen by heating suitable oxygen compounds.
Preparation: 1) By the action of heat on oxygen rich compounds.
i) From oxides and peroxides:
ii) From certain compounds (salts):
2. By the action of some chemical reagents on oxygen rich compound:
3. By decomposition of steam by chlorine:
4. By electrolysis of water either acidified with H2SO4 using Pt – electrodes or by making it alkaline with NaOH or Ba(OH)2 using nickel electrodes.
5. Manufacture: By fractional distillation of liquid air.
Properties: i) It is a colourless, odourless, tasteless slightly heavier than air, sparingly soluble in water, soluble in pyrogallol.
ii) Reaction with metals and non metals:
a)
b)
iii) Other reactions: (In presence of catalyst)
Test: i) With NO it gives radish brown fumes of NO2
ii) Absorbed by alkaline pyrogallol.
Uses:
i) For breathing.
ii) In welding and cutting : oxyhydrogen acetylene torch is used.
iii) In iron and steel industry: to increase the content of blast in the Bessemer and open hearth process.
Anomalous behaviour of oxygen:
The oxygen differs from the rest of the elements due to its
i) small size
ii) higher electronegativity
iii) absence of d-atomic orbitals in valence shell.
iv) tendency to form multiple bonding.
Points of difference:
i) It is a diatomic gas and it readily form multiple bonds, while other elements are solid and have puckered eight membered rings.
ii) It is paramagnetic while others are diamagnetic.
iii) Oxygen show oxidation states of -2, – 1 and + 2 while other members show oxidation states of – 2, + 2, + 4 and + 6.
iv) H2O, the hydride of oxygen form H-bond while other do not.
v) The compounds of oxygen are more ionic than those of other members of this family. O2– ion is also very common, S2– ion is quite common but Se2– and Te2– ions are uncommon.
Ozone (O3):
Discovered by Van Marum in 1758 by passing electric discharge through air. The term ozone was given by Schonbien in 1840. The molecular formula, O3 was given by Sorret, in 1866 and he pointed out that ozone is an allotrope of oxygen. O3 is present in stratosphere of the atmosphere. This prevents the U.V. radiations coming from the sun from reaching the earth.
Preparation:
i) It is formed in atmosphere by the action of UV rays on O2.
ii) By slow oxidation of phosphorus in air.
iii) By the reaction of SO2 with H2O2.
iv) Lab method: Ozone is prepared by passing a silent electric discharge through cold, dry oxygen.
This is an endothermic reaction. According to Le chatelier principle energy must supplied to produce ozone, the instrument used for this purpose is called ozonizer. In laboratory, two kinds of ozonizers are commonly used.
a) Siemen’s Ozonizer: It consists of two concentric glass tubes sealed together at one end. The innersides and the outersides of the tubes are coated with tin foils. The inner and outer coatings are connected with the two terminals of an induction coil. A current of dry oxygen at low temperature is passed in from one end. When the current is put on, the oxygen is partially converted into ozone. Ozonized oxygen come out from other end and is collected. It contains about 10% ozone. The percentage can be increased upto 15% if temperature is lowered to 50
b) Brodie’s Ozonizer: Brodie’s ozonizer also works in the similar manner to sieman’s ozonizer. The ozonizers differ in their structures. In this zonizer the tin foil of siemen’s ozonizer, is replaced by dil sulphuric acid in which ‘pt’ – electrodes are dipped. These electrodes are connected to an induction coil. Oxygen passing through the annular space is subjected to silent electric discharge, and is partially connected into ozone.
Manufacture: By sieman-Halske’s Ozonizer:
On large scale, O3 is prepared by siemen Halske’s ozonizer. It consists of cast iron box containing a number of aluminium rods in glass cylinders which are cooled by water. Each ‘Al’ rod rests on a glass plate and thus is insulated from the box. The ‘Al’ rods are charged to a high potential of 8000-10,000 volts. Air is passed in the lower compartment where it is subjected to the action of silent electric discharge as it rises up through the annular spaces. Ozonised air passes out through the exit at the top and collected. This contains about 5% of ozone.
Recovery of pure ozone from ozonized oxygen:
The ozonized oxygen is passed through a spiral cooled by liquid air. Ozone condenses as its condensation temperature is – 112.40C. The liquid ozone, thus obtained may contain some dissolved oxygen. Thus liquid is fractionally distilled to get pure ozone.
Physical properties:
i) It is a pale blue, pungent smelling and poisonous gas.
ii) It is heavier than air.
iii) It can be liquefied to pale blue liquid at – 112.40C. At – 249.70C, it forms violet black crystals.
iv) It causes headache and nausea, when inhaled in small amount.
v) It is lightly soluble in water but more soluble in terpentine oil, gracial acetic acid or CCl4.
Chemical properties:
1) Decomposition: It is thermodynamically unstable and dissociates into O2.
MnO2, Pt – black, Ag, PbO2 etc., decompose ozone at ordinary temperature, i.e., they catalyse its decomposition.
2) Oxidising nature: It acts as a strong oxidizing agent. The potential equation is
.
The oxidation potential in acidic medium is . There are only three substances which have oxidation potentials higher than + 2.07V. These are fluorine, atomic oxygen and OF2. Due to high value of oxidation potential it acts as a strong oxidizing agent.
Examples:
i) It oxidizes the black lead sulphide to white lead sulphate:
Similarly, CuS, ZnS and CdS are oxidized to CuSO4, ZnSO4 and CdSO4 respectively.
ii) It oxidizes HCl into Cl2, HBr into Br2, HI into I2 and HF into F2.
iii) It oxidizes moist KI to I2
iv) Silver metal is blackened (tarnished) due to alternate oxidation of the metal and reduction of oxide.
v) It oxidizes the acidified ferrous sulphate into ferric sulphate.
vi) Ozone reacts with KOH and forms potassium ozonide, KO3, which is an orange coloured solid and contains paramagnetic O33–
vii) It oxidizes dry iodine to yellowish powder, I4O9.
Similarly, moist sulphur, phosphorus and arsenic are oxidized to their corresponding oxyacids.
viii) It oxidized acidified stannous chloride to stannic chloride.
ix) Tailing of mercury: The loss of metallic luster and meniscus of mercury metal, in the presence of ozone and as a result, mercury stick to the glass surface is called “tailing of mercury”.
The impure ‘Hg’ regains its luster and meniscus on washing with water.
x) It oxidizes nitrites into nitrates, sulphites into sulphates, assenites to arsenates, manganate to permanganate and ferrocyanide to ferricyanide.
3) Reducing nature: It acts as strong reducing agent.
Examples:
(i) It reduces H2O2 to H2O.
(ii) Ozone reduces Ag2O to Ag
Ag2O + O3 → 2Ag + 2O2
(iii) Ozone reduces BaO2 to BaO.
4) Bleaching property: It is a good bleaching agent. The bleaching action is due to its oxidizing action on the organic matter.
Coloured substance + [O] → colourless.
It bleaches oil, ivory, starch, waxes, wood pulp etc.
5) Addition reactions: Unsaturated organic compounds undergo addition reactions with O3 giving ozonides.
Structure of O3: Trambaruls (1953) and Hughes (1956) have pointed out from the studies of ozone by electron diffraction that ozone molecule is ‘V’ shaped with a bond angle of 11.680 and O – O bond length 1.27Å.
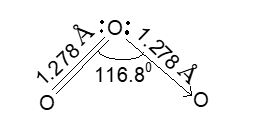
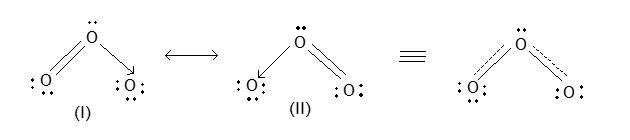
The bond length is intermediate between that for a single bond i.e., 1.48Å as in H2O2 and for a double bond i.e., 1.21Å as in O2. Ozone is, therefore, considered to be a resonance hybrid of the following two resonating forms.
Uses:
i) As an insecticide and bactericide.
ii) In sterilization of water.
iii) To purify the atmospheric air in cinema theatres, mines etc.
iv) In the manufacture of artificial silk and camphor etc.
v) To identify the unsaturation in carbon compounds.
vi) In the manufacture of artificial silk, synthetic silk, synthetic camphor and potassium permagnate.
Tests of Ozone:
i) It has a strong rotten smell.
ii) Metallic mercury loses its fluidity in contact with ozone.
iii) It turns an alcoholic solution of benzidine brown.
iv) It turns an alcoholic solution of tetramethyl base violet.
v) It turns starch iodide paper blue.
Illustration 8: Locate the oxidant and reductant in the reaction of H2O2 with O3.
Solution: H2O2 acts as oxidizing agent and O3 acts as reducing agent.
Sulphur:
The name sulphur has been derived from Sanskrit word sulveri, means killer of copper. The elementary nature of sulphur was established by Lavoisier in 1777.
Occurrence: It occurs free in volcanic regions. In combined state it occurs as
i) Gypsum : CaSO4. 2H2O
ii) Galena : Pb5
iii) Zinc blende : ZnS.
iv) Copper pyrite : Cu2S Fe2S3
v) Iron pyrite : FeS2
Allotropic forms: Its important allotropic forms are :
(i) Crystalline: Rhombic, monoclinic.
(ii) Amorphous: Plastic, milk of sulphur, colloidal sulphur.
Properties: Ordinary sulphur is pale yellow solid, soluble in water but dissolves in CS2, C6H6 and turpentine oil.
Uses:
i) In the manufacture of SO2, SO3, CS2, H2SO4, matches, gunpowder, fire works, etc.
ii) For valcanising the rubber.
iii) In medicine for internal as well as external applications.
iv) As a disinfectants for houses and for destroying bacteria, fungi, insects etc. Vapours of ‘S’ are poisonous for bacteria, etc but not for human beings.
Compounds of sulphur:
1) Hydrogen sulphide:
It occurs in volcanic gases, sewage gases, coal gas and in several spring waters. It is found in small amount in atmosphere, where it comes from the heating of coal and decay of animal and vegetable matter containing sulphur compounds.
Preparation (1) Lab Method: By the action of dil H2SO4 or HCl or iron sulphide: (Kipp’s apparatus is used)
Physical properties:
i) It is a colourless poisonous gas with odour like rotten eggs, fairly soluble in water.
ii) It is inhaled in small quantities but may cause death when inhaled for a long time.
iii) It can be easily liquefied by pressure. The boiling point is about -600c. It freezes to a transparent solid at – 85.60C.
Chemical properties:
I) Combustibility : The gas burns with blue flame in oxygen.
In restricted supply of oxygen, sulphur is formed due to incomplete combustion.
II) Thermal decomposition: This gas is not so stable as water. It decomposes on heating, the decomposition starts at 3100C and completes at 17000
III) Acidic nature: The aqueous solution of H2S acts as a weak dibasic acid. It ionizes as follow:
It forms two series of salts, hydrosulphides and sulphides.
IV) Reducing nature: It acts as a strong reducing agent as it decomposes evolving hydrogen.
i)
ii)
iii)
iv)
v)
vi)
vii)
viii)
V) Reaction with metal and metal oxides:
VI) Reaction with salts: Hydrogen sulphide reacts with salts of various metals forming corresponding sulphides the metal sulphides can be divided into three groups.
i) Sulphides precipited in acidic medium: Sulphides of Hg, Ag, Pb, Cu, Bi, Cd, As, Sb and Sn are ppt in acidic medium.
ii) Sulphides precipitated in alkaline medium:
These are the sulphides of Zn, Co, Ni Mn and Fe.
iii) Sulphides which are precipitated neither in acidic nor in alkaline medium: These are the sulphide of Cr, Al, Mg, Br, Sr, Ca, K and Na.
VII) Formation of Polysulphides: These are obtained by passing H2S through their hydroxides.
Uses:
i) In qualitative analysis for the detection of basic radicals.
ii) As a reducing agent.
iii) It is used for the preparation of metallic sulphides many of which find use in paint industry.
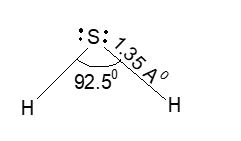
Structure of H2S: The shape of H2S molecule is i.e., V-shaped structure with bond length (H – S) 1.35Å and bond angle (H – S – H) 92.50.
Tests of H2S:
i) It has unpleasant odour like rotten egg.
ii) It turns leadacetate Pb (CH3COO)2 paper black.
iii) It gives a violet colouration with a solution of sodium nitroprusside.
Note: H2S is called sulphuretted hydrogen. It is poisonous and its largest amount prones fatal. Antidote for H2S is dilute chlorine solution which destroys the effect of H2S by oxidizing it to sulphur.
Illustration 9: What is Wackenroder’s liquid or solution?
Solution: It is obtained by passing hydrogen sulphide gas through saturated aqueous solution of SO2 till its smell is disappears and it is turned milky. When H2S gas passed through H2SO3 acid, the reaction called Wackenroder’s reaction.
Illustration 10: Why is H2S a better reducing agent than water?
Solution: Because ‘H-S’ bond energy is lower than H – O bond energy, Moreover, H2O is extensively associated and stable molecule.
Sulphurous acid (H2SO3):
Preparation: It is obtained by dissolving SO2 in water.
The solution gives a smell of SO2 which is evolved completely on heating. It is, thus believed that the acid is present in equilibrium with free gas.
It forms two series of salts, bisulphites and sulphites. The salts are fairly stable.
Structure: H2SO3 is believed to exist in two form which are always in equilibrium with each other.
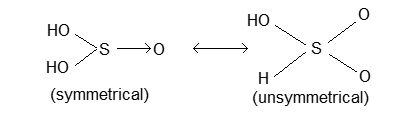
Uses: It reduces acidified KMnO4 or K2Cr2O7 to Mn2+ or Cr3+ respectively, halogen acids and iodates to iodine.
Its reducing character is believed to be due to tautomerism between the following two interconvertible forms.
i) It behaves as both reducing as well as oxidizing agent and also bleaches the articles due to reduction.
Sulphuric acid (H2SO4):
It is also kwon as oil of vitriol and king of chemicals the prosperity of any country is measured by the amount of H2SO4, it consumes.
Prep:
i)
ii)
iii)
iv)
v) v)
vi)
Manufacture: H2SO4 is manufactured these days by the following two processes.
i) Lead chamber process: The various steps involves are (Principle)
a) Production of SO2: By burning ‘S’ or iron pyrites.
b) Catalyst: ‘No’ acts as a catalyst in the lead chamber process.
c) Reaction in lead chamber:
i) Berzelius mechanism:
Thus, NO and NO2 as oxygen carrier.
ii) Davy and Lunge Mechanism:
d) Conditions: Temperature 500C, excess of steam, lead chamber, since Pb is not attached H2SO4.
Procedure and plant: The various parts of the plant and their functions are described as follows:
i) Pyrite burners: This is a specially designed bricked furnace where iron pyrite or ‘S’ is burnt.
ii) Dust chamber: This is a chamber where dust particles present in gaseous mixture coming from burner, separate due to gravity, the dust particles separation can also be done by using Cottrell electrostatic precipitator.
iii) Nitre pot: From the dust chamber, the gaseous mixture is passed through a nitre pot, where KNO3 is heated with H2SO4, then oxides of nitrogen are produced.
In modern plants, oxides of nitrogen are produced by the catalytic oxidation of NH3.
IV) Glover’s tower: This is a steel tower and is lined inside with sheet of lead or acid resisting bricks and is packed with quarts or flint stones. In this tower the acid from lead chamber and nitrated acid from Gay Lussac tower are allowed to flow down slowly and the gaseous mixture is allowed to enter from the base of the tower. Following changes occur in this tower.
i) The gases are cooled down from about 4000C to 800
ii) Chamber acid (dil) is concentrated.
iii) The nitrated acid from Gay Lussac tower is denitrated
iv) Some SO2 is converted into H2SO4
About 80% H2SO4 is collected at the bottom of the tower.
V) Lead Chamber: The gaseous mixture is changed into 65-70% H2SO4 in presence of steam admitted from the top of the lead chamber.
This chamber acid is pumped to the top of Glover’s tower for concentration.
VI) Gay – Lussac tower: Here the residual gases mainly air, oxides of nitrogen, from lead chamber and conc. H2SO4 from Glover’s tower, give nitrated acid. This nitrated acid is pumped on the top of the Glower’s tower. The waste gases are allowed to escape through chimney on the extreme right of the plant.
Purification: The acid obtained from lead chamber process contains chief impurities of PbSO4, As2O3, NO and NO2 which are removed as follows.
PbSO4 is insoluble in water and precipitated (settle down) on dilution of acid and filtered off.
The impurity of As2O3 is precipitated as As2S3 by passing H2S.
NO and NO2 are removed by heating with (NH4)2SO4.
Usually, the chamber acid is not purified and is used as such for most of the purposes. Whenever pure H2SO4 is needed the acid manufactured by the contact process is used.
Concentration of Chamber Acid: The acid (H2SO4) obtained from Glover’s tower contains about 80% H2SO4 and is known as brown oil of vitriol (B.O.V).
It can be further concentrated and this concentrated acid is called rectified oil of vitrol (R.O.V). The concentration is done by evaporation of water present in the acid with the help of hot gases. The two methods are employed for the concentration of chamber acid.
a) Cascade process: A series of ferrosilicon dishes are arranged one below the other, on a slope, heating is done by mean of hot gases passing below the dishes. As the acid flows downwards, it is subjected to stronger heat so that when it reaches the lowermost dish it has been concentrated to the maximum.
b) Gaillard process: The dilute acid is allowed to flow down in the form of a spray in a tower lined with acid resisting stone. Hot air enters from the base of tower. The dilute acid comes in contact with hot air and loses water content. By the time the acid reaches the bottom and becomes concentrated to about 95%.
2) Contact Process (Principle): This process involves the following steps.
i) Production of SO2: SO2 is prepared by burning sulphur or iron pyrites in excess of air.
;
It is purified by treating with steam to remove dust particles. The impurities of arsenic is removed by Fe(OH)3, water is removed by conc. H2SO4. The gases are filtered through coke filters and purity is tested by Tyndal box.
ii) Conversion of SO2 to SO3: It is done in contact or catalyst chamber after being preheated to 4500
This is a key reaction for the process.
iii) Absorption of SO3 into 92% H2SO4 and Dilution of oleum with water: SO3 is observed by conc. H2SO4 (98%) and then water is added to produce the acid of desired concentration.
H2S2O7 is Oleum or pyrosulphuric acid or fuming sulphuric acid.
It is to be noted that if SO3 is absorbed by water, it liberates large amount of heat producing acid fog.
Conditions favouring the maximum yield of sulphur trioxide: The conditions for the maximum yield of SO3 are derived by using Le – Chatelier’s principle as follows:
(i) Low temperature: Optimum temperature of 450 – 5000C is essential, to get the maximum yield of the product.
(ii) High pressure: A pressure of 1.5 to 1.7 atm osphere is sufficient for the oxidation.
(iii) Catalyst: A catalyst increases the speed of reaction. Platinised asbestos was used as catalyst. But it is easily poisoned by the impurities present in the gases and therefore, has now been replaced by Vanadium pentaoxide (V2O5). It is comparatively cheap and is not poisoned by the impurities.
(iv) Purity of gases: The gases must be purified before subjecting them to oxidation in the presence of catalyst.
Description of the plant: The various parts of the plants and their functions are described below:
1) Sulphur or pyrite burners: It is a kind of furnace in which sulphur or iron pyrite is burnt to form SO2.
2) Purification unit: The gaseous mixture coming out of sulphur burners is generally impure. The gases are purified as follows:
i) Dust chamber: Steam is introduced to remove dust particles.
ii) Coolers: The hot gases are cooled to about 1000C by passing them through cooling pipes.
iii) Scrubber: Gases are introduced into a washing tower (packed with quartz) also known as scrubber which dissolves mist and any other soluble impurities.
iv) Drying tower: It absorbs water vapours from the gases and they become completely dry.
v) Arsenic purifier: This is a small chamber fitted with shelve containing freshly precipitated Fe(OH) The impurities of As2O3 present in the gases are absorbed by Fe(OH)3.
3) Testing Box: The gases coming out, from the purification unit are tested in this box with the help of a strong beam of light. If some impurities are present, they will scatter light and the path will become visible. In case the gases are impure, they should passed through the purifying unit again.
4) Preheater and contact tower: The dry and pure mixture of SO2 and air is preheated to about 4000C are allowed to enter contact chamber. It consists of iron cylinder containing a number of vertical pipes packed with platinised asbestos heated to about 4500C, SO2 is oxidized to SO3 by O2 of the air. As this is a key reaction for the process, the heat evolved in this exothermic reaction maintains the temperature of the catalyst.
5) Absorption tower: SO3 escaping from the converter is led to the bottom of the tower where concentrated H2SO4 (98%) is sprayed from the top. SO3 gets absorbed by H2SO4 to form oleum or fuming H2SO4. Oleum on dilution gives H2SO4.
It may be noted that SO3 is not directly absorbed in water to form H2SO4 because the process is accompanied by formation of dense fog of the acid particles. Therefore, it becomes quite in convenient for the workers.
Physical Properties:
1. It is a colourless syrupy liquid. It contains 98.3% H2SO4. Its specific gravity is 1.84 at 150 (Normality = 36 N)
2. It’s boiling point is 3380C – constant boiling mixture containing 98.37. H2SO4 and 1.7% water.
3. It freezes into colourless crystals at 10.50
4. It is highly corrosive in nature and furmes strongly in moist air.
5. It is highly soluble in water. Its various hydrates such as H2SO4. H2O, H2SO4. 2H2O, H2SO4.3H2 H2SO4.4H2O are known.
6. For the dilution water should not be added to conc. H2SO4 but conc., H2SO4 should be added slowly to cold water with constant stirring.
Chemical properties:
i) Dissociation:
ii) Acidic nature: Dilute H2SO4 is a strong dibasic acid. It ionize as
It forms two series of salts, with bases.
iii) Reactions with carbonates & Bi carbonates:
iv) Displacement reactions:
a) Reactions with metals: Electropositive metals evolving (displace) the hydrogen.
Similarly, Sn, Zn, Al, Mn, Mg, evolving hydrogen from dil H2SO4.
Conc. H2SO4 reacts with all metals except Au and Pt with evolution of SO2.
b) It displaces more volatile acids from their metal salts:
5) Oxidising nature: It acts as a strong oxidizing agent. The potential equation is
a) Reaction with non-metals:
i) it oxidizes C to CO2:
ii) It oxidizes ‘S’ to SO2:
iii) It oxidizes ‘P’ to H3PO4:
b) Reactions with metals: Metals like Cu, Ag, Hg, etc. are first oxidized by conc. H2SO4 and then the oxides combine with acid to form corresponding sulphates.
c) HI is oxidized to I2:
d) Naphthalene is oxidized to phthalic acid in presence of Hg as catalyst:
6) Dehydrating nature: It acts as a powerful dehydrating agent due to its great affinity for water. Its corrosive action on skin is also due to dehydration of skin. It absorbs water from organic compounds.
7) Miscellaneous reactions:
i) Reaction with PCl5:
ii) Precipitation reaction:
iii) Sulphonation:
iv) Reaction with P2O5:
v) Reaction with KClO3:
vi) Reaction with ferrocyanide:
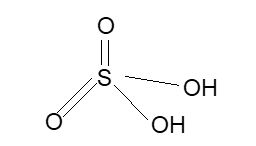
Structure: It has tetrahedral structure
Uses:
1. It is used as oxidizing, dehydrating agent.
2. In the manufacture of explosives such as nitroglycerine, gun cotton, TNT, picric acid etc.
3. In the refining of petroleum.
4. In lead storage batteries.
5. In the textile paper and dyeing industry.
Comparison between the lead chamber and contact process:
|
|
Lead chamber process |
|
Contact process |
|
1) |
‘NO’ is used as a catalyst. |
1) |
Solid V2O5 or Pt-asebestos is used as a catalyst. |
|
2) |
Impure acid is obtained. |
2) |
Pure acid is obtained. |
|
3) |
80% acid is obtained. |
3) |
Acid of any concentration. |
|
4) |
It is a cheaper method |
4) |
It is a costier method the cost has been reduced by the use of platinum. |
Illustration 11: Sulphuric acid is viscous and has high boiling point explain.
Solution: The high boiling point and viscosity of conc. H2SO4 is due to association of H2SO4 molecules via hydrogen bonding.
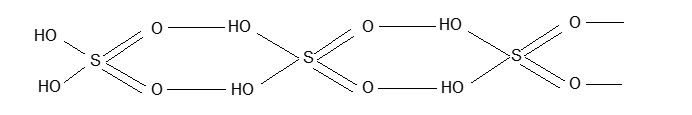
Illustration 12: What happens when conc. H2SO4 falls on skin?
Solution: Boils are formed due to corrosive action of H2SO4. It is because of dehydration of skin which burns and produces itching sensation.
Sodium Thiosulphates (Na2S2O3.5H2O): It is also known as ‘hypo’.
Prep: (i) By boiling Na2SO3 with flowers of sulphur in absence of air.
The unreacted ‘S’ is filtered off and the filterate is concentrated to crystallize Na2S2O3.5H2O.
ii) By heating sodium hydrogen sulphide and sodium hydrogen sulphite together.
iii) By the oxidation of sodium sulphide or sodium polysulphide with air.
iv) By the reaction of sodium sulphide with SO2.
Properties: Physical properties:
i) It is a colourless, crystalline, efflorescent substance.
ii) It is highly soluble in water.
i) Action of heat: It looses all the molecules of water (i.e., water of crystallization) when heated to about 2150 On further heating, it undergoes thermal dissociation giving rise to H2S, SO2 and S.
ii) Reaction with dilute acids:
iii) Reaction with AgNO3 solution: Two kinds of reactions may take place.
First kind of reaction:
Second kind of reaction:
v) Reaction with salts :
This reaction is used in volumetric analysis.
vi) Reaction with moist chlorine: It absorbs halogens
Hence, this reaction is used to remove last trace of chlorine in some industrial process i.e., in textile industry. Hence, it is known as antichlor.
vii) Reaction with iodine:
viii) Reaction with H2SO4:
Uses:
1. It is used as an antichlor compound in textile industry.
2. As fixer in photography to remove AgBr left.
3. In volumetric analysis in laboratory.
4. As an antiseptic in medicine.
5. In metallurgy for extraction of Ag and Au.
Illustration 13: What is the use of ‘hypo’ in photography?
Solution: Hypo (sodium thiosulphate) is used in photography to remove unexposed AgBr as a soluble complex.
Illustration 14: Why is AgBr used in photography?
Solution: Out of all silver halides, AgBr is the most sensitive to light and undergoes photo reduction to metallic silver instantaneously on exposure to light.
Illustration 15: What is antichlor? Give an example?
Solution: Antichlor is a substance which removes chlorine. e.g., Hypo.
FORMULAE AND CONCEPTS AT A GLANCE
1. Elements of group 16 or VIA are called chalcogens i.e., ore forming elements.
2. These elements have six electrons in their outermost shell, i.e., ns2np4 configuration.
3. Oxygen is a gas while other members of group 16 are solids.
4. Oxygen atom is capable to form multiple bonds (pp – pp) with other oxygen atom on account of small size
5. Atomic radii, atomic volume and density increase gradually from O to Po.
6. Electronegativity decreases gradually down the group. Oxygen is second most electronegative atom.
7. These elements from hydrides of H2M type.
8. H2O is liquid while other hydrides are colourless poisonous gases with bad odour.
9. The thermal stability of the hydride decreases down the group. This is due to an increase in M – H bond length.
10. Acidic strength hydrides increases from H2O to H2Te.
11. All these hydrides have V-shape structure.
12. S, Se, Te and To form oxides on burning in air.
13. S, Se, Te form similar oxyacid.
14. Sulphur and selenium also form oxyhalides. These oxyhalides are called thionyl and selenyl halides respectively.
15. Ozone is present in sufficient amounts in the atmosphere at higher altitudes.
16. Ozone is a pale blue gas with a characteristic strong smell.
17. In ancient days sulphuric acid was called as oil of vitriol.
18. Thiosulphuric acid is a dibasic acid.
19. Caro’s acid and Marshall’s acid contain peroxylinkages.
20. IUPAC name of H2S is sulphane.
21. Gun powder is a mixture of sulphur, charcoal and KNO3.
22. Liquid oxygen mixed with finely divided carbon is used in place of dynamite in coal mining.
23. A mixture of ozone and cyanogens is used as a rocket fuel.
24. S2Cl2 is used in the vulcanization of rubber.
25. H2SO4 is known as king of chemicals.
26. Thiosulphate has S = S linkage.
27. Led chamber process is cheaper method than contact process.
28. Hypo is used in photography to remove unexposed AgBr.
29. SF6 is used in high voltage transformers because of its insulating property.
30. The name sulphur has been derived from Sanskrit word sulveri meaning killer of copper.
SOLVED PROBLEMS-1
Prob 1. Why is oxygen a gas at room temperature, whereas sulphur is a solid?
Sol: Oxygen molecule (O2) is a non-polar covalent molecule. The only possible molecular interaction in oxygen is due to weak van der Waal’s forces. Thus, O2 is a gas at room temperature.
Where as sulphur is octa atomic molecule (S8). It has a puckered ring structure and is a non-polar molecule. Its size is large and therefore, the van der Waal’s force of attraction is stronger than that of O2. Hence, S8 is a solid at room temperature.
Prob 2. Oxygen almost invariably exhibits an oxidation state of – 2 but the other members of the family exhibit negative as well as positive oxidation states of +2, + 4 and + 6.
Sol: All the elements of oxygen family, have a general configuration of ns2np4 of the outer most orbit. They all tend to attain 8 electrons by accepting two more electrons, i.e., all show -2 oxidation state.
Prob 3. A mixture of three gases A, B and C is passed first through acidified K2Cr2O7 solution, when A is absorbed turning K2Cr2O7 solution green. Remainder is passed through excess of lime water which turns milky resulting in absorption of B. The residual gas ‘C’ is absorbed in alkaline pyrogallol solution. The mixture A, B and C does into turn lead acetate paper black. Identify A, B and C.
Sol: ‘A’ is a SO2 gas, as it turns acidified K2Cr2O7 solution green.
‘B’ is a CO2 gas, as it turns lime water milky and ‘C’ is oxygen which is absorbed in alkaline pyrogallol.
Prob 4. O2 is paramagnetic, whereas is diamagnetic. Explain.
Sol: Molecular oxygen is paramagnetic because there are two unpaired electrons in the p antibonding orbitals, but in ion these unpaired electrons get paired up with the result is diamagnetic.
Prob 5. SF6 is known but OF6 is not. Explain.
Sol: OF6 is not known because for the formation of OF6 the oxygen atom should have six half-filled orbitals. This is not possible due to absence of d-orbitals in the valence shell of oxygen. But in case of SF6, it is possible because sulphur has empty d-orbitals of suitable energy.
Prob 6. Arrange the hydrides of group 16th in increasing order of
(a) boiling point (b) bond energy (c) thermal stability (d) acid strength
Sol: (a) Boiling point :
(b) Bond energy :
(c) Thermal stability :
(d) Acid strength :
Prob 7. SO2 is more powerful reducing agent in an alkaline medium than in acidic medium.
Sol: The potential equation for reducing property is
Addition of acid favours reverse reaction whereas presence of OH– favours the forward reaction. Hence SO2 is more powerful reducing agent in an alkaline medium.
Prob 8. What is tailing of mercury? What is the chemical reaction taking place in it?
Sol: The loss of metallic luster and meniscus of ‘Hg’ metal, in the presence of Ozone and as a result mercury sticks to the glass surface is called tailing of mercury. The chemical reaction is
Prob 9. Explain, why the tendency to show – 2 oxidation state diminished from sulphur to polonium?
Sol: Atomic size increases from sulphur to polonium, therefore, tendency to gain two electrons decreases.
Prob 10. Sulphur disappears when boiled with sodium sulphite. Why?
Sol: When sodium sulphite is heated with sulphur, we get sodium thiosulphate which is soluble in water that is why sulphur disappears.
SOLVED PROBLEMS-2
Prob 1. Dry bleach is done by
(A) Cl2 (B) SO2 (C) O3 (D) H2O2
Sol: (C) Dry bleach is done by ozone.
Prob 2. The most stable allotrope of sulphur is
(A) rhombic sulphur
(B) monoclinic sulphur
(C) plastic sulphur
(D) flowers of sulphur
Sol: (A) Rhombic sulphur is the most stable allotrope of sulphur.
Prob 3. Which of the following reactions depicts the oxidizing behaviour of H2SO4?
Sol: (D)
Prob 4. When SO2 is passed through acidified solution of H2S
(A) H2SO3 is formed
(B) H2SO4 is formed
(C) H2SO5 is formed
(D) sulphur is precipitated
Sol: (D)
Prob 5. Ozone is
(A) an isobar of oxygen
(B) an isotope of oxygen
(C) a polymer of oxygen
(D) an allotrope of oxygen
Sol: (D) Ozone is an allotrope of oxygen.
Prob 6. In the manufacture of sulphuric acid by contact process, most favourble conditions are
(A) use of catalyst, optimum temperature and excess of air
(B) no catalyst, low temperature and low pressure
(C) catalyst, low temperature and low pressure
(D) catalyst, high temperature and high pressure
Sol: (A) In the manufacture of sulphuric acid by contact process, most favourble conditions are use of catalyst, optimum temperature and excess of air.
Prob 7. Identify the incorrect statement with respect to ozone
(A) ozone is formed in the upper atmosphere by a photochemical reaction involving dioxygen
(B) ozone is more reactive than dioxygen
(C) ozone is diamagnetic whereas dioxygen is paramagnetic
(D) ozone protects the earth’s inhabitants by absorbing gamma radiations
Sol: (D)
Prob 8. When P2O5 is heated with conc. H2SO4, the later gets converted into
(A) H2S (B) SO2 (C) SO3 (D) a mixture of SO2 and SO3
Sol: (C)
Prob 9. All of the following have a tetrahedral shape except
(A) (B) (C) (D)
Sol: (B) XeF4 as square planar shape.
Prob 10. Calculate the weight of 60% H2SO4 required to decompose 50g of chalk.
(A) 29 g (B) 29.4 g (C) 49 g (D) 50 g
Sol: (B)
weight of H2SO4 required to decompose 50g of CaCO3
weight of 60% H2SO4 required =








