Introduction:
There are many properties of substances particularly of solids and liquids which depend upon the nature of the surface. Surface represents the boundary which separates two bulk phases. This is also called interface and is generally represented by separating the bulk phase by a hyphen or a slash. e.g. The interface of surface between a solid and a liquid may be represented as solid – liquid or solid/liquid interface. There is no interface between gases because they are completely miscible.
The branch of chemistry which deals with the nature of surfaces and changes occurring on the surfaces is called surface chemistry. Adsorption on solid or on solution surfaces, colloidal properties, heterogeneous catalysis, corrosion, etc, are important surface effects which are useful to understand many physical and chemical properties of the substances. Therefore, the study of surface chemistry is of great importance not only from the academic point of view but also from the point of view of its important applications in industry, analytical chemistry and our daily lives.
Sorption: Concentration of a substance on the surface and in the bulk of another substance is known as sorption. The surface for sorption means a layer of thickness of about 100nm from the outer surface of the substance. Sorption includes following two stanultaneous process. i) adsorption, ii) Absorptions.
Adsorptions: The Phenomenon of accumulation (or concentration) of a substance on the surface of a solid or a liquid is known as adsorption. In the process of adsorption two substances are involved. One is the solid or the liquid on which adsorption occurs. The second is the gas or liquid or the solute from a solution which get adsorbed on the surface. These two substances are called adsorbent and adsorbate respectively.
i) Adsorbent: The substance on whose surface the adsorption occurs is known as adsorbent.
ii) Adsorbent: The substance whose molecules get adsorbed on the surface of the adsorbent i.e. solid or liquid is known as adsorbate.
Examples of adsorption:
i) Adsorption of a gas by charcoal:
Finely divided activated charcoal has a tendency to adsorb a number of gases like ammonia, sulphur dioxide, chlorine, phosgene etc.
In this case, charcoal acts as an adsorbent while gas molecules acts as adsorbate.
For example:
Charcoal, silicagel, alumina get, clay etc; are highly good adsorbents because they have highly porous structures and hence large surface area.
ii) Adsorption of a dye by charcoal:
Animal charcoal is uses for decolorizing a number of organic substances in the form of their solutions.
iii) Adsorption of hydrogen on platinum or Nickel i.e. Hydrogenation of Oil. In this process hydrogen gas is adsorbate and Pt or Ni is adsorbent.
iv) Adsorption of acetic acid by animal charcoal
In this process acetic acid is adsorbate and the animal charcoal is adsorbent.
v) Decolonization of molasses by activated charcoal.
Absorption:
A phenomenon in which the molecules of a substance are uniformly distributed throughout the body of other substance is called absorption. It is a bulk phenomenon where as adsorption is a surface phenomenon.
e.g (i) If a chalk piece is placed into a solution of coloured ink & kept for source time, the chalk piece absorbs the coloured substance.
(ii) A sponge placed in water absorbs water into it
Difference between adsorption and absorption:
|
Adsorption |
Absorption |
|
1. It is the phenomenon of higher concentration of particles of gas or liquid on the surface than in the bulk of the solid |
1. It is the phenomenon in which the particles of gas or liquid get uniformly distributed throughout the body of the solid |
|
2. The concentration on the surface of the adsorbent is different from that in the bulk. |
2. The concentration is the same through out the material. |
|
3. Absorption occurs at uniform rate |
3. Adsorption is rapid in the beginning and its rate slowly decreases |
Types of Adsorption:
Based on the nature of the type of forces responsible for adsorption on the surface of the adsorbents, adsorption is classified into two types. i) Physical adsorption and ii) Chemical adsorptions.
i) Physical adsorption:
If the forces that are responsible for the adsorption of adsorbate molecules on the surface of the adsorbent are physical forces or van der Waal’s forces the adsorption is referred as physical adsorption or physiocorption.
e.g. (i) Adsorption of H2 or O2 on charcoal.
The attractive forces are weak and therefore, these can be easily overcome either by increasing the temperature in by decreasing the pressure. In other words physical adsorption can easily reversed is decreased.
ii) Chemical adsorption:
If the forces that are responsible for the adsorption of absorbate molecules on the surface of the adsorbebent are chemical forces, the adsorption is called chemical adsorption or chemisption.
In this type of adsorption, a chemical reaction occurs between the adsorbed molecules and the adsorbent on the surface.
This type of adsorption is reversible.
Differences between the physical and the chemical adsorption
|
SN. |
Property |
Physical adsorption |
Chemical adsorption |
|
|
1. |
Nature of adsorption |
Weak |
Strong |
|
|
2. |
Enthalpy of adsorption (in Kj mol-1) |
Low (20-40) |
High (40 – 400) |
|
|
3. |
Reversibility of adsorption process |
Reversible & occurs rapidly |
Irreversible and occurs slowly |
|
|
4. |
Temperature at which adsorption is more |
Low temperatures( below the b.pt of the adsorbate gas) |
High temperature (generally above the b.pt of the adsorbate gas) |
|
|
5. |
Effect of change in temp. |
Decrease with rise in temperature |
Increase with rise in temperature |
|
|
6. |
Specificity of adsorption |
Not specific generally takes place on all surfaces |
Highly specific. Takes place on specified surface only |
|
|
7. |
Effect of pressure on adsorption |
Increases with rise in pressure of adsorbate gas and finally attains a limiting value |
Pressure of the adsorbate as a negligible effect. |
|
|
8. |
Nature of adsorbate layers formed |
Multi layered |
Unilayered |
|
|
9. |
Energy of activation (Ea) |
In significant (i.e very low) |
Significant (i.e. relatively high) |
|
|
10. |
Dependence on the nature of the adsorbate & adsorbent |
Depends on the adsorbate (gas) only. Easily liquefiable gases are more readily adsorbed |
Depends on the adsorbate as well as adsorbent. No corelation can be given |
|
|
11. |
Forces |
The forces between the adsorbate molecules and the adsorbent are weak van der Waal’s forces |
The forces between the adsorbate molecules and the adsorbent are strong chemical forces |
|
|
12. |
Ease of desorption |
Easy since van der Waals forces are involved |
Not easy since chemical forces are involved. |
|
Adsorption of gases on solids:
The extent of adsorption of a gas on a solid surface depends on the following factors.
i) Nature of the gas (adsorbate): The adsorption depends on the nature of gas (adsorbate) adsorbed. The physical adsorption is non specific in nature and therefore, every gas gets adsorbed on the surface of any solid to a lesser or a greater extent. However, under given condition of temperature and pressure the easily liquiable gases such as etc are adsorbed to greater extent. Whereas are not readily liquefied and are adsorbed to a less extent with difficulty.
e.g. 1g of an activated charcoal adsorbs 400ml of and .
ii) Nature of adsorbent:
The extent of adsorption of a gas depends upon the nature of adsorbent. Activated charcoal can adsorb gases which are easily liquefied. Many poisonous gases are adsorbed by charcoal. Therefore it is used in gas mask for adsorbing these poisonous gases. Gases such as and are generally adsorbed on finely divided transition metals. e.g. Ni, Co. etc.
iii) Surface area of the adsorbent:
The extent of adsorption is directly proportional to the surface area or amount of gas adsorbed. Greater the surface area greater is the extent of adsorption i.e. the amount of gas adsorbed by unit. mass of the adsorbent (x/m) increases with the increase in surface area of the adsorbent. Therefore the porous and finely divided forms of adsorbent adsorb large quantity of adsorbate. However, the pores of the adsorbent should be large enough to allow the gas molecules to enter them. The solid in the finely divided state provide larger area as compared to solid lumps. The process of increasing the surface area of an adsorbent and making it a better adsorbent is called activation of the adsorbent.
iv) Pressure of the adsorbate (gas):
The extent of adsorption of a gas on a solid surface changes depending on.
i) Whether the adsorption is physiosorption or chemisorptions.
ii) The pressure of the gas at equilibrium state.
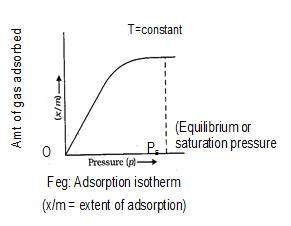
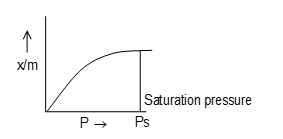
In the case of physiosorption, the gas forms a monolayer at low pressure and multiple layers at high pressure. In the process of physical adsorption it bears a simple relation to the pressure while in the case of chemisorptions this relation is complex. The relation between the amount (x) of gas adsorbed by per unit mass (m) of adsorbent (i.e., x/m) and adsorption equilibrium gas pressure (p) (or) concentration) for solutions at constant temperature is called an adsorption isotherms.
If it clear from the figure that extent of adsorption (x/m) increases with pressure and becomes maximum corresponding to pressure is called equilibrium pressure. Since, adsorption is a reversible process, the desorption also takes place simultaneously. At this pressure (Ps). The amount of gas adsorbed becomes equal to the amount of gas desorbed so that the extent of adsorption becomes constant even through the pressure is increased. This state is also called saturation state and Ps is called saturation pressure.
v) Effect of temperature:
Generally physical adsorption takes place at low temperature while chemisorptions takes place at high temperatures.
e.g. at 190C, adsorption of on iron metal is physical adsorption but at it is chemical adsorption because iron nitride is formed. So, as the temperature rises physical adsorption may change into chemical adsorption or desorption may also take place as in the case of noble gases on activated charcoal. The process of adsorption is exothermic. Hence, according to Le-chatelier principle, the extent of adsorption (x/m) decreases with rise in temperature. This is very true in case of physical adsorption. But in case of chemical adsorption the effect of temperature is complex. The extent of adsorption (x/m) increases first with increase in temperature reaches a maximum and then again decreases on further rising the temperature. The graphs showing the variation of x/m with temperature are known as adsorption isobars.
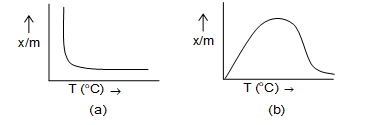
a) physical adsorption (b) chemisorptions
The adsorption isobars are used to distinguish physical adsorption from chemical adsorption as their shapes are differ.
Adsorption isotherms: The scientists have tried to explain the adsorption in terms of some empirical mathematically relations called adsorption isotherms. The most common types are given below:
1. Freundlich Adsorption isotherm:
In the case of adsorption of gases on solids, the relation between x/m and the pressure p of the gas at constant temperature is given by the equation.
where p = pressure
k, n = constant
This equation is called Freundlich adsorption isotherm.
The equation can also be written in logarithmic form as :
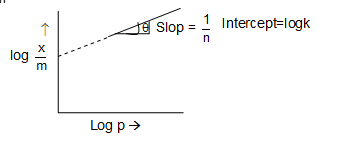
In case of solution, pressure (p) is taken as concentration c.
Draw back: One of the draw backs of Frendlich adsorption isotherms is that it fails at high pressure of the gas.
Langmuir adsorption isotherm:
Irving Langmuir in 1916 derived a simple adsorption isotherm on theoretical considerations based on kinetic theory of gases.
This is named as langmuir adsorption isotherm. Mathematically,
Assumption of Langmuir adsorption isotherm:
i) The solid surface is homogeneous and has a fixed number of adsorption sites.
II) Each adsorption site can adsorb one gas molecule.
In other words, the adsorption of molecules is limited to a mono molecular layer.
iii) Each adsorption site is equivalent and the ability of the gas molecule to bound there is independent of weather the neighbouring sites are occupied or not.
iv) The adsorbed layer is uniform all over the adsorbent.
v) The adsorbed gas behaves ideally in the vapour phase.
vi) At low temperature, physically adsorbed molecules are mainly localized and at high temperature chemically adsorbed molecules are localized.
Adsorption from solutions:
Freundlich adsorption and Langmuir adsorption isotherms have been found to be applicable in the adsorptions from solutions.
(Freundlich adsorption isotherm)
where, c = equilibrium concentration of the solution when adsorption equilibrium is established.
(Langmuir adsorption isotherm)
Application of Adsorption:
i) In gas masks
ii) In dyeing of cloths – mordants such as alums are used in dyeing of cloth they adsorb the dye particles which other wise, do not stick to the cloth.
iii) In dehumidizers : silicagel is commonly used to adsorb humidity or moisture from air this is necessary for storage of delicate instruments which might otherwise be damaged by moisture.
iv) Removal of colourouring matter.
v) Hetero geneous catalysis
vi) In ion exchange resins
Illustration 1: Which of the following is not correct statement?
(A) the extent of adsorption has no upper limit
(B) the extent of adsorption depends on the pressure of the gas
(C) the extent of adsorption depends on the temperature
(D) the extent of adsorption depends on the nature of adsorbent and adsorbate
Solution: (A) The extent of adsorption has upper limit.
Illustration 2: Which of the following is not applicable to chemisorption?
(A) Effect of pressure is given by Freundlich adsorption isotherm
(B) There is formation of monomolecular layer
(C) It occurs at high temperature
(D) It involves the formation of chemical bonds between adsorbent and adsorbate
Solution: (A) In chemisorption effect of pressure is not given by Freundlich adsorption isotherm.
Illustration 3: Which one of the following is a property of physisorption?
(A) Non-specific nature
(B) High specificity
(C) Irreversibility
(D) none of the above
Solution: (A) Physisorption is non-specific nature.
Illustration 4: Adsorption of gases on solid surface is generally exothermic because
(A) enthalpy is positive
(B) entropy decreases
(C) entropy increases
(D) free energy increases
Solution: (B) Adsorption of gases on solid surface is generally exothermic because entropy decreases i.e., Ds for the process is negative. As ΔG = ΔH –
or
Illustration 5: For adsorption of a gas on a solid, the plot of log x/m vs log P is linear with slope equal to : (n being whole number)
(A) k (B) log k (C) n (D) 1/n
Solution: (D) For adsorption of a gas on a solid, the plot of log x/m vs log P is linear with slope equal to 1/n.
Colloidal state:
Thomson Graham studied the diffusive property of many chemical substances through permeable membranes.
From the experiments he observed that some substances like salts, acids urea & bases, diffused through the membranes while the substance like glue, gum arabic, agar, gelatin etc did not diffuse through the membrane.
On the basis of this property (difference) he classified the chemical substances into two categories as
(i) Crystalloid and (ii) Colloids.
Cryptalloids:
The substances that diffused through the permeable membrane are called crystalloids. e.g., salts, acids, bases & urea etc.
Colloids:
Those substances that do not diffuse through the permeable membrane are called colloids. Colloid means – glue like (collos = glue, oil = like). e.g., Glue, gumarabic agar, gelatin etc. Now a days the term colloid has replaced by the word colloidal state or colloidal solution. These terms are not applicable to a single substance but only to a binary heterogeneous system of two substances.
The colloidal system contains two phases i.e. In colloidal chemistry solute is known as dispersed phase and that of solvent is dispersion medium.
Types of solution:
(i) True solution (Binary):
True solutions are binary solutions (system) in which particle size of the solute is 1 mμ o less than 1 mμ. The true solution is a homogeneous binary system.
e.g.,.aqueous solution common salt, sugar, acid, base etc.
(ii) Suspension:
It is a heterogeneous mixture which contains small insoluble particles. The particle size is more than 100 nm. e.g. Dirt particles in water. The particles of a suspension can neither pass through an ordinary filter paper nor through animal membrane.
(iii) Colloidal solution:
Colloidal solution is a heterogeneous binary system in which the particle size of the dispersed phases (solutes) ranges from 1 m μ to 1μ. e.g., starch paste, gelatin or glue when added to hot water and the mixture is shaken well, colloidal solutions of these substances are formed.
Thus colloidal solutions are intermediate between true solutions & suspensions.
Thus, a colloidal solution is a binary heterogeneous system and it contains two phases:
i) Dispersed phase:
ii) Dispersion medium:
Table: The important distinguishing feature of the three types of solutions.
|
Property |
True solution |
Colloidal solution |
Suspension |
|
1. Nature |
Homogeneous |
Heterogeneous |
Heterogeneous |
|
2. Particle size |
< 1 n m |
1 m μ to 1 μ |
> 100 nm |
|
3. Appearance |
Transparent (very clear) |
Generally transparent |
Opaque |
|
4. Separation |
|
|
|
|
5. Settling of particles |
Not possible |
Possible by centrifugation |
Possible under gravity |
|
6. Diffusions of particles |
Fast |
Slow |
Does not diffuse |
|
7. Brownian movement |
Negligible |
Show |
May show |
|
8. Tyndall effect |
Does not show |
Show |
Shows |
Classification of colloidal solutions (Sols):
(A) Based on affinity with solvents:
(i) Lyophilic sols : (Lyo = solvent, philic = loving) the colloidal solutions in which the particles of the dispersed phase have a great affinity (or cove) for the dispersion medium are called lyophilic colloids or sols. These solutions are called solvent loving. e.g., the common examples of lyophilic colloids are gum, gelation, starch, proteins, rubber etc.
In general, all high molecular weight carbon compounds in waterform, lyophilic sols.
These sols are quite stable and can not be easily coagulated. Hence these are reversible in nature.
In case, water acts as the dispersion medium, the lypophilic colloid, is called hydro philic sol.
(ii) Lyophobic sols: (Lyo = solvent, phobic = hating) the colloidal solutions in which there is no affinity between particles of the dispersed phase and dispersion medium are called lyophobic colloids or sols. These solutions are solvent hating. Low molecular weight inorganic substances (metals, salts, hydroxides etc). from lyophobic sols.
These are generally not very stable and readily coagulated or precipitated on the addition of small amount of electrolytes, by heating or by shaking.
These are irreversible in nature. e.g the colloidal sols of metals like Ag and Au, hydroxide like, metal sulphides like etc., are the common examples of lyophobic sols.
In case, the dispersion medium is water, the lyophobic sol is called hydrophobic sol.
Table – Differences between lyophilic and lyphobic sols
|
Property |
Lyophilic Colloids |
Lyophobic Colloids |
|
1. Ease of preparation |
Easily formed by direct mixing |
Formed only by special methods. |
|
2. Particle nature |
The particles are true molecules and are big in size |
The particles are aggregates of many molecules. |
|
3. Visibility |
The particles are not easily visible even under ultra microscope |
The particles are easily detected under ultra micro scope. |
|
4. Stability |
These are very stable |
These are unstable |
|
5. Reversible or Irreversible |
These are reversible in nature |
These are irreversible in nature. |
|
6. Action of electrolytes : |
These are not easily precipilated by small amount of electrolytes. Very large quantity of electrolyte is required to cause coagulation. |
These are readily precipited by the addition of small amount of suitable electrolyte |
|
7. Viscosity |
The viscosity of the sols is much higher than that of the dispersion medium |
The viscosity is nearly the same as that of the disperses medium |
|
8. Surface tension : |
Usually lower than that of the dispersion medium |
Almost the same as that of the dispersion medium. |
|
9. Tyndall effect : |
Do not show |
Shows. |
|
10. Charge or particles : |
The particles do not carry any charge the particles may migrate in any direction or even not under the influence of an electric field. |
The particles move in a specific direction i.e. either towards anode or cathode depending upon their charges |
Illustration 6: Which of the following is correct for lyophillic sol?
(A) irreversible sol
(B) formed from inorganic substances
(C) readily coagulated by addition of electrolyte
(D) self stabilized
Solution: (D) Lyophilic sols are self stabilized and they are not coagulated by addition of electrolytes.
Illustration 7: Surface tension of lyophillic sols is
(A) lower than that of H2O
(B) more than that of H2O
(C) equal to that of H2O
(D) none of these
Solution: (A) Surface tension of lyophillic sols is lower than that of H2O (dispersion medium).
Illustration 8: The stability of lyophillic colloid is due to which of the following?
(A) Charge on their particles
(B) Large size of their particles
(C) Small size of their particles
(D) A layer of dispersion medium
Solution: (D) The stability of lyophilic colloid is due to a layer of dispersion medium..
(B) Based on types of particles of dispersed phase: Colloids are of three types: (i) Multi molecular colloids (ii) Macro molecular colloids and (iii) Associated colloids
(i) Multi molecular colloids:
When, on dissolution, atoms or smaller molecules of the substances having diameter < 1 nm, aggregate together to form particles of colloidal dimensionals, the particles thus formed are called multimolecular colloids (sols).
Therefore, in these sols the dispersed phase (solute) consist of aggregates of atoms or molecules with molecular size less than 1nm.
e.g.,. sol of gold atoms and sol of sulphur (S8) molecules. In these sols, the particles are held together by van der Waal’s forces.
ii) Macro molecular colloids:
The substances having big sized molecules. (i.e. macro molecules) which on dilution form solution in which the dispersed phase particles have size in the colloidal range are called macro molecular. These macromolecules forming the dispersed phase are generally polymers having very high molecular masses. Naturally occurring macro- molecules are starch, cellulose, proteins, enzymes, gelatin etc. Artificial macromolecules are synthetic polymers such as, nylon, polythene, plastics, polystyrene etc.
Since these macro molecules have large sizes comparable to those of colloidal particles, the solution of such molecules are called macro molecular colloidal solutions. Their solutions are quite stable and resemble true solution in many respects. Thus, the common examples of macromolecular colloids are starch, cellulose, proteins, plastics etc.
iii) Associated colloids:
There are certain substances which behave as normal, strong electrolytes at low concentration but at higher concentration they behave like a colloidal solutions due to the formation of aggregated particles. The aggregated particles thus formed are called micelles or associated colloids or sols.
Soaps and detergent are common examples of associated colloids & sols.
When sodium stearate (soap) is dissolved in water it gives .
The stearate ions associate to form ionic micelles of colloidal size and they have long hydrocarbon part (lyophobic end) and small –COO- part (lyophilic).
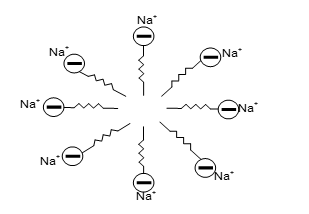
Aggregation (cluster) of several ions to form ionic micelles.
The formation of micelles takes place at particular temperature called kraft temperature and above a certain concentration called critical micellization concentration (CMC). e.g.
CMC for soap is about . When the concentration of the soap solution is below its CMC it behaves as normal electrolyte while above this concentration, it is aggregated to behave as micelles.
Some other examples of micelles are:
i) Sodium palmitate:
ii) Sodium lauryl sulphate:
iii) Cetyl tri methyl ammonium bromide:
Difference between multi molecular, macromolecular and associated colloids.
|
Multimolecular colloids |
Macromolecular colloids |
Associated Colloids |
|
i) They consist of aggregates of atoms or molecules which generally have diameter less than 1nm |
(i) They consist of large molecules (generally polymers) |
(i) Behave as colloidal size particles at higher concentrations |
|
ii) They have usually a lyophilic character |
(ii) They have usually lyophobic character |
(ii) Their molecules contain both lyophilic and lyophobic group. |
|
iii) The atoms & molecules are held by weak van der Waal’s forces. |
(iii) The molecules are flexible and can take any shape |
(iii) They behaves as normal electrolyte at low concentration and behave as colloidal sol only at high concentrations. |
General Methods of preparation of sols:
1) Preparation of Lyophilic sols:
These colloidal sols are readily formed simply mixing the dispersed phase and dispersion medium under ordinary conditions. e.g., The substances like gelatin, gum, starch, egg albumin etc., pass readily into water to give colloidal solution.
They are reversible in nature because these can be precipitated and directly converted into colloidal state.
2) Preparation of Lyophobic sols:
Lyophobic sols can be prepared by mainly two types of methods.
(A) Condensation methods (B) Dispersion methods
(A) Condensation methods:
Under condensation methods following methods are employed.
1) Physical methods:
i) By exchange of solvent:
Colloidal sols of certain substances such as sulphur, phosphorus which are soluble in alcohol but insoluble in water can be prepared by pouring their alcoholic solutions in excess of water.
ii) By excessive cooling:
A colloidal solution of ice in an organic solvent like chloroform of ether can be prepared by freezing a solution of water in the solvent. The molecules of water which can no longer be held in solution, separately combine to form particles of colloidal size.
2) Chemical methods:
The following chemical methods may be used to prepare lyophobic colloidal sols.
i) Oxidation:
A colloidal sol of sulphur is obtained by bubbling gas through the solution of bromine water, etc.
ii) Reduction:
The colloidal sols of metals are obtained by reduction of their compounds.
the gold sol, thus prepared, has a purple colour and is called purple of cassius.
iii) Hydrolysis:
A colloidal solution of ferric hydroxide is prepared when a concentrated solution of ferric chloride is added (drop wise) to hot water.
iv) Double decomposition:
sol is obtained by passing through dilute solution of arsenious oxide in water.
(B) Dispersion methods: Under dispersion method. The following methods are employed.
1) Bredig’s arc method:
This method is used to prepare sols of metals such as platinum, silver, copper or gold. The metal whose sol is to be prepared is made as two electrodes immersed in dispersion medium such as water
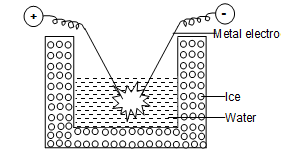
The dispersion medium is kept cooled by surrounding it with freezing mixture.
An electric arc is struck between the electrodes. The tremendous amount of heat is generated by the arc vaporizes the metals which are condensed immediately in the liquid to give colloidal solution.
2) Peptisation:
The process of converting a freshly prepared precipitate into colloidal form by the addition of a suitable electrolyte is called peptisation. The electrolytes used for the purpose are called peptizing agents.
Cause of Peptization:
When an electrolyte is added to a freshly prepared precipitate, the suitable ions from the added electrolyte are adsorbed by the particles of the precipitate.
The charged particles repel one another and form colloidal solution.
e.g., On treating a precipitate of iron (iii) oxide with a small amount of solution, a reddish brown coloured colloidal sol is obtained.
Similarly, a precipitate of silver chloride can be peptised by shaking it with a dilute solution of silver nitrate to give a colloidal solution of silver chloride.
Illustration 9: Peptisation denotes
(A) digestion of food
(B) hydrolysis of proteins
(C) breaking and dispersion into colloidal state
(D) precipitation of a solid from colloidate state
Solution: (C) Peptisation denotes breaking and dispersion into colloidal state.
Purification of colloidal solution (sols):
The colloidal solutions prepared by the above methods usually contain impurities especially electrolytes which can destabilize the sols. These impurities must be eliminated to make the colloidal solutions stable. The following methods are commonly employed for the purification of colloidal sols.
1) (a) Dia lysis:
The process of separating the particles of colloids from those of crystalloids by means of diffusion through a suitable membrane is called dialysis. It’s principle is based upon the fact that colloidal particles can not pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it. For the purpose the colloidal sol is filled in a bag made up of cellophane or parchment paper. Now the bag is suspended in a freshwater container. The impurities slowly diffuse out of the bag leaving behind pure colloidal solution. The water is changed frequently to avoid accumulation of the crystalloids other wise they may start diffusing back into the bag. Thus, dialysis can be used for removing HCl from the ferric hydroxide sol.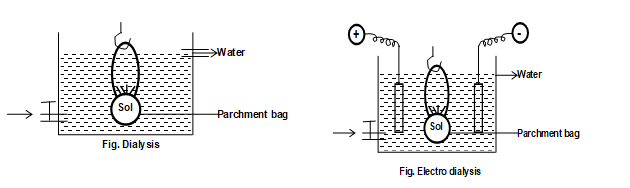
(b) Electro dialysis:
The ordinary process of dialysis is slow. To increase the process of purification the dialysis is carried out by applying electric field. This process is called electrodialysis.
Note: Kidneys in human body act as dialysers to purify blood which is of colloidal nature. The most important application of dialysis process is the artificial kidney machine used for the purification of blood of the patients whose kidneys have failed to work.
ii) Ultra filtration:
In this method, colloidal solutions are purified by carrying out filtration through special type of graded filters called ultra filters.
These filter papers allow the electrolytes to pass through them but do not allow colloidal particles.
These filters are made from ordinary filter papers by impregnating them with colloidal particles.
As result, the size of the pores gets reduced.
Ordinary filter papers cannot be used for the purpose since the colloidal particles can easily pass through the proses of these papers.
iii) Ultra centrifugation:
In this method, the colloidal sol is taken in a tube which is placed in a high speed centrifugal machine.
On centrifusing, the colloidal particles settle down. The impurities remain down in the sol is called centrifugate. The settled colloidal particles are mixed with water to form the colloidal solution again.
Properties of colloidal sols: The main characteristic properties of colloidal sols are given below:
1) Physical properties:
i) Heterogeneous character: The colloidal sols are heterogeneous in nature consisting of two phases:
(a) dispersed phase and (b) dispersion medium.
ii) Stable nature:
The colloidal solution are quite stable. Their particles are in a state of motion and do not settle down at the bottom of the container. However particles of certain colloidal sols, which have comparatively large size may settle down but very slowly.
iii) Filtrability:
The size of colloidal particles is less than the pores of the filter paper and therefore, they easily pass through a filter paper. Colloidal particles however can’t pass through the parchment paper of an animal membrane or ultra filter paper. This forms the basis of separation of colloidal particles from those of crystalloids.
2) Mechanical properties:
i) Brownian movement:
Robert Brown, a botanist discovered in 1827 that the pollen grains placed (suspended) in water do not remain at rest but move about continuously and randomly in all directions.
Later on it was observed that the colloidal particles also are moving at random in zig-zag motion. This zig-zag motion of colloidal particles is called Brownian movement after the name of its discoverer, Robert Brown.
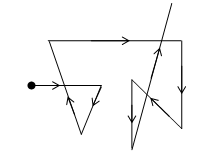
Brownian movement of colloidal particles
Brownian movement arises because of the impact of the molecules of the dispersion medium with colloidal particles (stated by Wiener). It has been postulated that the impact of the particles of the dispersion medium on the colloidal particle are unequal which ultimately causes its Zig-Zag motion.
Cause of Brownian movement:
However, as the size of the particle increases, the probability of unequal impacts decreases and the Brownian movement becomes slow. Ultimately, when the dispersed particle becomes bigger enough to acquire the dimensions of suspension, no Brownian movement is observed.
Significance of Brownian movement:
i) Ceaseless motion: Brownian movement provides a direct demonstration of ceaseless motion of molecules as postulated by kinetic theory.
It counter the force of gravity acting on colloidal particles and hence help in providing stability to colloid.
ii) Diffusion: The sol particles diffuse from a region of higher concentration to lower concentration region. However, due to bigger size, they diffuse at a lesser speed.
iii) Sedimentation: The colloidal particles settle down under the influence of gravity at a very slow rate. This phenomenon is called sedimentation and is used to determine the molecular mass of macromolecules. The rate of sedimentation can be accelerated by the use of a high speed centrifuge called ultra centrifuge.
3) Optical properties (Visibility & Tyndall effect):
When a strong beam of light is passed through a true solution placed in a beaker, in a dark room the path of light does not become visible. However, if the light is passed through a sol, placed in the same room, the path of the light becomes visible ( i.e. get illuminated), which is seen under by bluish light ultra microscope placed at right angle to the beam
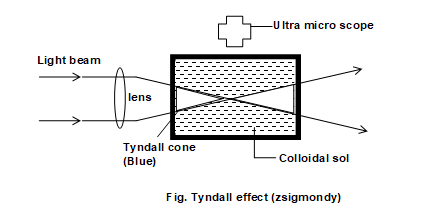
This phenomenon was studied for the first time by Tyndall and therefore, it called Tyndall effect and the path of illuminated beam is called Tyndale cone.
The cause of tyndall effect is the scattering of light by the colloidal particles i.e. these particles first absorb the incident light and then a part of this light gets scattered by them.
Since, the intensity of the scattered light is at right angled to the plane of the scattered light is at right angles to the plane of the incident light the path becomes visible only when seen in that direction.
Note that the same phenomenon is noticed due to scattering of light by dust particles when a beam of sunlight enters a dark room through a slit. The true solutions do not exhibit tyndall effect because the particles in them are two small in size and hence donot cause any scattering.
Illustration 10: Scattering of light takes place in
(A) electrolysis
(B) colloidal solutions
(C) electroplating
(D) solutions of electrolytes
Solution: (B) Scattering of light takes place in case of colloidal solutions.
4) Electrical properties:
i) Electrophoresis:
The particles of the colloidal solution are electrically charged and carry same type of charge; either positive or negative.
The dispersion medium has an equal and opposite charge making the system neutral as a whole.
Due to similar nature of the charge carried by the particles, they repel each other and do not combine to form bigger particles. That is why a sol, is stable and particles do not settle down.
Arsenious sulphide, gold, silver and platinum particles in their respective colloidal sols are negatively charged while particles of ferric hydroxide, aluminium hydroxide are positively charged.
The presence of the charge on the sol particles and its nature whether positive or negative can be determined with the help of a phenomenon known as electrophoreses.
The movement of movement of colloidal particles under an applied electric field is called electrophosesis. If the particles accumulate near the negative electrode, the charge on the particles is positive on the other hand, if the sol particles accumulate near the positive electrode, the charge on the particle is negative.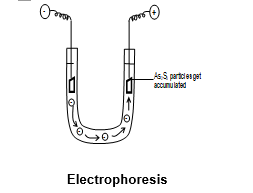
It has been observed that is a negatively charged sol. Under the influence of electrical field particles have accumulated near the positive electrode in the U-tube (fitted with Pt- electrodes) Similarly, when an electric current is passed through positively charged sol, it is observed that they move towards negatively charged electrode and get accumulated there.
Thus, by observing the direction of movement of the colloidal particles, the sign of the charge carried by the particles can be determined.
ii) Electro osmosis:
When the movement of the colloidal particles is prevented by some suitable means and the molecules of the dispersion medium are allowed to move under the influence of applied field, the phenomenon is called electro-osmosis. Thus electro-osmosis is the phenomenon of the movement of the molecules of the dispersion medium under the influence of electric field whereas colloidal particles are not allowed to move.
Some common positively and negatively charged colloidal sols:
|
Positively charged sols |
Negatively charged sols |
|
i) The hydroxides like |
i) the sulphides like etc. |
|
ii) The oxide : TiO2 |
ii) Metals like Cu, Ag, Au and Pt. |
|
iii) Basic dyes like methylene blue, haemoglobin etc. |
iii) Acid dyes like eosin, congo red etc. |
|
iv) Protein in acidic medium |
iv) Sols of starch, gum, gelatin, clay, charcoal etc. |
5. Coagulation of colloidal sols:
Presence of small concentrations of appropriate electrolytes is necessary to stabilize the colloidal solutions. However, if the electrolytes are present in higher, concentration, then the ions of the electrolyte neutralize the charge on the colloidal particles.
Now these colloidal particles may unite together to form bigger particles which are then precipitated.
Thus, the phenomenon of precipitation of a colloidal solution by the addition of excess of an electrolyte is called coagulation or flocculation.
Hardy Schulze rule:
The coagulation capacity of different electrolytes is different. It depends upon the valency of the active ion or flocculating ion, which is the ion carrying charge opposite to the charge on the colloidal particles.
According to this rule, greater the valency of the active ion or flocculating ion, greater will be its coagulating power.
The main points of Hardy schulze rule are:
i) The ions carrying the charge opposite to that of sol particles are effective in causing coagulation of the sol.
ii) coagulation power of an electrolyte is directly proportional to the valency of active ion i.e. ions causing coagulation.
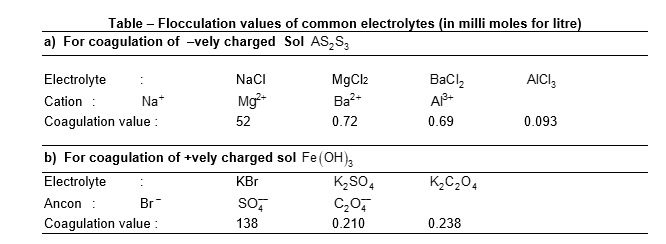
e.g. to coagulative negative sol. of , the coagulating power of different cations has been found to decrease in the order as .
Similarly, to coagulate a positive sol. such as the coagulating power of different anions has been found to decrease in the order as .
Coagulation value or flocculation value:
The minimum concentration of an electrolyte which is required to cause the coagulation or flocculation of a sol is known as coagulation value or flocculation value. It is usually expressed in milli moles per litre. Smaller the coagulation value of the electrolyte larger is its coagulating or precipitation power. i.e.
eg has 548 times more coagulation power than NaCl, in case of sol.
6) Protection of colloids:
Lyophobic sols can be easily precipitated by the addition of a small amount of electrolytes.
They can be prevented from coagulation by the previous addition of some stable lyophilic colloids like gelatin, albumin, etc. Thus, the process of protecting the lyophobic colloidal sols from precipitation by the electrolytes due to the previous addition of some lyophilic colloid is called, protection. The lyophilic colloid which is added to prevent coagulation of the colloidal sol is called protecting colloid. e.g. If a small amount of gelatin is added to gold sol, it is not readily precipitated by the addition of NaCl. Here, gelatin acts as protecting colloid.
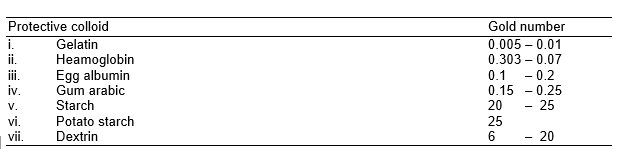
The protecting power of different lyophilic colloids (protective colloids) is expressed in terms of Gold number.
Gold number:
Zsigmondy introduced a term called gold number to describe the protective power of different colloids. This is defined as the minimum amount of the protective colloid in milligrams required to first prevent the coagulation of a/o mL of a given gold sol when 1 ml of a 10% solution of NaCl is added to it. The coagulation of gold sol is indicated by change in colour from red to blue. The gold numbers of few protective colloids are given below:
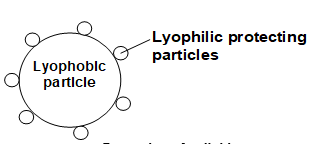
Protection of colloid
It may be noted that smaller the value of the gold number, greater will be protecting power of the protective colloid. Therefore, reciprocal of gold number is a measure of the protective power of a colloid. Thus, gelatin is the best protective colloid out of the list given in to table.
Congo Rubin number: Ostwald proposed that it is the amount of protective colloid in mg which prevents the colour change is 100ml of 0.01 % congo rubin dye solution to which 0.16g equivalent of KCl are added when observed after 10-15 minute.
Examples of some colloidal sols:
1) Smoke: This is an aerosol because carbon particles disperse in air and form colloidal sol.
Therefore smoke is solid in gas (air) sol or aerosol.
2) Cloud: This is an aerosol because water (drops) disperse in air and forms the colloidal solutions
Therefore, cloud is liquid in gas (air) sol or aerosol.
3) Milk: This is liquid in liquid type of colloid because liquid fat is dispersed in water.
This type of liquid in liquid colloid is called emulsion.
4) Blood: This is a sol in which albuminoid substances are dispersed in water. Therefore this is a solid in liquid sol.
5) Starch solution: This is an aqua sol or hydro sol. It is a colloidal dispersion of starch particles (solid) in water (liquid).
Note:
a) Liquid dispersed in liquid : It is called emulsion
Solid dispersed in gas : It is called an aerosol
Liquid dispersed in solid : It is called gel
b) Dispersion medium : Name of the colloidal sol
Water : Hydrosol
Alcohol : Alcosol
Benzene : Benzosol
Air : Aerosol
Illustration 11: Milk can be preserved by adding a few drops of
(A) formic acid solution
(B) formaldehyde solution
(C) acidic acid solution
(D) acetaldehyde solution
Solution: (B) Formaldehyde kills the micro-organism which spoils the milk by producing lactic acid which coagulate the milk.
Emulsion: Emulsion is a colloidal system in which both the dispersed phase and the dispersion medium are liquid:
e.g. milk is a natural emulsion in which the liquid fat (granules) is dispersed in water. The size of the fat particles is larger than the size of the colloidal particles of sols normally found.
Classification of Emulsion:
Emulsions are classified into two classes depending on the fact which is dispersed phase and which is dispersion medium.
i) Oil in water emulsion:
In this case, the dispersed phase is oil (immiscible liquid) and the dispersion medium is water.
e.g., milk and Vanishing cream.
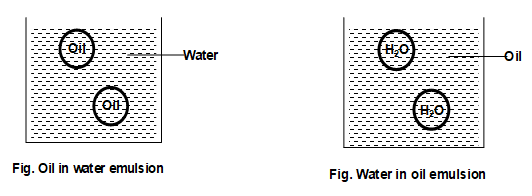
ii) Water in oil emulsion:
In this case, the dispersed phase is water and the dispersion medium is oil (immiscible liquid).
e.g. stiff greases, cod liver oil cold cream etc.
Emulsifying agents:
Generally, emulsions are not stable. If the emulsion is kept for some time, the droplets of the dispersed phase (oil or water) get aggregated and separate out as a separate layer. The two components (oil and water) of the emulsion are separated into two immiscible liquids. So another substance (third substance) is added to the emulsion to keep the emulsion stable. This substance is known as emulsifier or emulsifying agent. Thus, the third substance added in small amounts to an emulsion to keep the emulsion stable is emulsifier or emulsifying agent. e.g., soap, egg albumin, solid HgI2, graphite powder, gelatin, etc are common examples of emulsifying agents.
Application of Emulsion:
i) In washing processes of clothes & crockery: Cleansing or washing action of soap is due to the emulsification of grease.
Mechanism of cleansing action:
Soap anions form spontaneously the micelle. The hydrocarbon chains (tails) are in the interior of the miscelle and or ions are on the surface the grease or dirt is absorbed into the interior of the miscelle. The dirt on the cloth is thus removed into the miscelle.
Note: It may be noted that the two types of emulsions can be interconverted by simply changing the ratio of the dispersed phase and dispersion medium.
ii) In the digestion of fats in intestine. The fat in the intestine forms soap with the alkali of the intestine.
This converts the remaining fat into emulsion and stabilizes it.
iii) In the preparation of lotions, creams and ointments in pharmaceuticals and cosmetics.
iv) In the preparation of oily type of drugs for easy adsorption into the body.
v) To break oil and water emulsions in oil wells.
vi) In the extraction of metals, the concentration of ores is carried out through emulsification process (froth floatation)
vii) Milk, which is an important constituent of our diet is an emulsion of fat in water.
Identification of emulsions:
i) Dilution test:
Add water to the emulsion. If the emulsion can be diluted with water, this means that water acts as the dispersion medium and it is an example of oil in water emulsion.
In case, it is not diluted, then oil acts as dispersion medium and it is an example of water in oil emulsion.
ii) Dye test: An oil suitable dye is shakes with the emulsion. If colour is noticed on looking at a drop of the emulsion it is oil in water type emulsion.
In case the entire background is coloured, it is an example of water in oil type emulsion.
Gels:
A gel is a colloidal system in which a liquid is dispersed in a solid. Under certain conditions, the lyophilic sol may be coagulated to give a semi solid jelly like mass. Which encloses all the liquid present in the sol. The process of gel formation is called gelation and the colloidal system formed is called gel. e.g. gum arabic, gelatin, processed cheese, silicic acid, etc.
Classification of Gel: Gels may be classified into two types.
i) Elastic gels: They possess property of elasticity. They change into solid mass on dehydration which can again be converted into gel by addition of water followed by heating and cooling. They show the phenomenon of imbibition. e.g. – gelatin, agar, starch etc.
ii) Non- elastic gels: They do not possess property of elasticity. They change into solid mass on dehydration which becomes rigid and can’t be converted into original by heating with water. They do not show the phenomenon of imbibition. g.. silica gel
Note: Gel may shrink on keeping by loosing some of liquid held by them. This is known as syneresis or weeping of gel.
Surfactants:
Surfactants are those substances which are preferentially adsorbed at the interface like air-water, oil-water, solid-water interface. Thus, the substance which is responsible for micellisation and emulsification is called surfactant. Surfactants are divided into three categories.
1) Cationic sulfactants:
Such substances on ionization gives a cation having hydrophobic and hydrophilic group.
e.g., i) cetyl pyridium chloride
ii) Cetyl trimethyl ammonium Chloride
iii) Octadecyl ammonium chloride –
2) Anionic surfactant: Such substances give anion which act as surfactant. e.g.
i) Soium Palmitate –
ii) Sodium eleate-
iii) Salt of Sulphonic acid having molecular formula – etc.
3) Non – ionogenic surfactants: These surfactants do not ionize or dissociate in aqueous medium but these molecules also have hydrophobic and hydrophilic ends.
High molecular mass alcohol adds to several molecules of ethylene oxide to form hydroxyl surfactant
Illustration 12: Which one of the following is not a surfactant?
(A)
(B)
(C)
(D)
Solution: (B) , being a primary amine is not a surfactant.
Isoelectric point of colloids: The concentration at which the colloidal particles are neither positively charged nor negatively charged. (i.e. are electrically neutral) is known as isoelectric point of a colloid. e.g. For gelatin, isoelectric point is obtained at pH = 4.7. At this point the colloids do not migrate under the influence of electric field. At this point the lyophilic colloids are expected to have minimum stability
Application of colloids:
(1) Technical applications:
i) Sewage disposal
ii) Smoke precipitator
iii) Rubber industry
iv) Artificial rain
v) Photographic plates.
vi) Chemical warfare
vii) Tanning
viii) Colloidal medicine
ix) Purification of drinking water.
x) As disinfectants – the disinfectants such as dettol and Lysol give emulsions of the oil-in-water, when mixed with water.
xi) Metallurgical operations.
xii) Paints, inks, plastics, lubricant, cement etc.
Other applications (Natural applications)
i) Blue colour of sky
ii) Fog, mist and rain
iii) Food articles – milk, butter, ice cream, fruit juice etc.
iv) Blood-it is a colloidal solution of albuminoid substances. The syptic action of alum and ferric chloride solution is due to coagulation of blood forming a clot which stops further bleeding.
v) Soil – fertile soils are colloidal in nature in which humes acts as a protective colloid. On account of colloidal nature, soil absorbs moisture and nourishing materials.
vi) Formation of delta.
Catalyst and Catalysis: (Cata-Wholly, Lysis – to lose): A substance which alters the rate of a chemical reaction to which it is added without itself being consumed is called a catalyst, and this process is called catalysis. e.g., Decomposition of
Here, is used as catalyst and this process is called catalysis.
The word catalyst was introduced by Berzelius in 1836.
Characteristics of a catalyst:
i) A catalyst does not initiate a reaction. It only alters the rate of reaction.
ii) A catalyst is not consumed in the reaction and is chemically unchanged permanently.
iii) Small amount of catalyst is generally enough to charge the speed of a reaction.
iv) A catalyst functions under optimum conditions of temperature, pressure and pH etc.
v) A catalyst does not shift the position of equilibrium in reversible chemical reactions. But it helps to attain the equilibrium quickly.
vi) Catalysts are highly specific in nature.
vi) Catalyst provides a new path way of lower activation energy (Ea) (Rate of reaction ∝ 1/Ea .
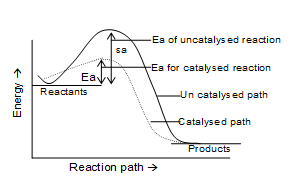
Comparision of activation energies of a catalysed and uncatalysed reaction
viii) Catalyst does not change ΔE of the reaction.
. (Remains same)
ix) Finely divided substances function as effective catalyst compared to coarsely divided substances.
x) Some times changed products are obtained if the catalyst is changed.
xi) Change of temperature may increase in decrease the catalytic activity of a substance.
e.g., The activity of metals and some metal oxides act as catalysts increases with increase of temperature. But some catalyst such as bio logical catalysts (enzymes) lose their activity at higher temperature(s).
xii) Some substances are added to reduce the rate of reaction instead of increasing the rate of reaction, are called negative catalysts.
xiii) The activity of a catalyst increases in some cases in presence of another substance. This another substance is called catalyst promoter.
xiv) A catalyst may lose its activity power if some other harmful substances are present in the reaction even in traces. This is called catalytic poisoning and the harmful substances are called catalytic poisons.
Classification of Catalysis:
Catalysis is classified into two types based on the physical states of the catalyst and states (phases) of the reactants.
1. Homogeneous catalysis:
The phenomenon of catalysis in which the catalyst and the reactants are present in same phase is known as homogeneous catalysis.
In other words, the catalysis pertaining to the catalytic reactions in which the catalyst is present in the same phase or state as that of the reacting substances is called homogeneous catalysis.
e.g. i) Gas phase reactions:
a) The catalytic oxidation of in presence of oxides of nitrogen NO an .
b) Oxidation of CO into in presence of NO(g) as catalyst.
ii) Liquid phase reaction:
a) Hydrolysis of ester in presence of mineral acid to give ethyl alcohol and acetic acid,
b) Hydrolysis of sucrose solution (can sugar) into glucose and fructose in presence of mineral acid (Inversion of sucrose)
Mechanism of homogeneous Catalysis:
i) Reaction:
Mechanism:
ii) Reaction
Mechanism:
2) Heteo geneous catalysis:
The phenomenon of catalysis in which the catalyst is present in a phase or state different from the phases or states of the reactants. In other words, catalysis pertaining to the catalytic reactions in which the catalyst is present in a phase or state different from that of the reactants. In heterogeneous catalysis, catalyst is generally a solid and the reactants are generally gases. Sometimes, liquid reactants are also used. This is also known as surface catalysis because the reaction starts at the surface of the solid catalyst. e.g.,
i) Gas phase reactions:
a) Hydrogenation of ethene in presence of Ni as catalyst.
b) Combination of hydrogen and oxygen in presence of finely divided Pt as catalyst :
c) Manufacture of from and in contact process using platinised asbestos or as catalyst.
d) Synthesis of by Haber’s process in presence of Fe(s) as catalyst.
ii) Liquid phase reaction:
a) Decomposition of in presence of or Pt(s).
Mechanism of heterogeneous catalysis:
The heterogeneous catalysis is a surface phenomenon.
It involves the following steps.
i) Diffusion of the reactants at the surface of the catalyst.
ii) Adsorption of the molecules of the reactant at the active sites.
iii) Occurrence of the chemical reactions on the surface of the catalyst.
iv) Desorption of product molecules from the surface
v) Diffusion of the products away from the surface of catalyst.
The role of heterogeneous catalyst can be explained in terms of adsorption of reactant on the surface of the catalyst. The adsorption helps the reaction in the following ways.
i) Adsorption increases the concentration of reactants on the surface of the catalyst. Due to increase in concentration of the reactants, the reactions proceed rapidly.
ii) Adsorbed molecules get dissociated to form active species like free radicals which reacts faster than molecules.
iii) The adsorbed molecules are not free to move about and therefore they colloid with other molecules on the surface.
iv) The heat of adsorption evolved acts as energy of activation for the reaction (chemisorption).
Let us consider, the mechanism of hydrogenation of ethene molecule in presence of Ni as catalyst.
Reactions:
Mechanism: This occur through the formation of an adsorption complex b/w the reactants and the catalyst.
i) and ethene molecules approach the metal surface and get adsorbed to the metal surface.
ii) molecules get spilt up into H atoms which get chemically bound to the metal catalyst i.e. metal atoms forming M-H bonds as H-H(g) + 2M(s) → 2 M-H
iii) The H atoms moves over the surface of catalyst and one of them combine with ethene molecule to form which remain attached to the metal surface.
iv) Finally another hydrogen atom moves over the surface and combine with molecule, which leave the surface. The over all reaction occurs as
Acid base catalysis: A large number of reactions catalysed by acids or base are known eg.
a) Hydrolysis of an ester in presence of an acid
b) Cyanides are hydrolysed by acids or alkalies
Such reactions follow acid – base catalysis and act as catalyst.
Modern concept of acid base catalysis:
According to this concept
i) a reaction which is catalysed by all substances having tendency to lose protons.
ii) a reaction which is catalysed by a base is also catalysed by all substances having tendency to gain proteins.
Auto catalysis:
If one of the intermediate products formed in reaction itself functions as a catalyst for the reaction the catalysis is called auto catalysis and the catalyst is called auto catalyst. i.e. one of the products act as a catalyst.
e.g., (1). Oxidation of oxalic acid by acidic .
The speed of the reaction is slow in the beginning but increases rapidly due to formation of ions which acts as auto catalyst.
ii) Decomposition of
As formed functions as auto catalyst.
Illustration 13: In Zeigler-Natta polymerization of ethylene the active species is
(A) AlCl3 (B) Et3Al (C) CH2CH2 (D) Ti3+
Solution: (D) Ti3+ forms TiCl3 compound having active site vacant which accommodate one alkyl group.
Enzymes or Biological catalyst: About 3000 enzymes have been identified. Catalyst are very essential for our existence. We can not deny the fact that nature is the master designer and user of catalysts. Living cells contain thousands of different kinds of proteins called enzymes which act as catalysts in our body. Every organism depends upon enzymes to sustain life. Thus, enzymes are biological catalysts produced by living cells which catalyse the biochemical reactions in living organism. Chemically all enzymes are proteins with molar mass ranging from 15000 to 1000,000g/mol. Without enzymes the living processes would be very slow to sustain life. e.g., In absence of enzymes our digestive tract would take about 50 years to digest a single meal. The enzymes differ from other types of catalyst in being highly selective and specific.
Table : Some common enzymes and their functions.
|
Enzyme |
Function |
|
i) Amylase |
i) Starch → Glucose |
Characteristics of enzyme:
i) High efficiency: Enzymes increases the speed of reactions upto 10 million times as compared to the uncatalysed reactions.
ii) Extremely small quantities: Extremely small quantities of enzymes as small as millionth of a mole can increase the rate of reaction by factors of
iii) Specificity: The enzymes are highly specific in nature. Almost every biochemical reaction is controlled by its own specific enzyme. e.g. maltase catalyses the hydrolysis of maltose, No other enzymes can catalyse its hydrolysis.
iv) Optimum temperature and pH: The enzymes are active at moderate temperature and pH (resound 7)
v) Control of activity of enzymes: The action of enzymes are controlled by various mechanism and are inhibited by various organic and inorganic molecules.
vi) Activity of enzymes is increased by activators or coenzymes.
Note 1: In some cases, the enzyme activity can be reduced or inhibited by the presence of certain compounds, known as enzyme enhibitors.
Note 2: Activator = metal ions
Coenzyme = metal ions or small organic group.
Mechanism of enzyme catalysed reactions:
Enzymes can increase the rate of biochemical reactions up to 10 million times. They are highly selective and specific and act on certain molecules called substrate. Bio-chemists are trying to explain the exact molecular basis of enzyme catalysis and propose the following mechanisms.
If concentration of ‘S’ increases, the order of reaction changes from first order to zero order. Specific binding of the enzyme (E) to substrate (S) accounts for the high specificity of enzyme catalysed reactions.
The specific binding or fitting of enzyme and substrate is explained on the basis of two models.
i) Lock and Key Mode:
Since catalytic properties of enzymes are present at certain specific regions on their surfaces. These are called active sites or catalytic sites. According to lock and key model, the active site of enzyme have characteristic shape (like lock) and fit suitably shaped specific substrate molecule (like Key) i.e., substrate fits into the slot of enzyme as key fits into a lock. The shape of the active site or catalytic site of any given enzyme is such that only a specific substrate can fit into it, in the same way as one key can open a particular lock.
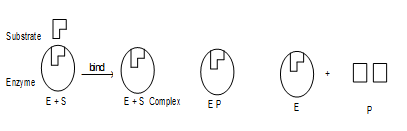
ii) Induced fit Model:
Modern X-ray crystallographic and spectro scopic methods have shown that in many cases unlike an ordinary lock, the protein molecule (enzyme) slightly changes the shape when the substrate lands at the active site. The ability of the enzyme to undergo the correct distortion also determines whether the key will fit or not. This refinement of the original lock and key model is known as induced fit model. According to this model, the substrate induces the active site to adopt a perfect fit rather than a rigidly shaped lock and key. Therefore, we can picture this model as hand in a glove, in which the glove (active site) does not attain its functional shape until the hand (substrate) moves into place.
Application of Enzymes:
i) Enzyme deficiencies and prevention of diseases:
a) The defiency of phenylalanine hydroxylase enzyme causes a congenital diseases called phenyl ketone urea. This disease cause accumulation of compounds in the body which results into severe brain damage and retardation in children. This can be prevented by a diet with low phenyl alanine content.
b) Deficiency of enzyme tyrosinase causes albinism. These diseases can be prevented by the supply of enzyme through diet.
ii) Curing diseases: Certain enzymes are also useful for treating heart disease. An enzyme streptokinase is used to dissolve blood clot.
iii) Industrial applications: The enzymes are widely used in industrial processes. For e.g.,
a) In breweries for the manufacture of beer, wine etc, by the fermentation of carbohydrates.
b) In food processing industries for preparing sweet, syrup, etc.
c) In the production of chese by coagulation of milk.
Nature of solid Calalyst: The solid catalyst may be metals, alloys, metal oxides, or metal sulphides.
The effectiveness of a catalyst depends upon the two important aspects. Activity and selectivity.
i) Activity of a catalyst:
The ability of a catalyst to increase the rate of a chemical reaction is called activity. The activity of a catalyst depends on the strength of chemisorption to a large extent.
The reactant must adsorb strongly reasonably to the metal surface to be active but must not adsorb so strongly that they become immobilize and other reactants do not get space on the catalyst surface for adsorption.
It has been observed that for hydrogenation, the catalytic activity increases as we go from group 5 metals to group 11 metals with maximum activity shown by the elements of group 7-9, in periodic table.
ii) Selectivity:
The ability of the catalyst to direct a reaction to give a particular product is called selectivity. e.g., different catalysts give different products from same reactants.
b)
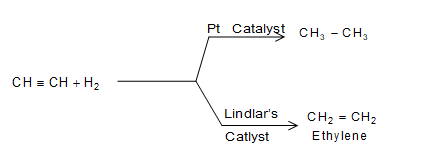
Thus, the action of catalyst is highly specific. A given catalyst can act as catalyst only in a particular reaction and not in all reactions. Thus, catahpt is highly selective in nature.
Shape selective catalysis by Zeolites:
The catalytic reaction which depends upon the porestructure of the catalyst and the size of the reactant and product molecules is known as shape selective catalysis. Zeolites are good shape selective catalysts because of their honey comb like structures i.e. porous structure. Zeolites are micro porous aluminosilicates of the general formula Mx/n , where n is the valency of the metal cation , and they are three dimensional network silicates in which some silicon atoms are replaced by aluminium atoms. They are found in nature as such and are also synthesized in the laboratories.
Zeolites to be used as catalysts are heated in vacuum so as to push out molecules of hydration. The loss of molecules of hydration causes cavities of molecular dimensions in the network structure and makes it porous. Thus, these are porous and have cavities of molecular dimensions.
Therefore, internal structure of a zeolite is a network of tunnels and cavities. The pore size in zeolites is generally varies between 260 pm and 740 pm.
The catalytic activity of zeolites depend upon the size of cavities (cages) or pores(apertures) present in them. Pores can trap only those molecules whose is size is small enough to enter or leave the cavities. Bigger or larger molecules cannot enter the cavities. Thus, zeolites can act as molecular sieves or selective adsorbents. Depending upon the sizes of reactant and product molecules relative to the sizes of cavities and apertures of the zeolite, reactions proceed in a specific manner. e.g. Alcohols are converted into gasoline using zeolite catalyst = ZSM-5. General formula of ZSM-5 is the alcohol is dehydrated in the cavities and the hydro carbons are formed. The shape selectivity in the reaction can be judged from the conversion of methanol and 1-heptanol to hydro carbon mixtures.
|
Product |
Starting from Methanol (%) |
Starting from 1-heptanol (%) |
|
Methane |
1.0 |
0.0 |
FORMULAE AND CONCEPTS AT A GLANCE
1. Presence of a substance in higher concentration at the surface than the adjoining bulk phases is called adsorption.
2. The substance on the surface of which adsorption takes place is called adsorbent.
3. The substance which is present in higher concentration at the surface of adsorbent is called adsorbate.
4. In physical adsorption adsorbate is held on the surface of adsorbent by van der Waal’s forces.
5. In chemical adsorption adsorbate is held on the surface of adsorbent by chemical bonds.
6. Enthalpy change accompanying the adsorption of one mole of adsorbate on the adsorbent is called enthalpy of adsorption it is always negative.
7. Extent of adsorption decreases with increase in temperature and increases with increase in pressure.
8. A graph of extent of adsorption vs pressure at constant temperature is called adsorption isotherm.
9. Freundlich adsorption isotherm ?
10. Langmuir Adsorption Isotherm
11. A graph of extent of adsorption vs temperature at constant pressure is called adsorption isobar.
12. The pressure beyond which extent of adsorption become independent of pressure is called saturation pressure.
13. Is state in which the particle size of the substance is in the range 1-1000nm is called colloidal state.
14. A colloidal solution containing solid as the dispers phase and liquid as the dispersion medium is called sol.
15. A colloidal solution in which there is great affinity between the disperse phase and the dispersion medium is called lyophilic sol.
16. A colloidal solution in which there is little affinity between the disperse phase and the dispersion medium is called lyophobic sol. Lyophobic sols are less stable.
17. A colloid in which colloidal particle are macromolecules is called macromolecular colloid.
18. A colloid in which colloidal particles are clustures are aggregates smaller molecules is called multimolecular colloid.
19. A colloid which is form when concentration of the solute exceeds a limit is called associated colloid.
20. Preparation of colloidal solution by shaking the substance with dispersion medium in the presence of some suitable electrolytes is called Peptisation.
21. Precipitation of a colloidal solution by induced aggregation of colloidal particles is called coagulation.
22. Minimum concentration of the electrolyte in milimoles per litre needed to cause coagulation of the sol is called coagulation value.
23. In case of electrolytes, the ion carrying charge opposite to that of colloidal particles is effective in causing coagulation. Greater the valency of the ion causing the coagulation greater in the coagulation power – Hardy-Shulze rules.
24. The zig-zag movement of colloidal particles is called Brownian movement.
25. Scattering of light by colloidal particles is called Tyndall effect.
26. The movement of colloidal particles towards one of the electrodes on passage of electricity through colloidal is called electrophoresis.
27. A parameter used for expressing protective power of a lyophilic colloid is called gold number.
28. Acceleration of rate of a reaction by using some additional substance is called catalysis.
29. Surface catalysis, acts through adsorption.
30. Zeolite is a shape elective catalysts.
31. Enzymes are complex proteinous substances produced by living bodies, which act as catalysts in the physiological reactions.
SOLVED PROBLEMS-1
Prob 1. How is the adsorption of a gas related to its critical temperature?
Sol: Higher is the critical temperature of a gas, greater is the ease of liquefication of gas i.e. larger the van der Waal’s forces of attraction therefore, greater is the adsorption.
Prob 2. Give reason why a finely divided substance is more effective as an adsorbent?
Sol: This, is because a finely divided substance has larger surface area and hence, larger adsorption occurs?
Prob 3. The coagulation of100ml of a colloidal solution of gold is completely prevented by the addition of 0.20g of starch to it before adding 1mL of 10% NaCl solution. Calculate the gold number of starch.
Sol: Amount of starch added to 100mL of gold sol required to prevent coagulation of 1 ml of 10% NaCl solutions.
= 0.20g
= 200 mg
or, starch required to be added to 10ml of gold sol to completely prevent coagulation by 1ml of 10% NaCl solution.
Gold number of starch = 20
Prob 4. Lyophiic sols are more stable than lyophobic sols why?
Sol: Lyophilic sols are more stable because they are highly hydrated in solution .
Prob 5. What is the difference between a sol and a gel?
Sol: In a sol, dispersion medium is liquid and dispersed phase is solid but in a gel, dispersion medium is solid and dispersed phase is liquid.
Prob 6. What does reciprocal of gold number indicate?
Sol: Reciprocal of gold number is a measure of protective power of a colloid. Smaller the value of gold number, greater will be its protecting power.
Prob 7. What happens when sodium chloride is added to freshly prepared ferric hydroxide solution?
Sol: A reddish brown colloidal sol of is obtained. This process is known as peptization
Positively charged colloidal sol
Prob 8. What is collodion?
Sol: In some cases peptization can also be achieved by organic solvents eg. Cellulose nitrate is peptised by ethanol. The colloidal sol so obtained is known as collodion.
Prob 9. Gold numbers of gelatin and gum arabic are 0.005 and 0.15 respectively, which of two has greater protecting power.
Sol: Gelatin has greater protecting power than gum arabic because smaller the gold number of protective colloid, greater will be its protecting power.
Prob 10. Which shape selective catalyst is used in getting patrol from alcohol ?
Sol: zeolite catalyst ZSM-5 is used in getting petrol from alcohol.
SOLVED PROBLEMS-2
Prob 1. Physical adsorption
(A) involves the weak attractive interactions between the adsorbent and adsorbate
(B) involves the chemical interactions between the adsorbent and adsorbate
(C) is irreversible in nature
(D) increases with increase in temperature
Sol: (A) Physical adsorption involves the weak attractive interactions between the adsorbent and adsorbate.
Prob 2. Chemisorption
(A) involves the weak attractive interactions between the adsorbent and adsorbate
(B) is irreversible in nature
(C) decreases with increase in temperature
(D) involves multilayer adsorption
Sol: (B) Chemisorption is irreversible in nature.
Prob 3. In the Freundlich adsorption equation , the value of n is
(A) always greater than one
(B) always smaller than one
(C) always equal to one
(D) greater than one at low temperature and is smaller than one at high temperature
Sol: (A) In the Freundlich adsorption equation , the value of n is always greater than one.
Prob 4. Which of the following statements is not correct?
(A) The extent of physical adsorption increases linearly with increase in pressure in the low pressure region
(B) The extent of physical adsorption attains a limiting value at the high pressure region
(C) In the intermediate range of pressure, the increase in adsorption is more than the increase in pressure
(D) Physical adsorption involves the reversible process, , where G, S and GS represent respectively, the unabsorbed gaseous molecules, adsorption sites and adsorbed gaseous molecules.
Sol: (C) In the intermediate range of pressure, the increase in adsorption is more than the increase in pressure is an incorrect statement.
Prob 5. Which of the following statements is not correct?
(A) Zeolite A is formed by linking sodalite cages through double four membered rings
(B) Faujasite zeolite is formed by linking the sodalite cages through double six membered rings.
(C) Zeolite structures contain tunnels or systems of interconnected cavaties which have precisely defined dimensions on the atomic scales.
(D) Zeolites are anion exchanges
Sol: (D) Zeolites are not anion exchanges
Prob 6. Which of the following statements is not correct?
(A) Peptization is the process by which certain substances are converted into the colloidal state when shaken in water containing a minute amount of an electrolyte
(B) Metal sols of gold, silver, platinum, etc, can be prepared by Bredig’s arc method
(C) Impurities present in a sol makes it more stable
(D) Dialysis is a process with the help of which impurities (made up of ions and molecules) present in a sol can be conveniently removed
Sol: (C) Impurities present in a sol makes it more stable is an incorrect statement.
Prob 7. Which of the following statements is not true for a lyophilic sol?
(A) It can be easily solvated
(B) It carries no charge
(C) The coagulation of this sol is reversible in nature
(D) It is not very stable in a solvent
Sol: (D) Lyophilic sol is stable in a solvent
Prob 8. Which of the following represents a multimolecular collidal particles?
(A) Sol of sulphur (B) Starch (C) Soaps (D) Proteins
Sol: (A) Sol of sulphur represents a multimolecular colloid.
Prob 9. Which of the following statements is not correct?
(A) Most heterogeneous catalytic reactions involve the solid surface of the catalyst
(B) Heterogeneous catalysts primarily function by lowering the activation energy of the reaction
(C) A solid catalyst present in the powder form is more effective as it has larger surface area
(D) The catalyst may be deactivated by heating it to a high temperature in vacuum
Sol: (D) The catalyst may be deactivated by heating it to a high temperature in vacuum is a wrong statement.
Prob 10. Which of the following statements is not correct?
(A) The efficiency of a solid catalyst depends upon its surface area
(B) Catalyst operates by providing alternate path for the reaction that involves a lower energy of activation
(C) Catalyst lowers the energy of activation of the forward reaction without affecting the energy of activation of the backward reaction
(D) Catalyst does not affect the overall enthalpy change of the reaction
Sol: (C) Catalyst lowers the energy of activation of the forward reaction without affecting the energy of activation of the backward reaction.








