Introduction:
Alkyl halides or haloalkanes are the class of organic compounds in which a halogen is bonded to an alkyl group. The general formula of alkylhalide, RX (where R is an alkyl group, and X is a halogen atom) is CnH2n+1X, e.g. CH3Cl, C2H5Cl. Unsaturated hydrocarbons also form halogen derivatives. For example:

Aromatic halogen compounds or haloarenes are the halogen compounds containing at least one benzene ring. Halogen derivatives of aromatic compounds are of two types.
Aryl halides: In this type of compounds, the halogen atom is directly linked to the carbon of benzene nucleus. They are also called nuclear substitution derivatives.
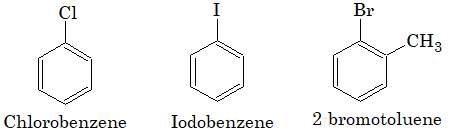
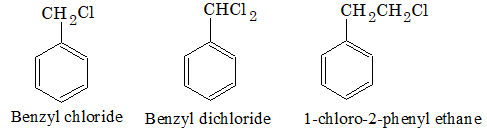
Arylalkyl halides: In this type of compounds, halogen is linked to the carbon atom of the side chain attached to benzene ring. They are also called side chain substitution derivatives.
Nomenclature of alkyl halides and aryl halides:
In common system, aliphatic halogen derivatives are named as alkyl halides. The words n, sec, tert, iso, neo and amyl are usually used in writing the common names. In IUPAC system, they are considered as derivatives of corresponding alkanes and are named as haloalkanes.
Common name IUPAC name
|
Methyl chloride |
Chloromethane |
|
| CH3CH2CH2Cl |
n -propyl chloride |
1-chloropropane |
|
iso-propyl chloride |
2-chloropropane |
|
|
CH3CH2CH2CH2Cl |
n-butyl chloride |
1-chlorobutane |
|
sec-butyl chloride |
2-chlorobutane |
|
|
iso-butyl chloride |
1-chloro-2-methylpropane |
|
|
tert-butyl chloride |
2-chloro-2-methylpropane |
The dihalogen derivatives having halogen atoms of similar type on the same carbon atom are known as geminal dihalides and are assigned common name alkylidene halides or alkylidene dihalides. The dihalogen derivatives having the two similar halogen atoms on adjacent carbon atoms are known as vicinal dihalides and are assigned common name alkylene halides or alkylene dihalides. Haloforms are named by prefixing the halogen and its position, if necessary, to the name of the parent aromatic compound.
Isomerism In Haloalkanes:
The kinds of isomerism exhibited by haloalkanes are discussed below.
Chain Isomerism:
The haloalkanes having four or more carbon atoms show chain isomerism. For example,
Position Isomerism:
The haloalkanes with three or more carbon atoms show position isomersim. For example, C3H7Br has two isomers.
Nature of C-X bond in alkylhalides:
Halogen atoms are more electronegative than carbon atom. The carbon halogen bond of alkylhalide is polarized with partial positive charge on carbon atom and partial negative charge on halogen atom .
Preparation of alkylhalides (specially ethylchloride):
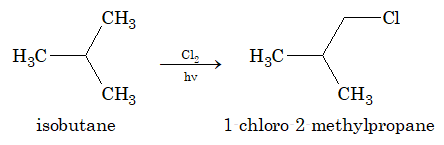
1. By Direct Halogenation of Alkanes:
When alkanes are treated with halogen, chlorine or bromine, in the presence of light or heat, a mixture of mono and poly substituted products is obtained.
In the above reaction, the substitution beyond monohalogenation can be suppressed by using alkane in excess. But the method is not practical because of the difficulties arise during separation of the mixture.
In case of higher alkanes, different isomeric products are formed even when monosubstitution is carried out.
In general, the ease of substitution of different types of hydrogen atoms follows the order–benzylic > allylic > tertiary > secondary > primary > vinylic, aryl.
The iodination of alkanes is reversible and is done by heating the alkane with iodine in the presence of oxidizing agents like conc. HNO3, HIO4 or HIO3. The function of using such agents is to oxidize HI, formed during the reaction, to iodine, and hence shift the equilibrium in the forward direction.
2. By Addition of HX on Alkenes (HX = HCl, HBr, HI):
In unsymmetrical alkenes the addition of halogen acids takes place according to the Markonikov’s rule. Anti-Markonikov’s addition is observed during addition of HBr in presence of peroxide (H2O2), known as peroxide effect or Kharasch effect.
3. From Alcohols:
a) Action of halogen acids: Primary alcohols need ZnCl2 (anhydrous) for preparation of alkyl halides. The mixture of HCl and anhydrous ZnCl2 is known as Lucas reagent.
This reaction is also known as Groves process.
Bromoalkanes can be obtained by heating alcohols with KBr or NaBr and conc. H2SO4. HBr is generated in situ.
Iodoalkanes are obtained by heating alcohols with KI and 95% H3PO4.
The reactivity order of alcohols is tertiary > secondary > primary and the reactivity order of halogen is HI > HBr > HCl.
b) By reaction with phosphorus halides:
Chloroalkanes are obtained by reaction of alcohols with PCl3 or PCl5.
Here ‘R’ can be replaced by group to prepare ethyl chloride.
[Note: PBr3 and PI3 being less stable, hence for bromides and iodides, P + Br2 or P + I2 mixture is used.]
c) Action of Thionyl Chloride or Darzen’s Method:
Here R is ethyl group.
[Note: SOBr2 is less stable and SOI2 does not exist hence bromides and iodides are not prepared by this method.]
4) By Halide Exchange:
(Finkelstein reaction) Iodides are usually prepared by this method.
luoroalkanes (which is difficult to prepare directly by action of alkanes) can be obtained by treating alkyl halides with salts like AgF , Hg2F2 , CoF3 or SbF3. This reaction is known as Swarts reaction.
5) From Silver Salts of Fatty Acids (Borodin–Hunsdiecker reaction):
Alkyl chlorides and alkyl bromides are obtained by the action of Cl2 or Br2 in CCl4 on silver salt of fatty acids. The reaction proceeds through free radical mechanism.
Iodoalkanes, cannot be obtained by this method because I2 reacts with silver salts of fatty acids to form ester.
This reaction is called Birnbaurn–Simoni reaction.
Illustration 1: The best method to prepare fluoroethane is
(A)
(B)
(C)
(D)
Solution: (C) Fluoroethane is prepared by halogen exchange method, i.e. Swarts reaction.
Illustration 2: In the chlorination of isobutane, which product will be formed in excess,
(A) (CH3)2 CHCH2Cl
(B) (CH3)3CCl
(C) Both of the above
(D) None of the above
Solution: (A)
Physical properties of alkylhalides (haloalkanes):
1. Physical state: CH3Cl , C2H5Cl , CH3Br are gases at room temperature. The alkyl halide upto C18 are liquids, while higher are colourless solids.
2. Boiling point: The boiling point of haloalkanes are in the order RCl < RBr < RI. It is because with the increase in size and mass of the halogen atom, the magnitude of van der Waals forces of attraction increases. Among isomeric alkyl halides, the boiling point decreases with increase in branching in the alkyl group. This is due to the reason that with increase in branching the molecule acquire less surface area due to attainment of spherical shape. As a result, interparticle forces become weaker, resulting in lower boiling point.
The boiling point of various halogen compounds increases with the increase in number of halogen atoms.
The boiling point of alkyl halides shows following order,
Alkyl iodide > Alkyl bromide >Alkyl chloride (for a given alkyl group)
Methyl halide < Ethyl halide < Propyl halide (for a given halide)
1o halide > 2o halide > 3o halide (for a given halide and alkyl group).
3. Solubility: The alkyl halides are polar in nature but still they are insoluble in water as they do not form H-bonds with water molecules.
4. Density: Alkyl fluorides and alkyl chlorides are lighter than water. Alkyl bromides and alkyl iodides are heavier than water. The density of halides thus follow the order, iodide > bromide > chloride > fluoride.
5. Action of flame: All halides burn on Cu wire with green edge flame. This is Beilstein test for halogens. The green or blue colour of flame is due to interaction of halogens with Cu wire.
6. Exposure to sunlight: Alkyl iodides are less stable and darken on exposure to light or on standing due to adsorption of liberated iodine.
[Physical properties of ethylchloride:
It is a colourless gas with pleasant odour. Its boiling point is 286 K and its density is 0.91 g/ml. It is soluble in organic solvents like carbon tetrachloride, but almost insoluble in water. As C – Cl bond is covalent it does not give Cl ions in solution. Hence it does not form precipitate with silver nitrate solution.]
Chemical properties of alkylhalides (haloalkanes):
The alkyl halides are highly reactive due to high electronegativity difference between carbon and halogen atom, which provides polarity in C δ+ –X δ – bond. Thus, carbon atom of C–X bond is easily attacked by a nucleophile and shows nucleophilic substitution.
The nucleophilic substitution may follow SN1 or SN2 mechanism. In addition to nucleophilic substitution alkyl halides also show elimination reactions.
SN2 mechanism: The rate of reaction is dependent on the concentration of alkyl halide as well as the nucleophile, i.e.Rate = K[RX][Nu–]. Hence, the reaction is bimolecular nucleophilic substitution. There is complete inversion of configuration as it involves attack of nucleophile from backside.
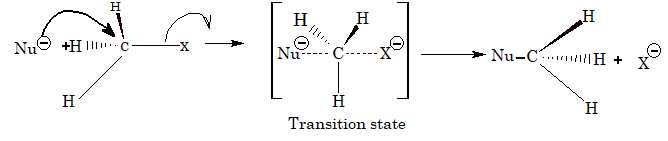
The order of reactivity of various alkyl halides towards nucleophilic substitution reaction by SN2 mechanism is,
1o > 2o > 3o
SN1 mechanism: The rate of reaction is dependent only on the concentration of alkyl halide, i.e. Rate = K [RX]. Hence, the reaction is unimolecular nucleophilic substitution.
The mechanism involves two steps:
Step 1: In the first step, the alkyl halide slowly dissociates into halide ion and carbocation.
Step 2: In the second step, carbocation formed immediately combines with the nucleophile to form the final substituted product.
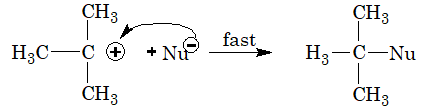
The order of reactivity of various alkyl halides towards nucleophilic substitution reaction by SN1 mechanism is, 3o > 2o > 1o
A racemised product is obtained by SN1 mechanism. This is due to the fact that the carbocation formed has planar structure. So, the nucleophile can attack this carbocation from both sides.
i) Nucleophilic Substitution of alkyl halides:
The halogen atom of alkyl halide is easily replaced by a nucleophile to give a large variety of nucleophilic substitution reactions.
1) Replacement by hydroxyl group (formation of alcohols):
2) Replacement by alkoxy group: (formation of ethers). In this reaction, alkyl halide is treated with alcoholic sodium or potassium alkoxide to form ether. This reaction is known as Willamson’s synthesis.
3) Replacement by cyano group (formation of cyanides or nitriles):
4) Replacement with Isocyanide (formation of isocyanide):
5) Replacement by amino group (formation of amines). This reaction is known as Hofmann’s ammonolysis.
If haloalkane is present in excess, then other two hydrogen atoms of amino group are also replaced by alkyl groups leading to the formation of secondary and tertiary amines. Tertiary amine so formed further combines with alkyl halide to give quaternary ammonium salt.
6) Replacement by nitro group. Treatment of alkyl halide with AgNO2 gives nitro alkane as a major product.
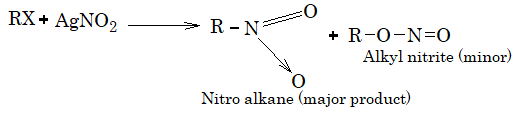
7) Replacement with nitrite group. Treatment of alkyl halide with potassium nitrite gives alkyl nitrite as a major product.
8) Replacement with–SH (hydrosulphide) group: Alkyl halides on reaction with hydrosulphide group forms thiols or mercaptans.
9) Replacement by mercaptide (SR): Alkyl halides on reaction with mercaptide ion gives thio ethers.
Thioethers can also be prepared by reaction of alkyl halides with sodium or potassium sulphide.
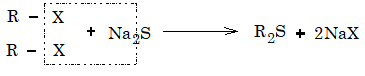
10) Replacement by alkynyl group. Higher alkynes can be prepared on reacting alkyl halides with alkynyl group.
The reaction is of limited practical use and can be used only in case of primary halides because the secondary and tertiary halides undergo elimination rather than substitution.
11) Replacement by carboxylate group: Alkyl halides on reaction with silver salts of carboxylic acid gives esters.
12) Replacement by hydride ion. Alkanes can be prepared from alkyl halides by this substitution reaction.
ii) Dehydrohalogenation Reactions:
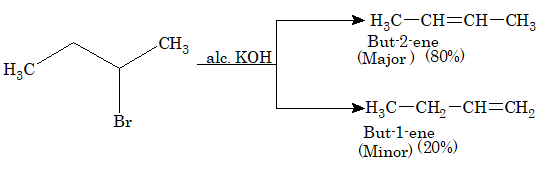
When haloalkanes are heated with alcoholic KOH, they undergo dehydrohalogenation to form alkenes. This is also known as α, β elimination reaction.
The reactivity of haloalkanes towards elimination reaction follows the order,
Tertiary > Secondary > Primary
Haloalkanes undergo elimination in two different ways to produce two different alkenes. The preferred alkene is one in which more number of alkyl groups are attached to the double bonded carbon atoms. This generalization is known as Saytzeff rule.
III. Reactions with Metals:
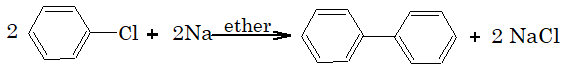
1) Reaction with sodium. This reaction is knows as Wurtz reaction and generally used to prepare symmetrical alkanes with more number of carbon atoms.
2) Reaction with magnesium.
3) Reaction with zinc powder.
4) Reaction with lithium.
5) Reaction with sodium amalgam.
6) Reaction with Pb–Na alloy.
iv) Reduction:
1) Reaction with H2 / Ni:
2) Reaction with zinc-copper couple (in presence of alcohol):
Similarly, Zn / HCl, Zn / NaOH, Na / C2H5OH, Sn / HCl or HI / red P at 430 K can also be used to reduce haloalkanes to alkanes.
v) Action of Heat:
Alkenes can be prepared by heating alkyl halides at a very high temperature.
Uses of ethylchloride:
i) As a refrigerant
ii) As a solvent
iii) As a local anesthetic
iv) As a ethylating agent in preparation of TEL
v) In the preparation of Grignard reagent (RMgX)
Illustration 3: Which of the following has highest boiling point?
(A) C2H5F (B) C2H5Cl (C) C2H5Br (D) C2H5I
Solution: (D) Boiling point of alkyl halides decreases in the order RI > RBr > RCl > RF
Illustration 4: Which of the following alkyl halide has the maximum density?
(A) C3H7I (B) C2H5I (C) CH3Br (D) CH3I
Solution: (D) CH3I has the highest density because of lowest hydrocarbon part (i.e. CH3) and heaviest halogen (i.e. I).
Illustration 5: Arrange the following halides in the decreasing order of SN1 reactivity
CH3CH2CH2Cl CH2 = CHCH(Cl)CH3 CH3CH2CH(Cl)CH3
I II III
(A) I > II >III (B) II > I > III (C) II > III > I (D) III > II > I
Solution: (C) Allyl carbocation is more stable than 2o carbocation which in turn is more stable than primary.
Illustration 6: Product-I C2H5Br Product II
(A) Product I is obtained by the elimination reaction.
(B) Product II is obtained by the substitution reaction
(C) The molecular formula of Product I is C2H4, while the molecular formula of Product II is C2H6O.
(D) Product I is the isomer of dimethyl ether, while Product II is the dehydrated compound of Product I.
Solution: (D)
C2H5Br C2H5OH (Product I)
(Nucleophilic substitution reaction)
C2H5Br C2H4 (Product II)
(Elimination reaction)
The isomer of C2H5OH (Product I) isomer is CH3OCH3
C2H5OH C2H4
Illustration 7: During debromination of meso-dibromobutane, the major compound formed is
(A) n-butane (B) 1-butene (C) cis 2-butene (D) trans 2-butene
Solution: (D) Debromination is a trans elimination reaction and hence meso-dibromobutane on debromination gives trans 2-butene.
Illustration 8: The reaction of 1-bromo-3-chlorocyclobutane with sodium metal under reflux condition gives
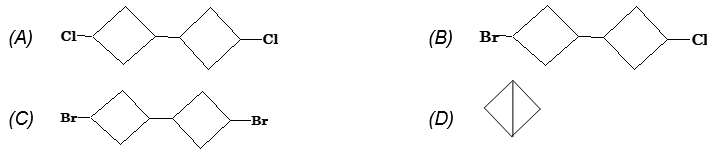
Solution: (D) Since, bromides are more reactive than chlorides in Wurtz reaction, therefore Na react on the side of Br gives.
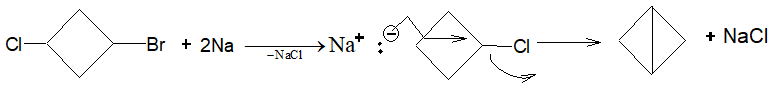
Illustration 9: The end product of the following reaction is
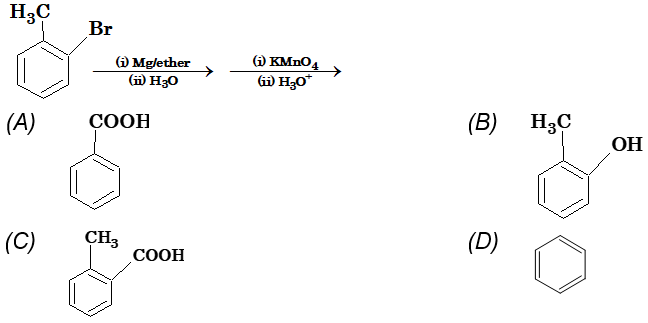
Solution: (A)
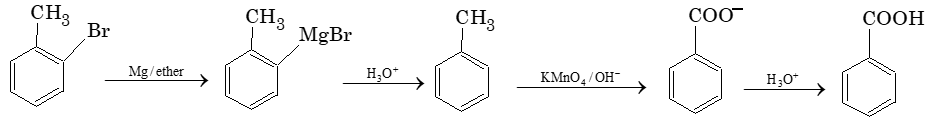
Nature of C – X bond in arylhalides (haloarenes):
i) In haloarene, the halogen atom is bonded to the sp2 – hybridized carbon atom of an aromatic ring. The sp2 orbital has more ‘s’ character than sp3 Due to this C – Cl bond length in haloarene is 169 pm while it is 177 pm in haloalkane (alkylhalide). As shorter bond in storonger haloarenes are less reactive towards nuclophilic substitution reactions.
ii) Resonance effect also makes aryl halides less reactive towards nuclophilic substitution reaction. Due to resonance effect, C – X bond acquires a partial double bond character and the cleavage of C – X bond becomes more difficult than the in haloalkane (alkylhalide).
iii) Benzene ring has more electron density. Therefore, a stronger nucleophile can not approach it easily.
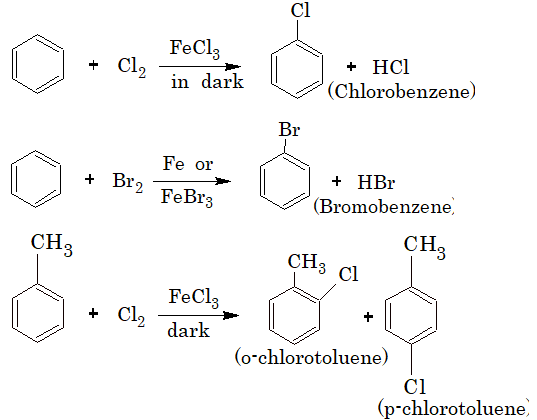
Preparation of arylhalides (haloarenes):
1) By Direct Halogenation:
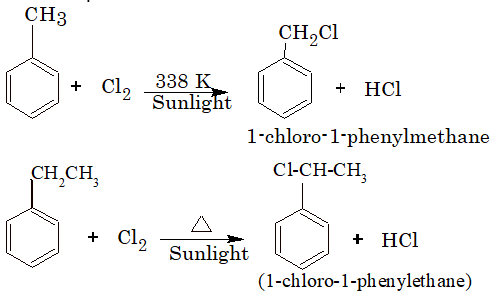
The alkyl benzene when heated with halogens in the presence of sunlight and in the absence of Lewis acids (halogen carrier) undergoes substitution at the alkyl chain resulting in the formation of aralkyl halides. For Example:
2) From Diazonium Compounds:
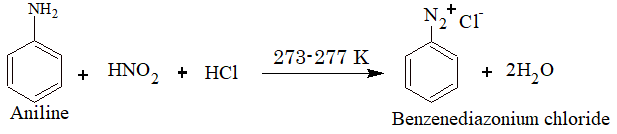
The reaction is known as diazotization.
The diazonium compound is treated with CuCl and HCl or CuBr and HBr to give the corresponding haloarene. This reaction is known as Sandmeyer reaction.
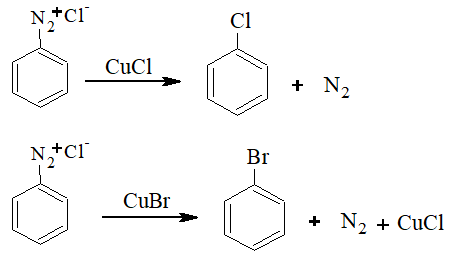
If instead of CuCl and CuBr, Cu powder and HCl or HBr is used, the reaction is called Gattermann’s reaction.
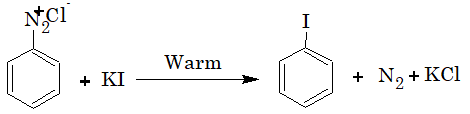
Iodoarenes are obtained by warming benzenediazonium salts with KI.
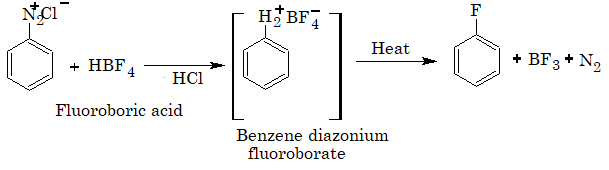
Fluoroarenes are obtained by the reaction of corresponding diazonium salts with fluoroboric acid to produce diazonium fluoroborate which on heating produces fluorobenzene. This reaction is called Balz–Schiemann reaction.
3) From Benzene (Commercial Method):
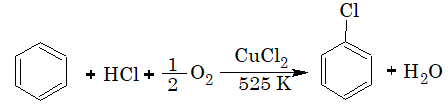
This is process is known as Raschig process.
4) By Hunsdiecker Reaction:
Physical properties of arylhalides (haloarenes):
1. Boiling point: The boiling point of monohalogen derivatives of benzene, which are all liquids, follow the order–iodo > bromo > chloro.
The boiling point of isomeric dihalobenzenes are nearly the same. However, their melting points are quite different.
2. Solubility: They are soluble in organic solvents like alcohol and ether but insoluble in water as they cannot form H-bonding with water molecules.
3. Density: These compounds are heavier than water, Their densities follow the order–Iodo > Bromo > Chloro.
Chemical properties of aryl halides:
Aryl halides are much less reactive towards nucleophilic substitution reaction than haloalkanes. However, they can be made to react under drastic conditions, such as high pressure and high temperature.
I. Nucleophilic Substitution Reactions:
1) Replacement by hydroxy group – Dow’s process:
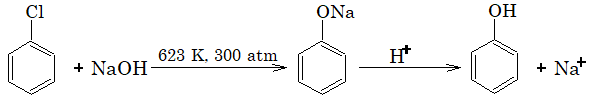
The reactivity of aryl halides towards nucleophilic substitution increases if some electron withdrawing group such as nitro, cyano or carbonyl group is attached to the ring.
2) Replacement by cyano group:
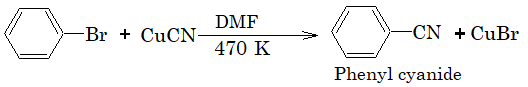
3) Replacement by amino group.

4) Replacement by alkoxide group:
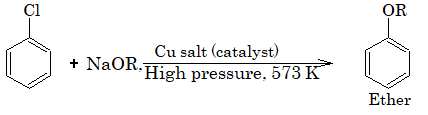
5) Replacement by – SH group:II. Reduction:
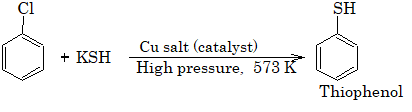
III. Reaction with Metals:
1) Reaction with magnesium:
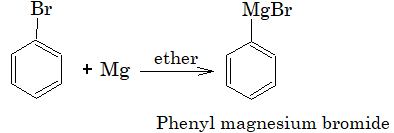
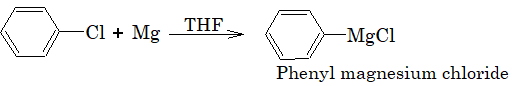
Aryl chlorides do not form Grignard reagent in presence of ether as solvent but it is possible if THF (Tetrahydrofuran) is used.
2) Reaction with sodium:
Aryl halides react with sodium in presence of ether.
When aryl halides are treated with haloalkane and sodium in presence of dry ether, undergo Wurtz Fitting reaction.
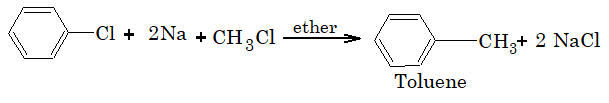
3) Reaction with lithium:
Aryl halides reacts with lithium in presence of dry ether to form organometallic compound.
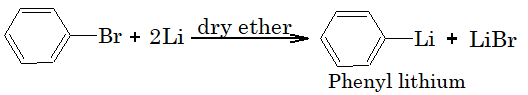
4) Reaction with copper powder (Ulmann Reaction):
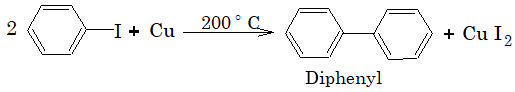
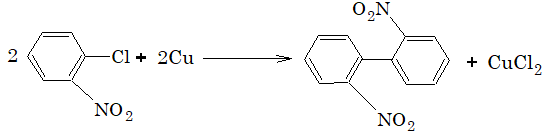
Aryl chloride and aryl bromide usually do not give this reaction, however if o and p-nitro substituted aryl chloride and aryl bromide are used, then it shows Ulmann reaction.
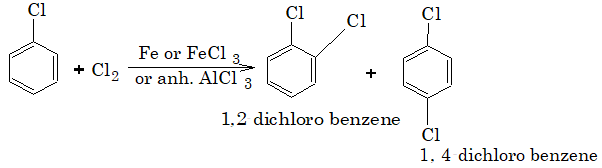
IV. Ring substitution reactions:
Aryl Halides undergo electrophilic substitution reaction in the benzene ring. The halogen atoms are o and p-directing groups but deactivates the ring at the same time. Therefore, substitution occurs relatively at slower rate in comparison to benzene.
1) Halogenation:
2) Nitration:
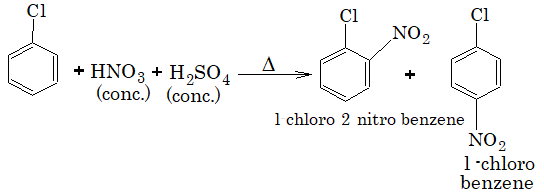
3) Sulphonation:
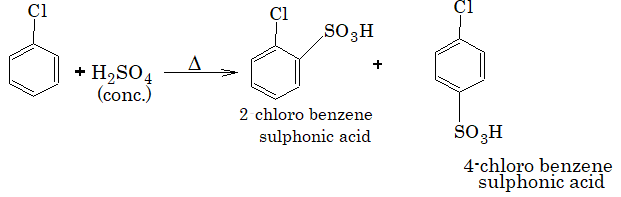
4) Friedel-Crafts alkylation:

5) Friedel-Crafts acylation:
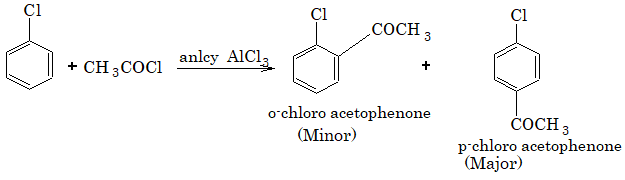
6) Action with chloral:
Two molecules of chlorobenzene reacts with (chloral) to form DDT.
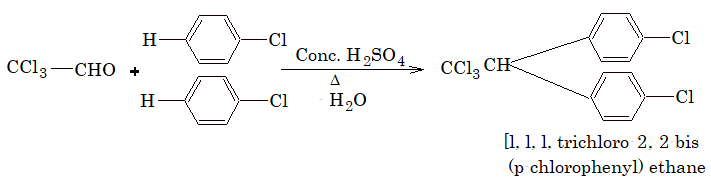
Illustration10: Ethyl benzene with bromine in presence of FeBr3 predominantly gives
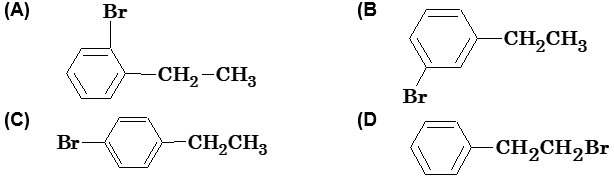
Solution: (C) In presence of Lewis acid (i.e. FeBr3), alkyl halides give ortho and para product but due to steric hindrance in ethyl benzene p-isomer is major product.
Illustration 11: Which of the following halogen containing compound will not undergo SN reactions easily?
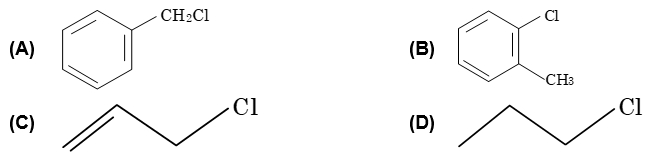
Solution: (A) cannot be substituted by other nucleophile due to double bond character and unstability of
Illustration 12: Toluene on reaction with N-bromo succinimide gives
(A) p–bromomethyl benzene
(B) o–bromomethyl benzene
(C) phenyl bromomethane
(D) m–bromomethyl benzene
Solution: (C)
Trichloromethane (Chloroform):
Methods of Preparation:
1. From methane:
Chloroform is prepared by chlorination of methane in the presence of light.
The mixture of CH3Cl, CH2Cl2, CHCl3, and CCl4 are separated by fractional distillation.
2. From chloral hydrate:
Chloroform (in pure form) can be prepared by distillation of chloral or chloral hydrate with concentrated aqueous NaOH solution or KOH solution.
3. From ethanol or acetone (lab method): Chloroform is obtained from ethanol or acetone by reaction with a paste of bleaching powder and water.
(a) In case of alcohol:
(b) In case of acetone:
4. From carbon tetrachloride:
By partial reduction of carbon tetrachloride with iron filings and water.
Physical Properties:
Chloroform is a colourless, heavy liquid. It has sweetish, sickly odour and taste. It is heavier than water and slightly soluble in water. Its boiling point is 334 K. Inhaling of chloroform causes unconsciousness and thus used as anaesthetic.
Chemical Properties:
1. Action of sunlight and air: It is oxidized by air to produce a highly poisonous compound called phosgene (COCl2).
To avoid the formation of phosgene, chloroform is kept in dark bottles to cut off active light radiations and filled upto brim to keep out air. Also a little amount of 1% ethanol is added which converts the toxic COCl2 as non-poisonous diethyl carbonate.
2. Hydrolysis: When boiled with aqueous KOH, chloroform is hydrolysed to potassium formate.
Mechanism:
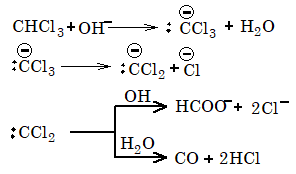
3. Chlorination:
4. Action of Ag powder:
5. Reaction with nitric acid:
Chloropicrin is used as an insecticide and war gas.
6. Reaction with acetone:
Chloretone is used as hypnotic (a sleep inducing drug).
7. Reaction with primary amines. (Carbylamine reaction):
Both aromatic and aliphatic primary amines give isocyanide with KOH (alcoholic) and chloroform. The isocyanide formed has a characteristic smell and this reaction is known as isocyanide or carbylamine reaction. It is used for the detection of primary amines.
8. Reduction with zinc and HCl:
9. Reaction with sodium ethoxide:
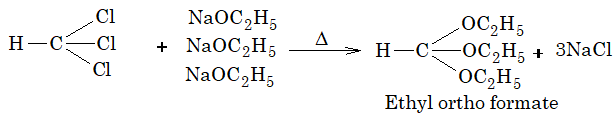
10. Riemer–Tiemann reaction.
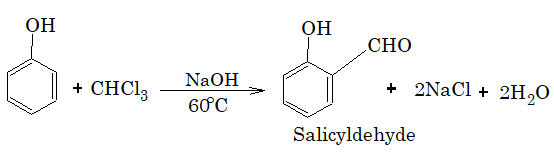
11. Reaction with silver nitrate.
Chloroform does not give a precipitate when treated with aqueous AgNO3. It is because the C–Cl bond in chloroform is covalent and does not ionize in aqueous solution to produce chloride ions.
Uses of Chloroform:
(i) As an anaesthetic.
(ii) As laboratory reagent–detection of primary (1o) amines.
(iii) As a solvent for fat, waxes, rubbers, varnishes.
(iv) As a preservative for anatomical specimens.
(v) In preparation of chloretone and nitrochloroform.
Illustration13: Chloroform on reduction with Zn and HCl (alc.) gives
(A) formic acid
(B) chloretone
(C) chloropicrin
(D) methylene dichloride 14:
Solution: (D)
Illustration 14: On warming with silver powder, chloroform is converted into
(A) acetylene
(B) hexachloroethane
(C) 1, 1, 2,2-tetrachloroethane
(D) ethylene
Solution: (A).
Triiodomethane (Iodoform):
Methods of Preparation:
Iodoform is prepared by the action of iodine and alkali on acetaldehyde, methyl ketones and alcohols (which produces unit on oxidation).
1. From alcohol:
The complete reaction is,
2. From acetone:
Physical Properties:
Iodoform is a yellow coloured, solid compound having melting point 382 K. It is insoluble in water but dissolves readily in organic solvents.
Chemical Properties:
Chemical reactions of iodoform is similar to that of chloroform.
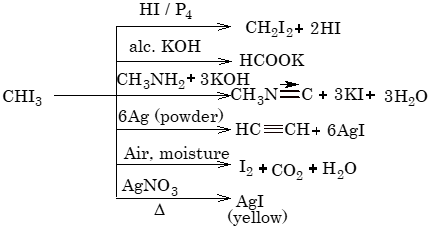
Uses of Iodoform:
(i) It is used as antiseptic for dressing wounds.
(ii) It is used for manufacturing pharmaceuticals.
Illustration 15: The compound which give negative Iodoform test is
(A) CH3CHO
(B) CH3CH2OH
(C) C6H5COCH3
(D) C6H5CH2CH2OH
Solution: (D) Compounds ontaining unit attached to
C or H are capable of giving Iodoform ( haloform test).
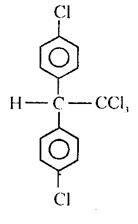
DDT:
p, p’ – dichloro-diphenyl trichloroethane (DDT) was the first chlorinated insecticide insects developed resistance to DDT. It is highly toxic to fish.
FORMULAE AND CONCEPTS AT A GLANCE
1. Compounds defived from alkenes by the replacement of one or more H atoms by corresponding number of halogen atoms are termed halogen derivatives of alkanes or haloalkanes. They do not occur in nature.
Haloalkanes are divided into mono, di, tri, tetra etc. according to the number of halogen atoms in the molecule.
2. The unsymmetrical alkenes follow Markownikoff’s rule during addition of HX forming 2o and 3o alkyl halides predominantly through the formation of the most stable carbocation.
The less stable intermediate carbocation changes into more stable carbocation by 1,2-hydride or 1,2-methyl or phenyl shift.
3. Action of halogen acids, HX (HI > HBr > HCl > HF) on alcohols in presence of anhydrous ZnCl2 yields alklyl halides. The increasing reactivity of alcohols towards HX is CH3OH < 1o < 2o < 3o < benzylic and allylic
The mixture (1 : 1) of concentrated HCl and anhydrous ZnCl2 is called Lucas reagent.
4. R-Cl or R-Br on heating with NaI in acetone or methanol solution forms R-I (This reaction is called Finkelstein reaction).
5. Alklyl fluorides can be prepared from corresponding chlorides by the action of mercurous fluoride (Hg2F2) or SbF3 (Swarts reaction).
6. Substitution reactions: The alkyl halides are highly reactive due to high electronegativity difference between carbon and halogen atom, which provides polarity in C δ+–X δ – bond. Thus, carbon atom of C–X bond is easily attacked by a nucleophile and shows nucleophilic substitution.
The nucleophilic substitution may follow SN1 or SN2 mechanism.
7. SN2 reactions are one-step reactions and occur through the intervention of a transition state. They are always accompanied by inversion of configuration also called Walden inversion. If the alkyl halide is optically active, the product is also optically active. However, the product may or may not have the same sign of optical rotation.
8. SN1 reactions are two step reactions and occur through the intermediate formation of carbocations. Since carbocations are prone to rearrangements, therefore, during SN1 reactions sometimes rearranged products are formed.
9. Elimination Reactions:
(i) Dehydrohalogenation of alkyl halides: On heaing with alcoholic KOH, alkenes are formed. This elimination proceeds by E1 or E2 process.
(ii) Unimolecular elimination (E1): The R-X dissociates first to form carbocation and halide ion.
(iii) E1 involves the formation of most stable carbocation intermediate due to 1,2-hydride or methyl or phenyl shift giving unexpected products.
(iv) Bimolecular elimination (E2): It involves the formation of intermediate TS.
(v) E2 eliminaiton is stereospecific and follows trans-elimination.
10. R-X reacts with lithium dialkyl cuprate (R2CuLi) to form alkanes (Corey-House synthesis).
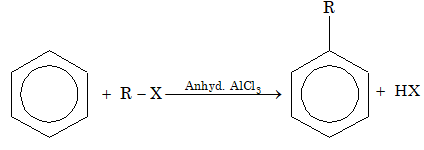
11. Friedel–Crafts reaction: R-X reacts with benzene in presence of anhydrous AlCl3 to form alkyl benzenes.
SOLVED PROBLEMS-1
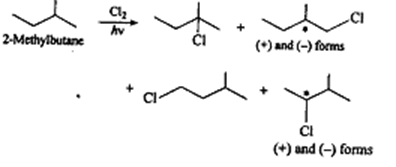
Prob 1. On monochlorination of 2-methyl butane, what will be the total number of chiral compounds
Sol. The number of monohalogenation products obtained from any alkane depends upon the number of different types of hydrogen it contains. The possible monochlorinated products of 2-methylbutane are
Prob 2. On treating a mixture of two alkyl halides with sodium metal in dry ether, 2-methylpropane was obtained. What are the alkyl halides?
Sol.
Thus, two alkyl halides are 2-chloropropane and chloromethane
Prob 3. Give the structures of two different alkyl bromides both of which yield the indicated alkene as the exclusive product of E2 elimination:
a) CH3CH=CH2 b) (CH3)2C = CH2 c) BrCH = CBr2
Sol.
a) and
b)
c)
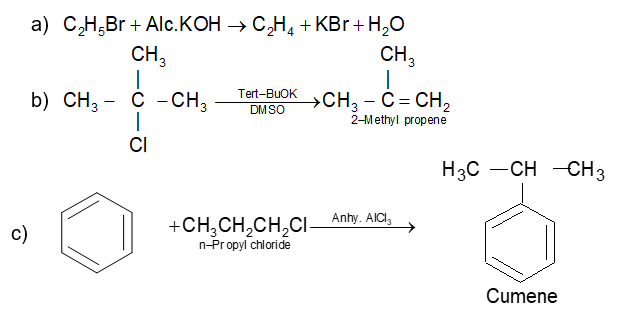
Prob 4. What happens when? Give equations only
a) Ethyl bromide is treated with alcoholic caustic soda.
b) 2-Chloro-2-methylpropane is treated with potassium tert-butoxide in dimethyl
c) Benzene reacts with n-propyl chloride in presence of anhydrous AlCl3.
Sol.
Prob 5. How will you synthesise?
a) Isopropyl bromide from n-propyl bromide
b) Vinyl bromide from ethyl alcohol
c) Methyl iodide from methane
Sol.
a)
b)
c)
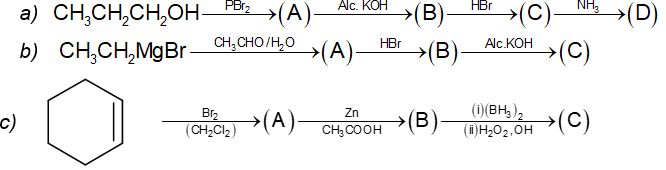
Prob 6. Complete the following by providing (A), (B) and (C)
Sol.
a) CH3CH2CH2Br (B) CH3-CH=CH2 (C)
b) (A) CH3CH2CH(OH)CH3; (B) CH3CH2CH(Br)CH3; (C) CH3CH = CHCH3
c) 
Prob 7. Identify the product (A), (B) and (C).
Sol. (A) C2H5Br (Ethylbronide); (B) C2H5OH (Ethylalcohol); (C) CHI3(Iodoform)
Prob 8. Explain the following
a) Chloroform is kept with a little ethyl alcohol in a dark brown coloured bottle.
b) Iodoform gives precipitate with AgNO3 on heating while chloroform does not.
Sol. a) When exposed to sunlight and air, chloroform slowly decomposes into phosgene and hydrogen chloride. Phosgene is extremely poisonous gas. As to prevent the decomposition, it is stored in dark brown coloured bottle and 1% ethyl alcohol is added. This retards the decomposition and converts phosgene into harmless ethyl carbonate.
b) C-l bond being less stable than C-Cl bond and thus undergoes fission on heating giving I– ions which combine with Ag+ ions to form a yellow ppt.
Prob 9. An halide C5H11X on treating with alcoholic KOH gives only pent -1-ene. What is halide?
Sol. Two structures expected (i) CH3CH2CH2CHXCH3 and
(i) CH3CH2CH2CH2X,
(ii) is correct because it gives only pent-1-ene while (i) gives a mixture CH3CH2CH = CHCH3 (major product) and CH3CH2CH2CH = CH2 (minor product)
Prob 10. Which hydrocarbon is consistent with the following formation? Molecular mass = 72 gives a single monochloride and two dichlorides on photochlorination.
Sol. Let the hydrocarbon be CnH2n+2, i.e., 12n + 2n + 2 = 72 or n = 5. The hydrocarbon is C5H12. As it forms a single monochloride, all the hydrogen atoms are equivalent. The compound is (CH3)4C, i.e., neopentane. Single chloro derivative is (CH3)3CCH2Cl and the two dichlorides are (CH3)3CCHCl2 and (CH3)2C(CH2Cl)2
SOLVED PROBLEMS-2
Prob 1. A mixture of 1-chloropropane and 2-chloropropane when treated with alcoholic KOH, it gives
(A) 1-propene
(B) 2-propene
(C) isopropylene
(D) a mixture of 1-propene and 2-propene
Sol: (A)
Prob 2. Consider the following reaction:
Identify the structure of the major product X
(A)
(B)
(C)
(D)
Sol: (B) Br is less reactive and more selective and thus, 3o free radical will be the major product.
Prob 3. In elimination reactions, i.e. in the formation of alkenes, the reactivity of halogens in alkyl halides follows the order
(A) I > Br > Cl (B) Cl > Br >I (C) Br > Cl >I (D) None of the above
Sol: (A) Iodine is better leaving group than bromine which in turn is better leaving group than chlorine. So, the reactivity order is, I>Br>Cl.
Prob 4. Ethyl bromide and isopropyl chloride can be distinguished by
(A) alcoholic AgNO3
(B) comparing their colours
(C) burning the compounds on spatula
(D) aqueous KOH solution
Sol: (A) C2H5Br gives yellow ppt. of AgBr whereas (CH3)2CHCl gives white ppt. of AgCl.
Prob 5. In alkyl nitrites, the oxygen of –O –N = O group is linked with carbon. An alkyl nitrite is
(A) an ester
(B) a nitro compound
(C) an amide
(D) a nitrile
Sol: (A) The compounds of oxyacids in which H atom of –OH group is replaced by an alkyl group are called inorganic esters.
Prob 6. A sample of chloroform before being used as an anaesthetic is tested by
(A) AgNO3 solution
(B) AgNO3 solution after boiling with alc. KOH
(C) Fehling’s solution
(D) Ammoniacal Cu2Cl2
Sol: (A) A white ppt. of AgCl is obtained if CHCl3 is impure. Pure CHCl3 does not give ppt. with AgNO3.
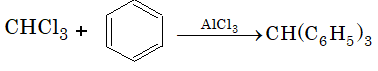
Prob 7. Chloroform when treated with benzene in the presence of anhydrous AlCl3, the product is
(A) chlorobenzene
(B) toluene
(C) mixture of ortho and para chlorotoluene.
(D) triphenyl methane
Sol: (D) This is Friedel–Crafts reaction.
Prob 8. 1,3-dichloro propane reacts with Zn and NaI gives (major product)
(A) propane (B) propene (C) cyclopropane (D) n-propyl Iodide
Sol: (C)

Prob 9. Haloform reaction cannot be used to prepare
(A) CHF3 (B) CHCl3 (C) CHBr3 (D) CHI3
Sol: (A) F2 reacts with NaOH to give oxygen difluoride (OF2) and not hypofluoride (OF–) ion needed for haloform reaction, hence CHF3 cannot be prepared.
Prob 10. Which process does not occur during formation of CHCl3 from C2H5OH and bleaching powder?
(A) Hydrolysis
(B) Oxidation
(C) Elimination
(D) Substitution
Sol: (C)
(Hydrolysis)
(Oxidation)
(Chlorination/Substitution)
(Hydrolysis)








