When two or more compounds combine in a stoichiometric ratio without any change in their ordinary valencies, they form addition or molecular compounds. These addition compounds can be divided into two categories. i.e.,
1) Double salts
2) Complex (or) coordination compounds
1) Double salts: These salts are formed by mixing of saturated solutions of the two (or more) compounds. They lose their identity in solution. When dissolved in water or melted, they furnish their constituent ions.
For example:
a) Mohr salt → Ferrous sulphate + Ammonium sulphate
b) Alum → Potassium sulphate + Aluminium sulphate
Karnallite → Potassium chloride + Magnesium chloride
The shapes and sizes of crystals of Mohr salt is different than that of ferrous sulphate and ammonium sulphate. In solution Mohr salts gives the tests of the NH4+, Fe2+ and SO42–.
2) Coordination or complex salts:
These are the compounds which retain their identities even when dissolved in water. On dissolving in water or melting they donot give all of their constituent ions.
For example:
Adding KCN into the ferrous cyanide solution results into potassium ferrocyanide.
When poto. Ferrocyanide is dissolved in water, it does give the test of CN– or Fe2+ ions instead it gives a characteristic test of a new ion [Fe(CN) 6] 4–, the ferrocyanide ion.
(i) An acceptor: Acceptor is the atom that can accept e– pairs from the donors. It is generally called as central metal ion or atom. Usually one or more neutral molecules or negative ions are attached to it by the dative bond. e.g., Fe2+ ion in [Fe(CN)6]4–.
(ii) A donor : A donor is an atom or molecule which can donate a pair of electrons. The molecules or ions having donor atoms are called ligands. They are attached to central metal atom.
e.g., CN– in [Fe(CN)6]4–.
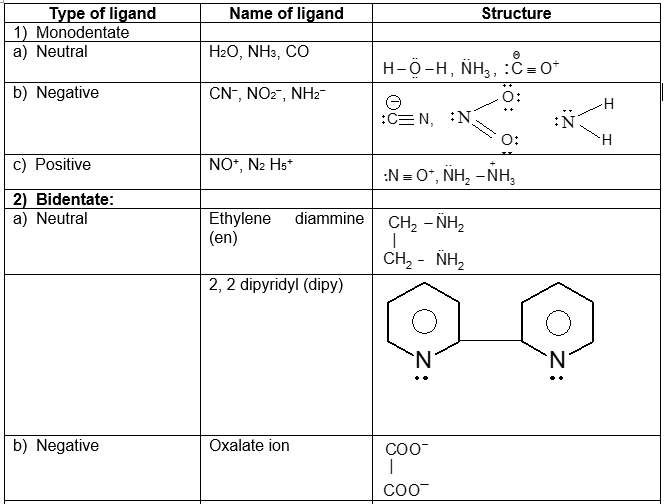
Ligands:
Neutral molecules (CO, NH3, H2O etc), cation (NO+, N2H5+ etc) and anions (Cl–, OH–, F– etc) which donate pair (s) of electron to any metal and forms covalent bond are called ligands. (Latin lizare = bind). The common donor atoms in most of the ligands are nitrogen, oxygen and sometimes carbon, halogens, phosphorus, sulphur etc. These atoms, who donate pairs are called donor atoms or donor sites of the ligand.
More examples:
|
Complex salt |
Central atom/ion |
Ligand |
Donor atom |
|
Fe2+ |
CN– |
C |
|
|
Ag+ |
NH3 |
N |
|
|
Cr3+ |
H2O |
O |
Types of ligands:
Depending on the no. of binding sites, the ligands have been classified as follows.
Chelating ligands: (Greek chele = claw):
When a polydentate ligand binds with a central metal atom by more than one binding site and thus forms a closed ring type structure involving metal atom, the resulting complex is called chelating complex and the ligand, chelating ligand.
e.g., Cu2+ complexes with ethylene diammine (bidentate), it binds by both the N atoms and form a chelate:
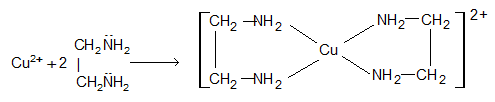
Characteristics of chelates:
1. Complexes of chelating ligands are more stable than non chelated complexes (chelating effect).
2. Chelating ligands with large groups form unstable rings in comparison to smaller groups (steric effect).
3. Chelating ligands without double bonds can form five membered ring (including metal as one of the member) while those with double bonds with six membered.
e.g., 5 membered.
6 membered → m
(ac = acetyl acetone)
Bridging ligand: A bridging ligand is one ligand which because of presence of more than 1 e– pair can bind with more than one metal atom at the same time.
e.g., etc.
Ambidentate ligand: A ligand which has more than one donor atoms, but at a time only either of them is used for complexation, is ambidentate ligand. e.g., -CN, – SCN, – NO2.
Note: Polydentate ligands have flexidentate character i.e., they are free either to use all of their available donor sites or only some of them.
e.g., EDTA through uses all the six donor sites while complexation but in some cases, it uses less than six also.
Coordination Sphere:
While writing the formula of complexes, the central metal atom and the non-ionizable ligands bound with it are closed in square bracket [ ]. Because this part is formed due to coordination between metal and ligands, it is called coordination sphere and the remaining part, outside the square bracket, which is bound by electrostatic forces and ionizes into cations or anions in solution is called ionization sphere.
e.g.,
Coordination Number:
It is the total number of ligands attached to the central metal atom in the coordination sphere. E.g., in, the C.N. of Pt is 6 and that of Ni in is 4.
Complexes in different coordination states and their stereochemistry.
|
Coordination No. |
Hybridization |
Example |
Stereochemistry |
|
2 |
sp |
Linear |
|
|
3 |
sp2 |
Trigonal planar |
|
|
4 |
sp3 |
Tetrahedral |
|
|
4 |
dsp2 |
Square planar |
|
|
5 |
sp3d |
Trigonal bipyramidal |
|
|
6 |
sp3d2 |
Octahedral |
|
|
7. |
sp3d3 |
Pentagonal pyramidal |
Werner theory:
Werner studied the structures of complexes like CoCl3.6NH3 and CuSO4.4NH3 and put forth his theory in 1893. The main points of his theory are:
1. Each complex compound contains two types of valences:
(i) Primary valency or principal valency or ionizable valency.
(ii) Secondary valency or non-ionizable valency.
2. Primary valency: it corresponds to the oxidation no. of central metal atom (ion) and is always satisfied by anions. It is represented by dotted lines.
3. Secondary valency: It is the coordination no. of the central atom (ion) and may be satisfied by either neutral or negative (rarely by positive) ligands. It is represented by thick lines.
4. Each metal has a fixed no. of secondary valencies (co-ordination no.) which depends on the size and charge of that central metal atom.
5. The ions that are attached by primary valencies are ionic in nature and dissociate into the solution while the secondary valencies being coordinate, do not.
6. Primary or ionic valencies are non directional while secondary are directional and they give rise to stereoisomerism in the complexes.
7. Each central metal atom tends to satisfy both of its valencies viz. primary and secondary.
8. Werner has pointed the coordination no. of a metal to be either four (tetrahedral or square planar arrangement) or six (in octahedral shape).
Note: Now, it has been established that coordination can be any integar between 2 to 9 for a metal atom.
While in attempt to satisfy their valencies, some groups (generally anions) are found to be joined as primary as well as secondary valencies.
On above basis Werner suggested following structures for various complexes of cobalt.
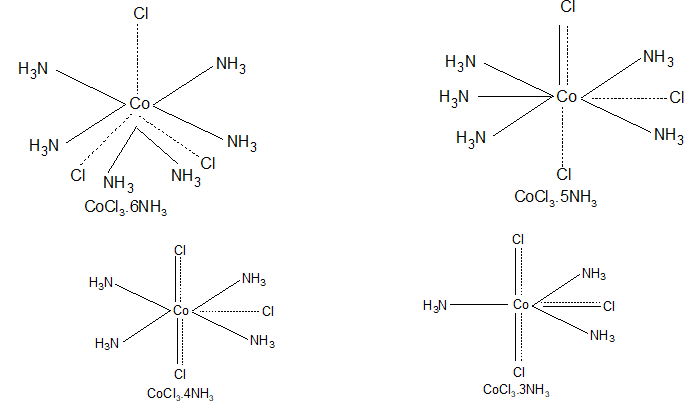
The valencies which are represented by thick as well as dotted lines have both ionic & covalent character and do not ionize in the solution. So the last compound i.e., CoCl3.3NH3 will not give white precipitate with AgNO3 solution.
|
Compound |
No. of ionizable chlorine atoms |
|
CoCl3.6NH3 |
3 |
|
CoCl3.5NH3 |
2 |
|
CoCl3.4NH3 |
1 |
|
CoCl3.3NH3 |
0 |
In modern terms the complex ion which is formed by coordination bonds called coordination sphere is written in square brackets and the ionizable ions outside the bracket. Hence the above four salts (complexes) can be written as
|
Werner formula |
Modern formula |
Cation |
Anion |
Total No. of Ions |
|
CoCl3.6NH3 |
[Co(NH3)6]Cl3 |
[Co(NH3)6]3+ |
3Cl– |
4 |
|
CoCl3.5NH3 |
[Co(NH3)5Cl]Cl2 |
[Co(NH3)5Cl]2+ |
2Cl– |
3 |
|
CoCl3.4NH3 |
[Co(NH3)4Cl2]Cl |
[Co(NH3)4Cl2]+ |
Cl– |
2 |
|
CoCl3.3NH3 |
[Co(NH3)3Cl3] |
Non electrolyte |
|
0 |
Effective Atomic Number (EAN):
It is the resultant no. of e– of the central metal atom (ion) of an complex after gaining e– from secondary valancies (ligands).
While coordinate bond formation there is gain of 2e– per biding of donor sites of the ligands.
Hence,
EAN = Atomic number of metal atom – No. of e– lost in cation formation + No. of e– gained from donor atoms.
EAN = Z – Ox. St. + 2L
L = Total no. of ligands or No. of donor sites of ligand(s)
Usually the EAN of an metal is the atomic no. of next inert gas of that very period, thus explaining the stability of the complexes so formed. Those with EAN not that of an inert gas are relatively less stable.
Illustration 1: EAN of Fe in K4 [Fe(CN)6 Atomic no. of Fe = 26
Solution: Oxidation no. of the central metal atom, Fe
4(+1) + (x) + (-1) 6 = 0 ⇒ x = + 2
No. of e– gained from 6CN– = 2 × 6 ⇒ 12
EAN = 26 – 2 + 12
= 36
Illustration 2: EAN of Cr in [Cr(NH3)6]Cl3
Solution: Z = 24
Ox. st. = + 3
2L = 12
EAN = 24 – 3 + 12
= 33 [three short of Kr]
Characteristics of coordination compounds:
1) Change in solubility: Solubility of a sparingly soluble salt may increase with the complex formation provided that the ion is involved in the complexation.
e.g., AgCl is precipitated in aq. solutions when AgNO3 is added to any solution having Cl– ions. This precipitate (sparingly soluble salt) becomes soluble if NH4OH is added to it due to complex, [Ag (NH3)2]Cl formation.
Similarly many other soluble ions bring about their precipitation with the formation of a complex.
e.g., Ni2+ are soluble in water and when, DMG (dimethyl glyoxime) is added to it, a red coloured precipitate, is obtained:
2) Change in conductivity:
Since, there is a change in the number of ions while complex formation, the conductivity changes abruptly.
e.g., (Conductivity decreases).
3) Change in colour: Almost all the complexation reactions are colour sensitive and show a variation in colour. Anhydrous CuSO4 is white, when it absorbs water or dissolved in water the colour changes to blue due to complexation of water molecules with CuSO4.
The colour change is observed due to changed energy states of the d orbitals of the central metal atom.
4) Change in pH: If complex formation is associated with either consumption or production of H+, the reaction is pH sensitive and change of pH informs complex formation.
e.g., when metal complexes with EDTA or Oxalate, the [H+] of the solution increases (due to change of -CO-O-H bond) and hence the pH decreases.
5) Change in chemical properties: When complex has been formed by the central metal atom and the ligand, both of them lose their chemical properties since metal atom achieves stable inert gas configuration by binding the ligand with it making them to behave as a different chemical identity. e.g., The ammonical solution of AgNO3, which involves formation complex [Ag(NH3)2]+ doesn’t give white ppt of AgCl with KCl solution, since Ag+ are no longer available.
Change in physical properties: The properties of the solutions of complexes which depend on the number of particles get changed because no. of particles decreases. The properties like osmotic pressure, lowering of vapour pressure, depression in freezing point and elevation of boiling point, shows a sharp decrease than the theoretical value. These properties are more widely used for the study of complexes in comparison to conductivity measurements which are not possible with neutral ligands.
Nomenclature of coordination compounds:
The International Union of Pure & Applied chemists has given a systematic nomenclature for coordination compounds in 1957 with few modifications in 1962 & 1971, they are exclusively used except some of more studied or familiar compounds, which will retain their trivial names e.g., K4[Fe(CN)6] as potassium ferrocyanide, K3[Fe(CN)6 potassium ferricyanide, Ni(CO)4 nickel carbonyl etc.
Following are some of the important rules for the nomenclature of inorganic complexes:
1. Order of listing central metal atom (ion) and ligand:
Ligands are named before the central metal atom, but while writing their chemical formula the order is reversed.
e.g., [Fe(CN)6]4–
Hexacyano ferrate (II) ion, cyano follows ferrate (II)
2. Order of listing ions:
If complex is salt (ionic), cation(s) is named before the anion(s)
e.g,
|
NaCl |
Sodium (I) chloride |
[Not a complex] |
|
[Cr(NH3)6]Cl3 |
Hexa ammonie chromium (HI) Chloride |
|
|
[K2[PtCl6] |
Potassium hexachloroplastinate (IV) |
|
Oxidation no. of central metal atom:
In every compound, the oxidation no. of central atoms is to be mentioned by Roman numeral written in parenthesis just after the name of the metal atom. Zero oxidation state is indicated by (O) and negative by putting negative sign before the Roman numeral (e.g., – I).
K3[Fe(CN)6] Potassium hexacyanoferrate (III)
Fe(Co)5 Tetracarbonyl iron (O)
Na{Co(CN)4] Sodium tetracyanocobaltate (-I)
Termination (ending) of names of complexes:
Anionic complexes, those having negatively charged coordination sphere, end with the name of metal suffixing -ate and in cationic or neutral complexes, the name of metal is retained.
K2[Ni(CN)4] Potassium tetracyanonickelate (II)
[Co(H2O)6](SO4)3 Hexa aqua cobalt (III) sulphate
[Pt(NH3)2Cl2] Diamminedichloroplatinum (II)
Order of ligands:
If different types of ligands are present in a complex, they are arranged in the order of:
i) Negative ligand
ii) Neutral ligand
iii) Positive ligand
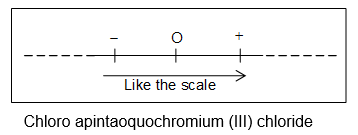
e.g., [Cr (H2O)5Cl] Cl2 Chloro apintaoquochromium (III) chloride
Note; Whole of the coordination sphere is always written as a single word without any break, hyphen or comma.
In case, these are several negative ligands in a complex, they are named alphabetically.
e.g., Na2 [Pt (NO2)2Cl4] Sodium tetrachlorodinitroplatinate(IV)
Similarly, if several neutral ligands are present, they are enlisted as:
i) H2O
ii) NH3
iii) then all others in alphabetically
e.g., [Pt(NH3)4 (NO2)Cl]SO4 Chloronitrotetraammineplatinum (IV) sulphate
Name of ligands: Neutral ligands are named as molecules with few exception enlisted under column B.
|
A : General |
B : Exceptions |
||
|
Ligand |
Name |
Ligand |
Name |
|
(CH2NH2)2 |
Ethylene diammine |
H2O |
Aquo |
|
|
|
NH3 |
Ammine |
|
(C6H5)3P |
Triphenyl phosphine |
CO |
Carboxyl |
|
(C2H5)2NH |
Diethyl ammine |
CS |
Thiocarbonyl |
Positive ligands end in ‘- ium’.
|
Ligand |
Name |
|
NO+ |
Nitrosylium |
|
N2H5+ |
Hydrazinium |
|
Cl+ |
Chloronium |
All negative ligands end with ‘0’.
|
Anion (–ide) |
Symbol |
Ligand name (–0) |
Anion |
Symbol |
Ligand name –ate) |
|
Chloride |
Cl– |
Chloro |
Carbonate |
CO32– |
Carbonato |
|
Oxide |
O2– |
Oxo |
Oxalate |
Oxalato |
|
|
Oxanide |
CN– |
Cyano |
Nitrate |
Nitrato |
|
|
Hydride |
H– |
Hydrido |
Acetate |
CH3COO– |
Acetato |
|
|
|
|
Nitrite |
Nitro or nitrite |
When group bond with N atom (N-donor) it is called nitro and when by O atom (O-donor), nitrito is the name.
Note: The spelling of ammine with two m’s differentiate it with organic amines. Its so since derived from ammonia.
Numerical prefixes:
(i) When more than one ligands of same kind, are present, their number is indicated by greek prefixes, di, tri, tetra, penta, hexa et.
(ii) If chelating ligands are the ligand already having di, tri, tetra etc in their names are present 2, 3, 4….. times, the prefixes bis, tris, tetra etc are used before the names of ligands, which are written in paranthesis.
e.g., Potassium trioxalatoalluminate (III)
Dichlorobis (ethylenediamine) cobalt (IV) sulphate
Point of attachment of ligands:
For ambidentate ligands, the donor atom which forms coordinate bond is designated by the symbol of its element in italics and hyphened between ligand and metal atom or so.
e.g.,
Ammonium hexathiocyanato-S-platinate(IV)
Tetramethylammonium tetrathiocyanato-N-cobaltate (II)
Molecules (solvent) of crystallization:
Generally water or any other solvent molecule, when present in the crystal of a complex as lattice component, is written after the name of complex and the no. of molecules is indicated by Arabic numerals between the complex and solvent molecule.
e.g., Aluminium (III) chloride – 4- ethanol
Dichlorotetra aquochromium (III) chloride-2- water
(Note) Bridged Polynuclear complexes:
Bridging ligands, which join the two centres of coordination, are prefixed by Greek letter μ. Each briding ligand is prefixed by μ, however if they are of same kind di, tri etc can be used before the name of the ligands.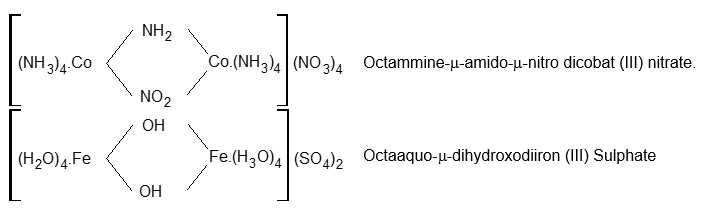
Illustration 3: Write the IUPAC names of the following complexes.
Cationic complexes
|
Problem |
Solution |
|
Hexaaquacobalt (II) sulphate |
|
|
Tetraamminezine (II) chloride |
|
|
Tetrachloropalladium (VI) sulphate |
|
|
Dichlorotetraaquochromium (III) perchlorate |
|
|
Nitritopentaammine cobalt (III) chloride |
Anionic complexes
|
Problem |
Solution |
|
Potassium dichloroargentate (II) |
|
|
Ba[(CuCl4] |
Barium tetrachlorocuprate (II) |
|
Na [Mn(CO)5] |
Sodium pentacarbonylmanganate (-I) |
|
(NH4)2 [TiCl6] |
Ammonium hexachlorotitanate (VI) |
|
Na[Fe(EDTA)] |
Sodium ethylene diammine tetra acetatoferrate (III) |
|
K [BF4] |
Potassium tetrafluroborate(III) |
|
[Pt (NH3)4Cl2] [PtCl4] |
Dichlorotetra ammine platinum (IV) tetrachloroplatinate |
Neutral complex
|
Problem |
Solution |
|
Fe(CO)5 |
Pentacarbonyl iron (O) |
|
[Pt (NH3)2Cl2] |
Dichlorodiammine platinum (II) |
|
[Co(en)3] |
Tris(ethylene diammine) cobalt (O) |
Isomerism in Co-ordination Compounds:
Similar to the isomerism exhibited by organic compounds, specific orientation of ligands in space around the central atom gives rise to isomerism in co-ordination compounds. The isomerism in co-ordination compounds can be classified as:
1. Structural isomerism 2. Stereo isomerism
1. Structural isomerism
a) Ionization isomerism:
Complexes having same empirical formula but producing different ions in solution state are ionization isomers and the phenomenon is ionization isomerism.
Such an isomerism is due to the interchange of groups between the co-ordination sphere of the metal ion and the ions outside the sphere.
Example:
and
Pentaamminebromocobalt(Ill) sulphate Pentaamininesulphatocobalt(III) bromide
(Violet) (Red)
The violet compound in aqueous solution gives precipitate with BaCI2 and not with AgNO3 solution, whereas red compound gives a precipitate with AgNO3 solution and no precipitate with BaCI2 solution.
b) Hydration isomerism: This isomerism arises when different number of water molecules are present within and outside the co-ordination sphere. For example, 3 hydration isomers of CrCl3 6H2O are;
c) Salt or Linkage isomerism: This isomerism arises when ligand has two possibilities in its mode of attachment to the metal atom. For example, the ligand —NO2 binds with metal atom through N or 0 in the following example.
d) Polymerisatlon isomerism: This isomerism arises when compounds have the same stoichiometric composition but different molecular composition (which is an integer multiple of stoichiometric composition), e.g., . This is not considered as true isomerism.
e) Co-ordination isomerism: This isomerism exist in polynuclear complexes (where both complex anions and complex cations are present) by partial or complete exchange of ligands. For example,
Stereoisomerism:
Two compounds are called stereoisomers when they contain the same ligands in their co-ordination sphere but differ in their l arrangement. Stereoisomerism is further classified as geometrical and optical isomerism.
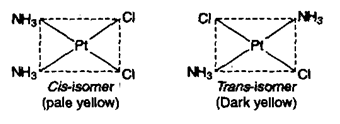
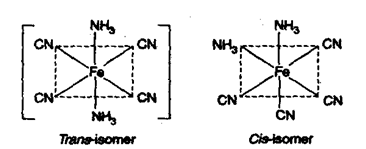
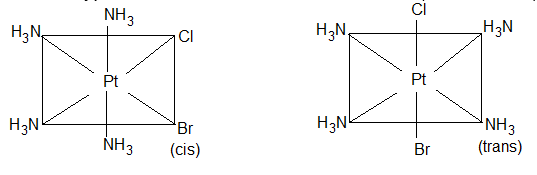
1) Geometrical isomerism: This type of isomerism arises due to different positions occupied by ligands around the metal When two similar ligands are adjacent to each other, then isomer is referred as cis-isomer and when they are at opposite position then trans-isomer. Geometrical isomerism of square planar (Co-ordination number = 4) and octahedral complex has been discussed below.
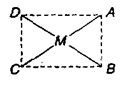
a) Square planar complex: Positions A – B, B – C, C – D and A – Dare adjacent to each other and are Cis-with respect to each other. Positions A – C and B – D are trans to each other. (i) Complexes of type do not show geometrical isomerism. (ii) Square planar complexes having formula show geometrical isomerism and exist in two forms.
[(NH3)2Cl2] resembling to formula exists in cis and trans forms.
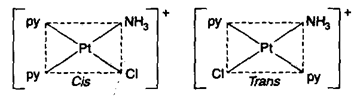
[Pt(Py)2NH3Cl]+ resembling to formula exists as
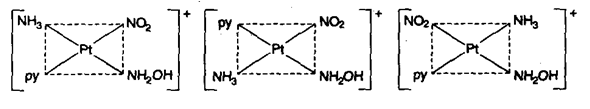
(iii) Complexes of MABCD type exist in 3-geometrical isomeric forms e.g.,
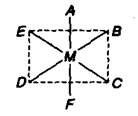
(b) Octahedral Complexes:
Positions A – B, B – C, C – D A – D etc. which are at 90° to each other are cis with respect to each other while A – F, B – D, C – E (at 1800 to each other) are trans positions.
(I) Complexes of type will show geometrical isomerism.
Example:
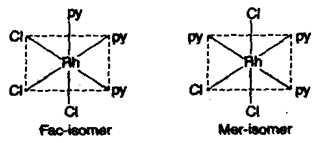
(ii) Facial (fac) and meridional (mer) isomers:
Such type of geometrical isomers are in compounds of type type. If three ligands of same type are at the corners of a triangular face then it will be fac-isomer. If they are at the corners of a square plane then it will be mer-isomer.

e.g., [RbCl3(py)3]
2) Optical Isomerism: Optical isomerism is due to the absence of elements of symmetry (plane of symmetry, axis of symmetry or centre of symmetry) in the complex. If the molecule possesses any of these symmetry elements, the compound will not be optically active. An optically active compound cannot be superimposed on its mirror image. It has the property of rotating the plane of polarized light either to its right (called dextro) or to its left (called Iaevo). If polarized light remains unaffected, the compound is inactive or racemic, i.e., it is composed of 50% dextro and 50% Iaevo form.
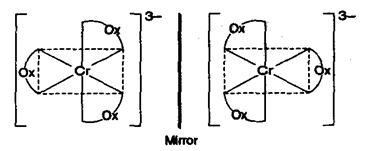
(a) Optical isomerism in octahedral complexes:
(i) Complexes of type type show optical isomerism where A is symmetrical bidentate ligand.
e.g.,Oxalato (symmetrical bidentate ligand)
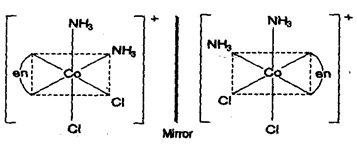
(ii) Complexes of type also shows optical isomerism. Here A = symmetrical bidentate ligand while B and C are monodentate ligands.
e.g.,
(iii) Complexes of hexadentate ligands like EDTA, also shows optical isomerism.
eg., [Co(EDTA)]–
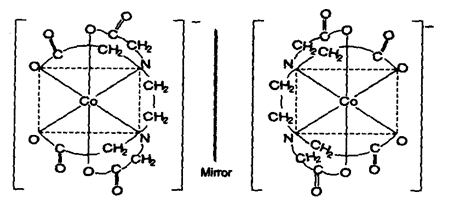
(b) Optical Isomerism In tetrahedral complexes:
Tetrahedral complexes also show optical isomerism but in complexes of type of the MABCD resolution is not possible. However complexes of type may be resolved where A is unsymmetrical bidentate ligand.
e.g., [Ni(gly)2] glycinate ligand.
In analogy with tetrahedral carbon, it is expected that a tetrahedral metal complex with four different groups attached to the metal ion, should exhibit optical activity. However, it has not been possible to isolate optically active dextro and laevo forms of such a complex because of its labile nature.
Illustration 4: How many geometrical isomers are possible for the following complex?
Solution: No isomers are possible.
Because this is an octahedral complex of the type MAB5.
Illustration 5: How many Geometrical isomers are possible for the complex ?Write the structures?
Solution: This is of the type of MA4BC and Cis, trans isomers (two isomers) are possible.
Bonding in Co-ordination Compounds:
Werner was the first to explain the bonding in complex compounds. Later on Sidgwick gave his concept. However, these were not able to explain all the facts about complexes. Four distinct approaches have been given to explain properties of complex compounds such as colour, geometry and magnetic properties. These are:
1. Valence bond theory
2. Crystal field theory
3. Molecular orbital theory
4. Ligand field theory
Valence bond theory:
Since, majority of the complexes are formed by the transition metals which have their ‘d’ orbitals incomplete, valence bond theory takes into account the hybridization of orbitals. Since, the d-orbitals of the penultimate quantum shell, in the case of transition metals, are near in energy to s- and p-orbitals of the outermost shell, various kinds of hybridization are possible.
Concepts of Valence bond theory:
• The central metal atom provides a number of empty orbitals equal to its co-ordination number for the formation of covalent bonds (or more accurately co-ordinate bonds) with ligand orbitals.
• The empty orbitals of the metal ion hybridize to give an equal no. of’ hybrid orbitals of equivalent energy.
• The metal atom or ion can use (n-1) d, ns, np, nd orbitals for hybridization to yield square planar, tetrahedral or octahedral geometry.
• These hybridized orbitals then overlap with ligand orbitals which can donate an electron pair for bending.
• The overlapping may result in a bond or possibly a ligand → metal co-ordinate bond.
• The ligand’s electron pair enters the metal ion orbital while still maintaining the electronic configuration originally present in ligand.
• Each ligand donates a pair of electrons to the central metal ion.
• The non-bonding metal electrons present in the inner orbitals do not take part in chemical bonding.
• If the complex contains unpaired electrons, the complex is paramagnetic in nature whereas, if it does not contain unpaired electrons, the complex is diamagnetic in nature.
• Under the influence of a strong ligand (like, NH CN– , CO, etc.), the electrons can be forced to pair up against the Hund.’s rule of maximum multiplicity.
Geometry (shape) and magnetic nature of the complexes (Application of valence bond theory):
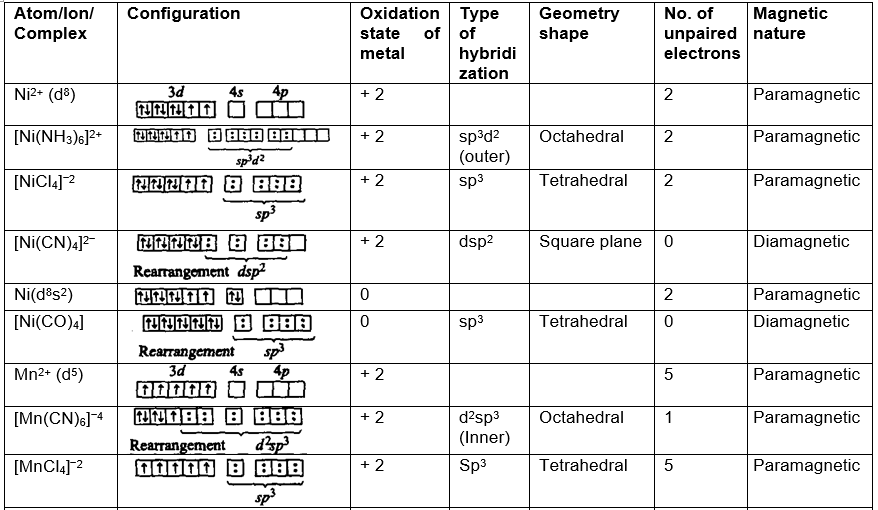
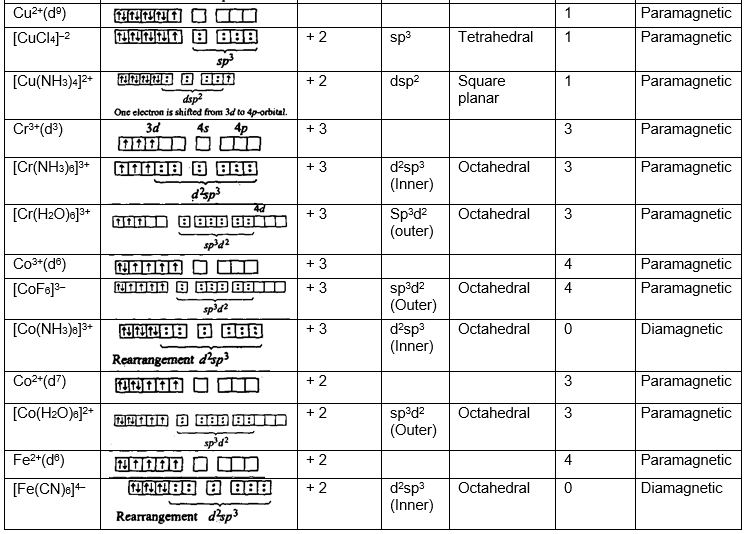
Limitations of valence bond theory: The theory could not explain:
(i) The colour of transition metal complexes.
(ii) The optical absorption spectra.
(iii) The detailed magnetic properties.
Crystal field theory:
• The ligands, are assumed to be point charges.
• The metal cation is surrounded by the ligands with the lone pair of electrons.
• The attractions between metal ion and ligands are purely ionic.
• The valency electrons of metal are repelled by the negatively charged ligands, so that they occupy those d-orbitals which have their lobes away from the direction of ligands. This effect of ligands is particularly marked on d-electrons and it depends on number of electrons, their arrangement and on nature of ligands.
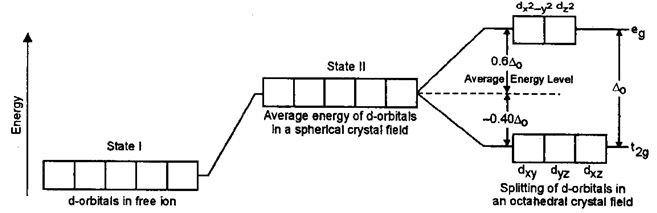
• The energy difference between two sets of d-orbitals is determined by Δ0 and called crystal field splitting energy.
• The field strength of some ligands is in the order of
• In octahedral field, d-subshell split into eg, t2g sets, here energy of eg, set is greater than the energy of t2g set
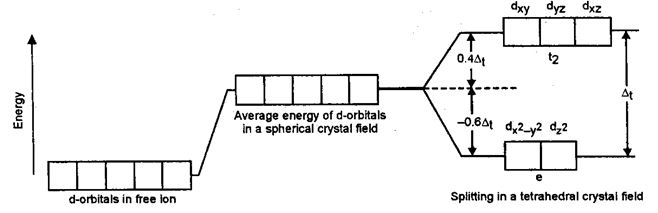
• In tetrahedral field t2g set have more energy than the eg set of orbitals (explained in solved subjective problem 9).
• We have Δt = Δ0 , where Δt is crystal field splitting energy in tetrahedral complex
Δ0 is crystal field splitting energy in octahedral complex
Inner orbital complex:
The complex, which is formed by the use of inner d-orbitals for hybridization is called inner orbital complex.
Strong ligands like CO, CN–, NO2– forces the unpaired electrons to pair up and forms inner orbital complexes.
Outer orbital complexes:
The complex which is formed by the use of outer d-orbitals for hybridization is called an outer orbital complex.
Weak ligands like I–, Br–, Cl–, F–, H2O ….. do not disturb the electronic configuration of the metal and forms outer orbital complexes.
Uses of co-ordination compounds:
1) Used in electroplating industry.
2) Micronutrients for plants, like Zn, Fe, Mg, etc, ….. are supplied in the form of soluble chelate complexes.
3) The formation of soluble complexes is used in the extraction of metals like Ag & Au.
4) Estimation of Ca+2 and Mg+2 in water is done volumetrically using complexing agent EDTA
5) The platinum complex, [Pt Cl2(NH3)2], known as cis platina is used in chemotheraupetic treatement of cancer.
6) Many bio-catalysts are complex enzymes containing metal ions.
7) Applications In qualitative analysis
(a) The separation of Ag+ and in the first group of qualitative chemical analysis is based upon the formation of a soluble complex of silver chloride with ammonia.
(b) The detection of copper in the presence of cadmium in the second group of chemical analysis can be made by cyanide complexes,
.
The copper complex is more stable than the cadmium complex and, therefore, when H2S gas is passed through a mixture of these two complexes, only Cd(II) gets precipitated as CdS (yellow) leaving a blue coloured solution of copper complex.
(c) Ferric ions can be detected by means of the red coloured sulphocyanide complex.
(d) Some complexes serve as the confirmatory tests for various cations and anions. For example,
(i) Test for sulphide: Sodium nitroprusside gives a red colour with sulphides.
(ii) Test for : Nessler’s reagent, K2[HgI4], form a brown complex (NH4)2[HgI4] with ammonium ions.
(iii) Copper salts give blue colour with ammonia owing to the formation of blue complex.
(iv) Potassium ferrocyanide gives a red coloured ppt. of copper ferrrocyanide with copper salts.
(v) Nickel gives a violet or a brick red ppt. with dimethylglyoxime (DMG).
(vi) Applications in quantitative analysis: Complex compounds have an important use for quantitative estimation of various cations and anions. For example, Ni can be estimated gravimetrically as nickel dimethyl glyoximate. EDTA (ethylenediamminetetraacetic acid) has been used in the estimation of zinc by volumetric methods.
Illustration 6: What is the order of wavelength of absorption in the visible region of the following?
Solution: The increasing field strength of the ligands is in the order of H2O < NH3 < NO2–
The energies absorbed for excitation will be in the order
and The wavelength order is
Illustration 7: is paramagnetic while Ni(CO)4 is diamagnetic though both are tetrahedral. Why?
Solution: In are weak and the electronic configuration of Ni+2 is
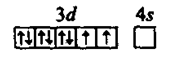
In Ni(CO)4, the electronic configuration of Ni(0) is

Illustration 8: Explain why Ni(CO)4 posses tetrahedral geometry, while [Ni(CN)4]–2 is square planar.
Solution:

Due to rearrangement of electronic configuration, sp3 hybridisation takes place, hence shape is tetrahedral.

Here, the hybridization is dsp2 and hence square planar.
Organometallic Compounds:
Organic compounds in which metal atom is directly linked to carbon atom are known as organometallic compounds. For example, NaC = CNa is an organometallic compound as Na is directly linked to carbon whereas C2H5ONa, Ti(OC2H5)4 are not organometallic compounds since the metal atom is linked to carbon through oxygen.
A wide varieties of metals have been used to prepare organometallic compounds, important ones are magnesium, zinc, cadmium, alkali metals (sodium, potassium and lithium) and lead. Some representative organometallic compounds are,
Alkyl or aryl magnesium halides (RMgX or ArMgX) are also called Grignard reagents.
ClassifIcation of organometallic compounds:
1) Ionic compounds of electropositive metals: These, compounds are mostly formed between the electropositive metals and the carbon compounds which are mostly acidic in nature. Thus, organometallic compounds of alkali metals and alkalme earth metals consist of ions or ion pairs.
These compounds are normally insoluble in hydrocarbon solvents. They are very reactive towards air and water. The stability of these compounds depends upon the structure of the carbon containing part of the compound.
2) σ – bonded complexes: In a a-bonded complex, a metal and a carbon atom of the ligand are joined together with a sigma bond. This means that the ligand contributes one electron and is therefore, called one electron donor. Tetramethyl tin, (CH3)4 Sn and trimethyl aluminium, (CR3)3 Al are σ-bonded organometallic compounds.
3) π-bonded complexes: These are organometallic compounds which involve the use of its π-bonds present in organic compounds. For example, Zeise’s salt, ferrocene and dibenzene chromium are organometallic compounds of this type. In all these compounds the π-electrons of the organic compound interact with the metal ion and thus, occupy one of the co-ordination sites. For example, in ferrocene and dibenzene chromium, the iron and chromium atoms are sandwiched between two aromatic rings. The number of
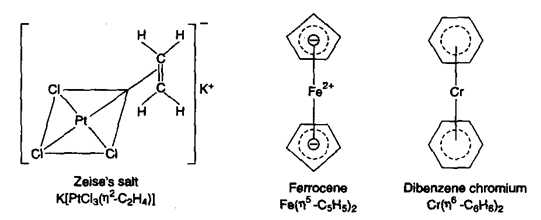
carbon atoms involved in the formation or π-complexes with metals is indicated by the power of h (pronounced as eta). For example, ferrocene is represented as indicating that 5-carbon atoms or cyclopentadienyl anion are involved in π-complexation with the metal. Similarly, one can write dibenzene chromium as indicating that all the six carbons of benzene are involved in n-complexation with chromium.
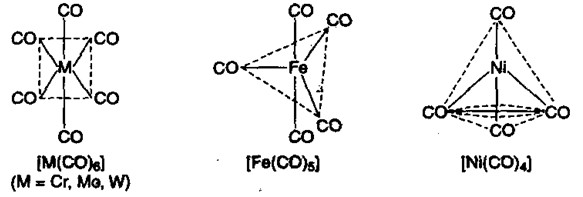
4) Metal carbonyls: These are the complexes where carbon of carbon monoxide donates a pair of electrons to the metal. Nickel carbonyl and iron carbonyl are the common examples.
In metal carbonyls the oxidation state of the metal is zero. These metal carbonyls may be monomeric, bridged or polynuclear.
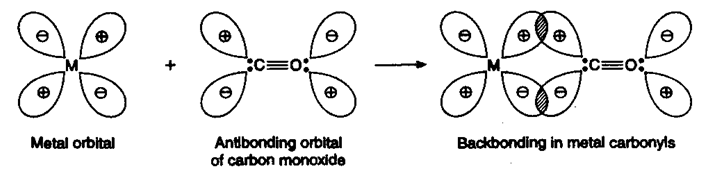
(i) Bonding in metal carbonyls: The metal-carbon bond in metal carbonyls has σ as well as p character.
(a) σ-Overlap: In a sigma bonded complex, the lone pair of electrons is present on the bonding orbital of carbon monoxide. This bonding orbital containing lone pair interacts with the empty d-orbital of the metal to form a metal-carbon bond as shown below:
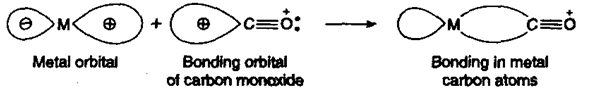
(b) π-Overlap: In addition to this, the antibonding orbitals of CO can also overlap with the filled d-orbitals of the metal resulting in back bonding as explained earlier. Thus, metal carbonyls become much more stable compounds due to multiple bonding in them.
Synthesis of organometallic compounds:
Some of the important methods to generate metal-carbon bond are given below:
1) By direct reaction of metals:
2) By using an alkylating agent: Grignard reagent and alkyl lithium on reaction with most of the metal and non-metal halides in the presence of ether as solvent yield other organometaflic compounds.
3) Preparation of π-complexes:
(a) Preparation of ferrocene : Ferrocene is prepared by the reaction of iron oxide with cyclopentadiene at 523 K.
It can also be prepared by treatment of cyclopentadienyl magnesium bromide with ferrous chloride.
(b) Preparation of Zeise’s salt:
(c) Preparation of dibenzene chromium:
4) Preparation of metal carbonyls: (a) Nickel carbonyl is formed when finely divided nickel reacts with CO at room temperature.
(b) Iron carbonyl is formed when iron reacts with CO at high pressure and temperature.
Applications of organometallic compounds:
(1) [Ni(CO)4] is formed during the purification of nickel in metallurgy.
(2) Grignard reagent and several other organometallics are good reagents for the synthesis of compounds.
(3) Ethyl mercury chloride is used to prevent the infection of young plants.
(4) Many organometallic compounds are found to be good catalyst for hydrogenation of unsaturated compounds.
(5) Triethyl aluminium in conjuction with TiCl4 is a well-known catalyst for Ziegler-Natta polymerisation.
(6) Organoarsenic compounds are used in the treatment of the disease syphilis.
(7) As homogeneous catalyst, e.g., (Ph3P3) RhCI (Wilkinson’s catalyst) for hydrogenation of alkane to alkane.
(8) Tetraethyl lead (TEL) is an important anti-knock compound present in leaded gasoline. TEL is a colourless, strongly smelling liquid (b.p, about 200°C), insoluble in water but soluble in ether, gasoline, etc. It is used as an ‘anti-knock’ for motor fuels. When mixed with petrol (1 part in 4000 parts) it raises the octane number of petrol and thus, prevents the knocking in internal combustion engine.
(9) Butyl lithium is an excellent catalyst for anionic polymerisation.
Illustration 9: Out of Zn(CN)2, Ni(CO)4, NiCO3, which is an organo metallic compound? Why?
Solution: In Ni(CO)4, Ni is attached to carbon of carbonyl group, NiCO3 is ionic
Ni(CO)4 is O.M.C.
Illustration 10: Cr(H2O)6]Cl3 has a magnetic moment of 3.83 B.M. Write the correct distribution of 3d electrons in the Cr present in the complex.
Solution: Magnetic moment value indicates that there are 3 unpaired e– s present in Cr.
As the complex is octahedral, the 3 electrons will be present in 3dxy, 3dyz, 3dxz orbitals.
The configuration is
SOLVED PROBLEMS-1
Prob 1. State the oxidation numbers of the metals in the following complex compounds.
a) b)
Sol: a) x + 1 (-1) + 0 + 2 (0) = + 2 x = + 3
b) x + 2 (-1) + 2 (-2) = -3
x = + 3
Prob 2. A metal complex having composition Cr (NH3)4Cl2Br has been isolated in two forms A & B. The A form reacts with AgNO3 to give a white ppt, readily soluble in dilute aqueous NH3. The B form gives a pale yellow ppt., soluble in conc. NH3. Write the formulae of A & B forms?
Sol: A form forms white ppt. Cl is out side the co-ordination sphere so that it forms AgCl (white ppt) and the coordination number of Cr is 6.
B form gives yellow ppt (AgBr) Br is out side the coordination, sphere
Prob 3. Why hexacyanocomplexes of metals in their +2 oxidation state are usually yellow, but the corresponding hexa aqua complexes are often blue or green?
Sol: CN– ligand is stronger and hence it generates large energy gap between the two sets of d-orbitals. Hence they absorb high energy indigo light and appears as yellow.
H2O ligand generates less energy gap between the two sets of d-orbitals, while the crystal field splitting. Hence they absorb orange (or) red light and appears as blue (or) grey.
Prob 4. A 0.01m complex of CoCl3 + NH3 (1:4 ratio) is found to give freezing point of -0.03720C. Then suggest the formula of the complex, (Kp of water = 1.86)
Sol: The depression in freezing point = 0.03720C
For 100% dissociation, we got I = 2 two ions are produced from the complex.
The possible formula is
Prob 5. Arrange the following in the increasing order of electrical conductivity
Sol: The conductivity of solution depends on the number of ions produced by the complex.
the order is
Prob 6. x, y and z are three complexes of chromium (III) with the molecular formula CrCl3H12O6. All the three complexes have water and chloride ions as ligands. Complex ‘X’ does not react with conc. H2SO4, where as complexes y and z lose 6.75% and 13.5% of their original weight respectively on reacting with conc., H2SO4. Identify x, y & z?
Sol: No reaction with H2SO4 NO H2O is outside the coordination sphere and coordination number of Cr should be 6.
b) For y Molecular wt. of complex = 266.5
100g complex looses 6.75 gr . wt (H2O)
266.5gr complex ?
formula of y is
c) for z 100g complex looses 13.5gm wt. (H2O)
266.5g complex ?
formula of z is
Prob 7. The EAN of a metal carbonyl M(CO)x is 36. The atomic number of metal is 24. The value of x is?
Sol: EAN = 24 + x (2) = 36 x = 6
Prob 8. What is Wilkinson’s catalyst?
Sol: A mixture of TiCl4 and Al(C2H5)3 is known as Wilkinson’s catalyst.
Prob 9. Draw a figure to show the splitting of degenerate of orbitals in an
a) Octahedral crystal field b) Tetrahedral crystal field?
Sol: a) In an octahedral crystal field, the ligands will approach the central metal, along the axis.
Hence, eg orbitals will suffer more electrostatic repulsions and hence their energy will be greater than t2g orbitals.
b) In tetrahedral field, t2g orbitals are pointing close to the direction in which ligands are approaching. As a result energy of t2g orbitals will be more than that of eg orbitals.
Prob 10. How the colour of complex ions can be explained?
Sol: In, complex ion, in the presence of ligand atmosphere, d-orbitals will split into set and eg set orbitals, each set having different energy. The electrons which are usually present in the lower set of orbitals absorbs suitable light and are promoted to set having more energy. These intra d-d transitions are responsible for the colour.
SOLVED PROBLEMS-2
Prob 1: AgCl dissolves in a solution of NH3 but not in water because
(A) NH3 is better solvent than H2O
(B) Ag+ forms a complex ion with NH3
(C) NH3 is a stronger base than H2O
(D) the dipole moment of water is higher than NH3
Sol: (B)
Prob 2: Which will not show geometrical isomerism?
(A) [Co (NH3)4]Cl2 (B) [Co (en)3]3+ (C) [Co (en)2 Cl2]Cl (D) [Cr (NH3)4Cl2]Cl
Sol: (B) It is of the type MA3
Prob 3: Which of the following compound is used to detect the presence of Fe3+ ions in solution?
(A) KCN (B) KNO3 (C) K4[Fe (CN)6] (D) K3[Fe (CN)6]
Sol: (C) It gives ferric ferrocyanide, which is having characteristic color (Prussian blue)
Prob 4: The geometry of Ni (CO)4 and Ni (PPh3)2Cl2 are
(A) both square planar
(B) tetrahedral and square planar respectively
(C) both tetrahedral
(D) square planar and tetrahedral respectively
Sol: (B) In Ni(CO)4, sp3 and in Ni (PPh3)2Cl2, dsp2 hybridization takes place.
Prob 5: The structure of iron penta carbonyl is
(A) square planar
(B) trigonal bipyramidal
(C) triangular planar
(D) none of the above
Sol: (B) In FeCO5, Fe undergoes dsp2 hybridization.
Prob 6: The two compounds sulphatopentaammine cobalt (III) bromide and sulphato pentaammine cobalt (III) chloride are
(A) linkage isomers
(B) ionization isomers
(C) co-ordination isomers
(D) no isomers
Sol: (D) Both are not having same ligands.
Prob 7: Formula of ferrocene is
(A) (B) (C) (D)
Sol: (D) Factual
Prob 8: The total number of possible isomers of the compound [Cu (NH3)4] [PtCl4] are
(A) 3 (B) 4 (C) 5 (D) 6
Sol: (B) i.e.,
Prob 9: In case of [Ti (H2O)6]3+ ion, the net CFSE value will be
(A) –0.4 o (B) –0.6 o (C) zero (D) 0.2 o
Sol: (A) One unpaired electron is present in lower energy orbital








