Introduction:
The study of the role of metals and non-metals in biological process and systems is an emerging branch of chemistry and is known as bio-inorganic chemistry.
Brief survey of the importance of metal ions in biomolecules:
About 40 elements are known to be involved in the life processes. Of these about 25 elements are found essential for healthy human life. These 25 elements can be divided as
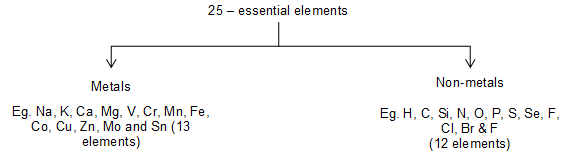
But of these elements Mn, Fe, Co, Cu, Zn and Mo are abundant. The approximate composition of some elements of human body weight (of 65 kg) is as follows>
Importance of metals in biological system:
Metals are found to pay important roles in bio-chemical processes.
For example – the ions of Fe, Co, Mn, Zn, Cu and Mg are associated with proteins.
The metal proteins which are also known as chromo proteins, are usually enzymes. In these enzymes metallic ions act as activators.
Functions of metal ions:
The important biochemical roles of some metal ions are given below.
(i) Na, K : These help to maintain osmotic pressure constant on either side of the cell wall. These are also necessary to maintain the sensitivity of the nerves and to control the muscle actions.
(ii) Mg, Ca : Mg2+ ions is the central ion in chlorophyll. Ca2+ ions are necessary for the formation of cell wall structures. These are also necessary for nerve impulse transmissions.
(iii) Zn : Zn – metalloenzymes catalyse peptide hydrolysis and maintain HCO3/CO2 equilibrium.
(iv) Fe: Biologically ion is the most important transition element. It is involved in several different processes.
a) As an oxygen carrier in the blood of mammals, birds and fishes (haemoglobin) i.e., Fe2+ is the central ion in haemoglobin and other hemes.
b) For oxygen storage in muscle tissues (myoglobin).
c) As an electron carrier in plants and bacteria (cytochromes).
(v) CO : CO3+ is the central ion in vitamin B12.
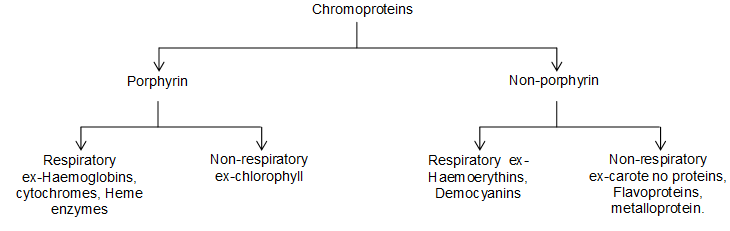
Proteins, enzymes and porphyrins are the important constituents of living cells. These contain Fe, Co and Mg besides some other metals. One class of proteins is called chromoproteins.
Chromoproteins:
These are coloured proteins. They contain prosthetic groups which are responsible for the colour.
These groups contain metals like Fe, Co and Mg.
Some prothetic groups are complex structures consisting of pyrrole rings united through methylene bridges (-CH2-) to form porphyrins.
Porphyrins are derivatives of porphin. Photoporphysin contains 4 methyl, 2 vinyl and two propionic acid groups in a porphyrin. The carbons not involved in -CH2– bridges are numbered 1 to 8.
The bridges are α, β, γ and δ.
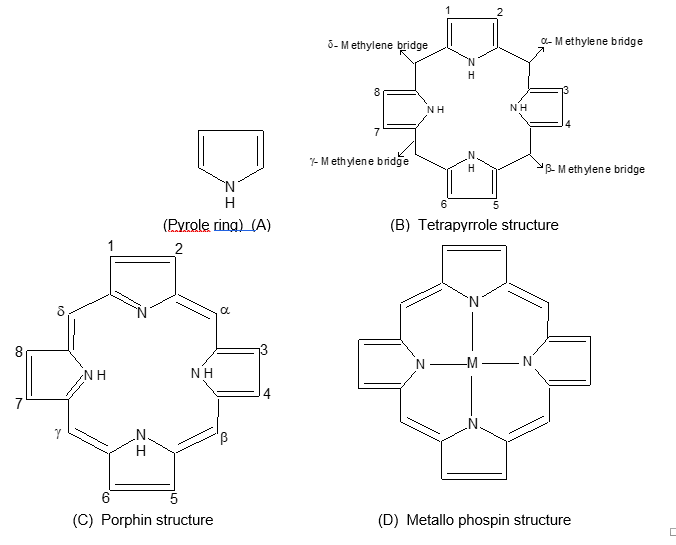
But chromoproteins may Br may not contain porphyrin ring structures. Moreover some are respiratory chromoproteins and others are not.
Classification of chromoproteins: Chromoproteins are classified as
Haemoglobin (HbO):
The human body contain about 4g of iron. About 70% of this is found as haemoglobin, the red pigment in the erythrocytes/red blood cells.
Thus, it is a globular proteins consisting of four polypeptide chains arranged tetrahedrally.
Functions of haemoglobin
1) As oxygen carrier: About 97% oxygen is carried out by haemoglobin.
Since haemoglobin (Hb) consists of a protein called globin and a pigment portion called heme. The heme portion contains four atoms of Fe, each capable of combining with a molecule of oxygen to form oxyhaemoglobin.
2) As CO2 carrier (Transport of CO2) : About 70% CO2 is transported. The dissolved CO2 in the blood reacts with water to form carbonic acid and dissociates into H+ and HCO3– as rapidly as formed. The blood removes the more soluble HCO3– ions and reduced haemoglobin removes the H+.
It is noted that HbO2 is weakly acidic and remains in association with K+ ions as KHbO2.
3) Coordination with CO, CN, etc:
Groups such as CO, CN– & PF3 can occupy the O2 site, coordination is still reversible but is much stronger. In the case of CN–, coordination is irreversible. These ligands reduce or prevent oxygen transfer and may result in death. Do you know why CO is poisonous?
CO gets bonded to Fe2+ of haemoglobin forming carboxy haemoglobin which is 300 times more stable then HbO2.
The bonding is very strong and it is difficult to destablised HbCO. Hence, CO is toxic in nature. In blood, when the conc. of HbCO reaches about 3 – 4 percent, the O2 carrying capacity is greatly reduced and increasing concentration of CO may even lead to death also.
4) Blood is well buffered between pH 7.36 – 7.42 through the process.
The affinity of haemoglobin for O2 decreases with the decrease in pH.

Chlorophyll:
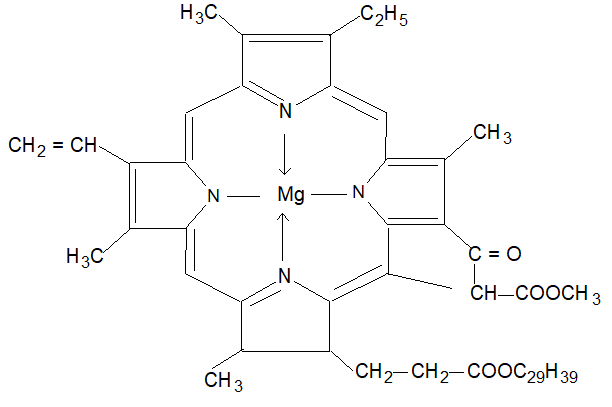
From the view point of life on this planet the most important complex formed by magnesium is chlorophyll.
The Mg2+ ion is at centre of a flat heterocyclic organic ring system called a porphyrin, in which four nitrogen atoms are bonded to magnesium as bonded to Fe in haemoglobin.
Thus chlorophyll is the green pigment present in the leaves of the plants. It is of two types namely chlorophyll ‘a’ and chlorophyll ‘b’.
Chlorophyll ‘b’ has formyl group (-CHO) on b-carbon of one of the pyrrole ring of phorphyrin in place of methyl group (-CH3) present in chlorophyll ‘a’.
Chlorophyll ‘a’, absorbs light in red region i.e., light of wave length 4300Å (violet blue) and 6800Å (red), from sunlight and makes the energy available for photosynthesis. In this process CO2 is converted into glucose.
Almost all life ultimately depends on chlorophyll and photosynthesis.
The basic structure of chlorophyll is shown below.
Functions:
(i) Chlorophyll is responsible for the synthesis of carbohydrates in plants.
(ii) It helps in maintaining O2, CO2 equilibrium in the nature. In photosynthesis CO2 is absorbed and O2 is released. The dioxygen in the atmosphere is a by product of photsynthesis.
Vitamin B12 is a co-enzyme and serves as a prosthetic group which is tightly bound to several enzymes in the body, but the precise role of vitamin B12 is not fully understood.
Pernicious anaemia is not simply due to deficiency of vitamin B12 but mainly results from the failure of the absorption of vitamin B12 due to lack of microprotein in gastric juice called the intrinsic factor.
Vitamin B12 promotes the formation of normal erythrocytes (RBC). However its mode of action was not clearly known until 1958 when Baker discovered that the 5¢ – deoxyadenosyl derivative of B12 is an essential co-enzyme in the conversion of glutamic acid to b-methyl aspartic acid in anaerobic bacterium.
Structure of Vitamin B12 (MF = C63 H88O14N14PCo): Source = liver of ox, pig, fish etc.
Using chemical analysis and X-ray crystallographic studies the structure of vitamin B12 was determined in 1957.
Dorothy Crowfoot Hodgkin was awarded the Nobel Prize for chemistry in 1964 for X-ray crystallographic work including solving the structure of this enzyme.
The vitamin B12 molecule has two characteristic components. The first part is a nucleotide like structure and the second part a corrin ring system which contains a Co (III) ion at the centre.
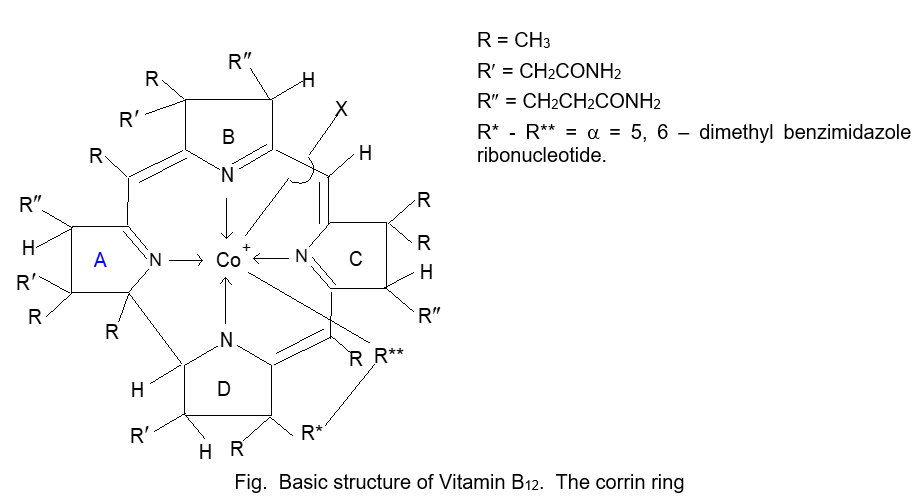
Functions of Vitamins B12:
(i) Vitamin B12 containing Co3+ ion is required to maintain normal bone Marrow function for producing erythrocytes (RBC).
(ii) Excess of cobalt produces excess of erythrocytes causing poly erythremia.
(iii) The deficiency of vitamin B12 causes permicious anaemia and hyper glycemia.
(iv) The lack of enough cobalt leads to nutritional type of anaemia.
(v) Synthesis of nucleic acids, synthesis of lipids from carbohydrates.
Chemistry in medicine:
Common drugs used in medicine:
The word drug is derived from the French word ‘drogue’ which means a dry herb.
Drug may be defined as a chemical substance used in the preventation, diagnosis, treatment or cur of a disease in man or other animals.
According to world health organisation (WHO): A drug may be defined as any substance or product which is used or intended to be used for modifying or exploring physiological system or pathological states for the benefit of the recipient.
An ideal drug should satisfy the following requirements:
(i) Its action should be localised only the site where it is expected to act with efficiency and safety.
(ii) It should not be toxic and it should have tolerable side effects.
(iii) It should not injure host tissued and the cells. It should not acquire the tolerance or resistance gradually.
Chemotherapy: The term chemotherapy is used for the science in which suitable chemicals are used for the treatment of disease.
Classification of Drugs: Drugs are broadly classified on the basis of their.
a) Chemical structure as a alcohols, amices, amino acids, enols, glycocides, ureasm etc, abd
b) Therapeutic action as
(i) Drugs acting on the CNS to depress or stimulate its action.
(ii) Drugs stimulating or blocking the peripheral nervous system.
(iii) drugs acting on cardiovascular haematopoietic and renal system – cardiovascular agent include cardiotonic, antihypertensive, hypochlestemic agents etc; haematological agents act on the blood, agents that effect real system are called diuretics.
(iv) Chemotherapentic drugs: These are the agents that all caused by certain species of metazoan, protzoa, fungi, bacteria, ricketissa and viruses.
Drugs active on the pathogenic agents are anti malarial agents, antiprotozoal agents, antiseptic, antifungal, antibacterial agents, sulfonamides, antituber culous and antilepral agents, antibiotics antiviral agents etc.
(v) Vitamins: These are compounds which are required by the animals for the maintenance and normal growth of life. They are generally supplied by the food. The deficiency of these causes disease in human beings.
(vi) Hormones: These are chemical substances produced by endocrine glands. These deficiency of hormone causes physiological disorders. The deficiency of a hormone causes physiological disease which is caused by giving that hormone.
Let us discuss some specific classes of drugs used in allopathic system. Some of the medicinal compounds are discussed below.
1. Analgesics (Body pain reliever):
The chemical substances which are used to relieve from body pains are called analgesics.
e.g., Asprin, paracetamol, methyl salicylate analgin, novalgin, naproxen, ibuprofen and dictofenac sodium or potassium.
2. Antipyretics (Body temperature reducer):
The chemical substances which are used to lower the temperature of the body in high fever are called antipyretics.
e.g., Asprin, paracetamol, and phenacetin.
1. Asprin (Acetyl saticylic acid):
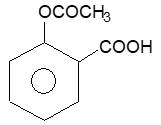
Prep: It was first prepared by Kolbe (1774) by refluxing salicylic acid with a mixture of acetic anhydride and acetic acid in the presence of sulphuric acid.
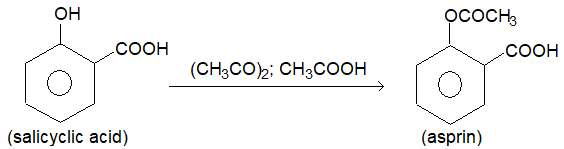
Asprin was first introduced as medicine by Dresser (1899) as analagesic to relieve from dull headaches, to othache etc and antipyretic.
2. Paracetamol:

Prep: Paracetamol is prepared by treating paraamino phenol with acetic anhydride in the presence of concentrated sylphuric acid.
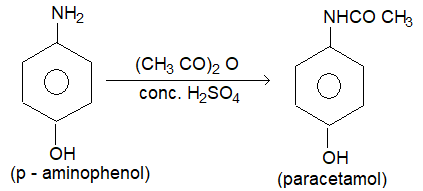
Paracetamol is sold as a medicine under several trade names like crocin, calpol and acetaminophin. This compound exerts analgesic and antipyretic effects, similar to asprin.
Paracetamol is a potent antipyretic and equianalgesic with asprin but devoide of significant anti inflammatory effect. This does not produce gastrointestinal irritation, acid – base imbalance, electrolyte disturbances, nor does it affect blood clotting. Because of these advantages is is preferred to asprin as analgesic or antipyretics.
Large doses of paracetamol, however, produce damage to liver. Paracetamol usage may produce anemia, skin reactions, etc.
3. Methyl salicylate:
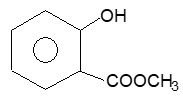
Prep: Like a typical carboxylic acid, salicylic acid is esterified upon treatment with methanol in presence of conc. H2SO4 to give methyl salicylate.
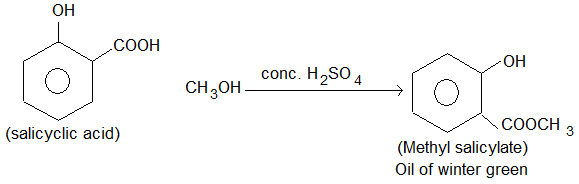
Properties: It is a plate yellow to reddish brown fluid liquid, strongly aromatic with sweet characteristic odour and soluble in 80% alcohol.
Therapeutic uses:
3. Antiseptics and Disinfectants:
a) Antiseptics: Antiseptics are the chemicals which either kill or prevent the growth of micro organisms. These are applied to living tissues. They can be applied to wound, cuts, ulcers and diseased skin surface. We all must be familiar with antiseptic creams like Furacin, soframycin, etc.
b) Disinfactants: Disinfactants also kill microorganisms but are not safe for living tissues. Disinfactants play a major role in water treatment and in public health sanitation. These are commonly, applied for inanimate objects such as a floors, drainage systems, instruments etc.
The same substance can act as disinfectants as well as antiseptic depending upon its concentration.
e.g.,
(i) Phenol: 0.2% solution of phenol acts as antiseptic and its 1% solution acts as disinfect.
(ii) Chlorine: Chlorine in concentration of 0.2 to 0.4 parts per million (ppm) is used as a disinfectant for drinking water.
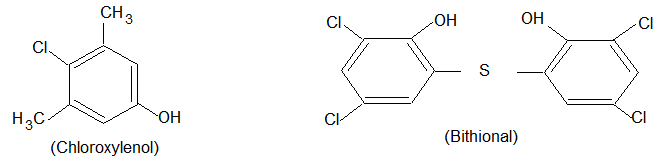
(iii) Dettol: Dettol is a commonly used antiseptic and it is a mixture of chloroxylenol and terpineol. Chloroxylenol has both antiseptic and disinfectant properties.
(iv) Bithional: It is added to soap to impart antiseptic properties. It eliminates undesirable odours resulting from bacterial decomposition of organic matter on the skin.
(v) Iodine: Iodine is a powerful antiseptic. It is employed as tinture of iodiene, an alcohol water solution containing 2 – 3 percent iodine.
(vi) Iodoform: It is used as an antiseptic powder for wounds.
(vii) Boric acid: A dilute aqueous solution of boric acid, is used as weak antiseptic for eyes. It also forms part of antiseptic baby talcum powders.
(viii) Hydrogenperoxide is also used as non-irritating strong antiseptic.
4. Antimalarials:
The chemical substances used for the treatment of malaria are called antimalarials.
The alkaloid quinine has been used as an antimalarial for a long time.
These days a number of synthetic drugs hae been prepared for the treatment of malaria. E.g., chloroquine, paraquine.
5. Transquilizers:
The chemical substances used for the treatment, of stress, mild & severe mental diseases.
These are used to relieve stress, fatigue, by inducing a lense of well being. They forms an essential component of sleeping pills.
Trnasquilizers are also (form an important category of the so) called psychotherapeutic drugs.
These drugs make the patient passive and help to control their emotional distress or depression.
The most commonly used transquilizers are barbituric acid and its derivatives such as veronal, Amytal, Nembutal luminal and Seconal. These are called barbimates.
These are hypnotic i.e., sleep producing agents.
In addition to the barbiurates, a large number of other non hypnotic transquilizers are known.
Some important compounds of this class are discussed below.
Chlordia zepoxide and memprobamate are relatively mild transquilizers suitable for relieving tension. Equanil is used in controlling depression and hypertension. Some other substances used as transquilizers are valibum, serotonin etc.
Antifertility Drugs: These are drugs which controls the female menstrual cycle and overlation i.e., pregnancy. It is estimated that 50 – 60 million women throughout the would take the pill as the primary form of contraception.
The contraceptive pill i.e., birth controlpill is essentially a mixture of synthetic estrogen and progestron derivatives, which are more potent than the natural hormones.
Mifepristone is a synthetic steroid that blocks the effects of progesterone and is used as a ‘morning after pill’ in many countries.
Antihistamines: These drugs are also called as antiallergic drugs and are sued to treat allergy e.g., skin rashes.
Since, the allergic reactions are caused due to liberation of histamine in the bodythat is why these drugs are called antihistamines.
Other than skin rashes these drugs are useful for conjunctivitis (inflammation of conjunctive of eye) and rhinitis (inflammation of moral mucosa).
In seasonal rhinitis and conjunctivitis, these drugs relieve sneezing, nasal discharge and etching of eyes, nose and throat.
Common drugs of this group are diphenyl hydramine, chlorpheniramie and promethazine.
Antibiotics: These are the chemical substances which are produced by microorganisms.
However, the development of synthetic method has resulted in a modification of this definion.
An antibiotics now refers to a substance (produced wholly or partly by chemical synthesis), which in low concentration inhibits the growth or destroys micro organisms by intervening in their meta bolic processes.
Antibiotic therapy may be regarded as “setting one thief against another”. This is because of antibiotic themselves are products of microbial growth.
The first antibiotic discovered by Alexander Flehming in 1929 from the mould Penicillium notation, was penicillin.
The antibiotics can be either bactericidal or bacteriostatic.
|
Bactericidal |
Bacteriostatic |
|
Penicilline |
Erythromycin |
|
Aminoglycosiders |
Tetracyclin |
Ampicilin and Amoxicillin are semi synthetic modifications of penicillin.
Broad spectrum antibiotics are medicines effective against several different types of harmful microorganism. E.g., Tetracycline, chloramphenicol and a mixture of potent antibiotics.
Sulpha drugs: These have great antibacterial powers and are used as medicines for various diseases. These are used against disease such as pneumonia, tuberculosis, diphtheria etc. Some important sulpha drugs are sulphanilamide sulphadiazine and sulphaguanidine etc. These are not in much use at present.
Illustration 1: Which of the following is not a broad spectrum antibiotic?
(A) Tetracycline (B) Chloromycetin (C) Penicillin (D) None of the above
Solution: (C) Penicillin is not a broad spectrum antibiotic since it is active against infections caused by gram positive bacteria only.
Anta acids: The chemical substances which neutralize excess acid in the gastric juices and give relief from acid in digestion, acidity, heat burns and gastric ulcers are called antacids.
These remove the excess acid and raise the pH to appropriate level in stomach are called antacids. Acid Gastritis is one of the commonest ailments associated with digestion. It is caused by excess of hydrochloric acid in the gastric juice. Magnesium hydroxide, aluminium hydroxide gel, sodium bicarbonate and aluminium phosphate are commonly used as antacid.
In recent years, a new class of drugs omeprazole and lansoprazole are most effective drugs which prevent the formation of acid in the stomach.
Dyes: A dye is a coloured substance that that can be applied in solution or dispersion to a substrate (i.e., giving it a coloured appearance).
Classification of Dyes: Dyes are classified either according to their constitution or method of application as follows:
Classification based on Application: Depending upon the process of application the dyes are classified as
a) Acid dyes: These contain sodium salt of sulphonic or carboxylic acid. It is used to dye wool, bilk, polyamide and acrylic fibres. Acid dyes do not have affinity for cotton examples include. Orange I.
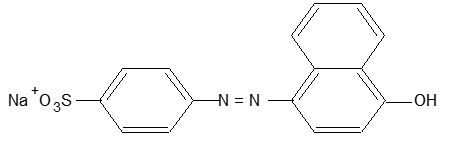
b) Basic dyes: These dyes are salt of a colour base. Such dyes are used to dye reinforced nylons and polyesters. Examples include aniline yellow, butter yellow and malachite green.
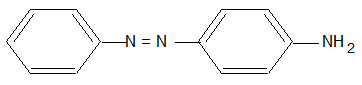
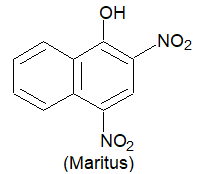
c) Direct Dyes: These are water soluble dyes and are directly applied to the fabric in an aqueous solution. These are practically suited for fabrics like cotton, rayon, wool, silk and nylon which form hydrogen bonds with water. Martius yellow and congo red are important examples of this class of dyes.
d) Disperse dyes: Such dyes are used for dyeing synthetic fibres like polyesters nylon and poly acrylonitrile. Many anthraquinone disperse dyes are suitable for application to synthetic polyamide fibres.
e) Fibre reactive dyes: These dyes directly combine with the hydroxyl or amino group of the fibre through its reactive group. Such dyes are used to dye cotton, wool or silks.
f) Insoluble azo dyes: This dye is produced in situ on the surface of fabric by means of coupling reaction between phenol, naphthol or rerorcinol adsorbed on the surface of the fabric and a diazonium salt.
These dyes have also been classified based on its structural units. Examples are nitrodyes (eg orange – I), phthalein dye (eg malarchite green), indigoid dye (eg indigo) and anthraquinone dye (or alizasin).
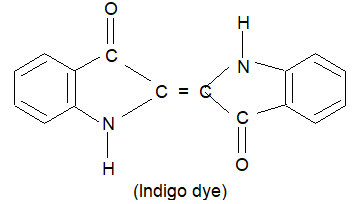
g) Vat dyes: Vat dyes are insoluble in water and can not be used directly for dying. Such a dye is used in its reduced form and after application to the fibre it is oxidised to impart colour to the fibre. Indigo and Indigosol O are versatile dyes which belong to this class.
h) Mordant dyes: This dye requires a metal ion (known as mordant) for dying a fabric. Example includes alizarin.
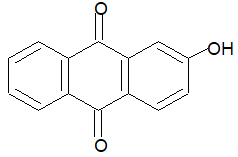
Alizarin imparts rose red, blue, brownish red, violet and red colour to the fabric in the presence of Al3+, Ba2+, Cr3+, Mg2+ and Sr2+ respectively.
Illustration 2: Alizarin gives red colour by mordanting it with the sulphate of a metal. The metal ion is
(A) Ba2+ (B) Al3+ (C) Cr3+ (D) Fe3+
Solution: (B). With Al3+ ions, alizarin gives red colour.
Illustration 3: Malachite green is a direct dye for silk and wool. It is prepared by condensing
(A) benzaldehyde and N, N–dimethylanilin
(B) carbonyl chloride and N, N–dimethylaniline
(C) benzenediazonium chloride with N,N–dimethylaniline
(D) None of the above
Solution: (A).
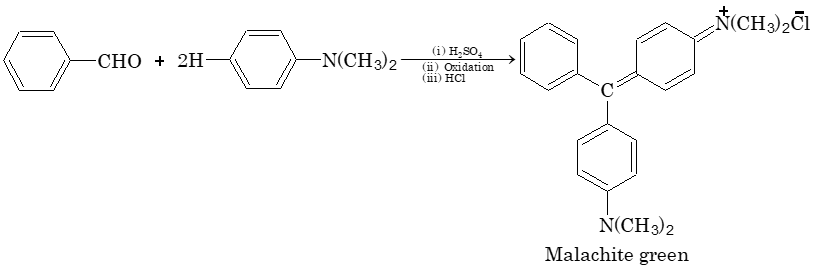
Illustration 4: Which of the following group is not an auxochrome?
(A) (B) (C) (D)
Solution: (D) is not an auxochrome.
Cosmetics:
The word cosmetics is derived from the Greek word kosmetikos. It means decorating, beautifying or improving complexion of skin. In India from the ancient times. Henna has been used to decorate hands and some other parts of the body. Some of the cosmetics which find use in daily life are discussed below.
1. Creams: There are used for facial make up and are classified as.
a) Cleansing cream: Removes facial make up, surface grime, lipirtic and oil.
b) Cold creams: Lubricate the skin and prevent roughness and chaffing.
c) Vanishing cream: Keep the skin cool and oily.
d) Bleach cream: Exert a bleaching effect on dark skin.
e) Sun burn creams: Save the skin from sun burn in summer.
2. Perfumes are the materials, used to provide fragrance. A perfume invariably consists of three ingradients a vehicle, fixative and odour producing substance. E.g., Ethanol and water mixture is the most common vehicle (solvent) used in perfumery, sandal wood oil finds use as fixative and terpenoids like linalool which occur in essential oils are natural odour producing compounds, while p-methoxy benzaldehyde, is a synthetic odour producing compound.
3. Talcum powder like face powders contain talc (Mg3(OH)2 Si4O10). Chalk, zinc oxide, zinc stearate and suitable perfumes act as the other main constituents of talcum power. Some times specific ingradients like antiseptic and cooling agents are added.
Badly talcum powders contain considerable amount of zinc stearate for adhesiveness and boric acid, for antiseptic purposes.
Illustration 5: The base of talcum powder is
(A) chalk
(B) magnesium hydrosilicate
(C) sodium aluminium silicate
(D) zinc stearate
Solution: Magnesium hydrosilicate.
4. Deodorants: These are applied primarily to mark the body odour results from the bacterial action following persipiration. A deodorant must therefore, possess anti-bacterial properties. Aluminium salts, ZnO, ZnO2 and (C17H35COO)2Zn also find use in deodorant preparations because they are astringents and as well as antiseptics.
Chemicals in food:
These include flovourings, sweeteners, dyes, antioxidants, fortifies and antifoaming agents.
a) Antioxidants: These are the important and necessary food additives. These compounds retard the action of oxygen on the food and thereby help in its preservation. These act as sacrificial materials and reduce the rate of involvement of free radicals in the aging process.
b) Artificial sweetners: These are another type of food additives. Saccharine is the first popular artificial sweeteners. It has proved to be a life saver for counters diabetics. Besides saccharin, the other commonly marketed artificial sweeteners are described here.
Aspartame is unstable at cooking temperatures. Alitame is more stable than aspartame during cooking.
c) Preservatives: The preservatives prevent spoilage of food due to microbial growth. The most common preservative used is sodium benzoate. Salt of propionic acid and fabric are also used as preservatives.
d) Edible colours: Edible colours used for food are essentially dyes. They are used to colour everything from meat to fruit. g., dyes are used dye orange peels so that oranges retain their colour.
There is a great deal of controversy over the potential harm the dyes may cause. Carotene are safe food edible colours.
Pheromones, sex attractants:
Sex pheromones are remarkably powerful and are usually emitted by the females, although there are some male insects which also produce them. It has been claimed that the sex attractant in some species can attract males from over two miles away. On can collect all the males in the vicinity, by baiting a trap with a small amount of sex attractant of an insect pest, since each insect has its own attractant. The gypsy moth attractants, attracts male moth in the area when a trap is baited with only 1 × 10–9g.
Detergent:
The wood ‘detergent’ means ‘cleansing agent’ and so the detergents are substances which remove dirt and have cleansing action in water.
According to this definition of detergents, soap is also a detergent and has been used for more than two thousand years. There are two types of detergents
i) Soapy detergents or soap.
ii) Non soapy detergents or soapeless soaps.
1. Soapy detergent or soap: A soap is a sodium or potassium salt of some long chain carboxylic acid i.e., fatty acid.
Sodium salt of fatty acids are known as hard soaps, which are prepared from cheap oils and fats with sodium hydroxide. On the other hand potassium salts of fatty acids are known as soft soaps, which are prepared from good quality oils with potassium hydroxide.
A soap has large non-ionic hydrocarbon group and an ionic COO– Na+ group. So for simplicity the structure of soap can be represented as ~~~~~~~ (-) Na+. Where represents the hydrocarbon group and (-) represents negatively charged carbonyl group.
2. Non-soapy detergents:
These are also called synthetic detergents or soapless soaps. So in our routine language when we say detergent, it means the synthetic detergent.
A synthetic detergents is the sodium salt of a long chain benzene sulphonic acid or the sodium salt of a long chain alkyl hydrogen sulphate.
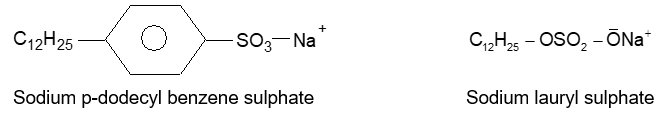
Washing powders available in the market contain about 15 to 30 percent detergents by weight and remaining part is other chemicals which are added to it.
(i) Sodium sulphate and sodium silicate are added to keep the washing power dry.
(ii) Sodium carbonate is added to maintain alkalinity which helps in removing dirt and also soften water.
(iii) Carboxy methyl cellulose is added to keep the dirt suspended in water.
(iv) A mild bleaching agent such as sodium perborate is added to produce whiteness in clothes.
Advantages of synthetic Detergents over soaps:
(i) Synthetic detergents can be used even in hard water whereas some of the soap gets wasted if water is hard.
(ii) Synthetic detergents can be used even in acidic medium as they are the salts of strong acids and are not decomposed in acidic medium.
(iii) Synthetic detergents have stronger cleansing action than soaps.
(iv) Synthetic detergents are prepared from the hydrocarbons obtained from petroleum used in preparation of soap.
(v) Synthetic detergents are more soluble in water than soaps.
Disadvantage of synthetic detergents:
Some of the early synthetic detergents were non-biodegradable, that is they were not broken down by bacteria. Thus, they caused water and soil pollution. This problem has been over come.
It has been found that if straight chain hydrocarbon is used in the detergent instead of a branched chain hydrocarbon then the detergent is biodegradable.
All the soaps, on the other hand are biodegradable.
Another problem is with the use of certain phosphate additives which are added to detergents. These phosphate additives acts as nutrients for algae which form a thick green scum over the river water and upset the animal life in the river. But action has now been taken for this problem also.
New height performance materials:
(i) Carbon fibres:
Carbon fibres are a new breed of high performance materials, which have attracted world wide attention and hold great promise for the future.
This is because of the fact that these fibres are stronger than steel, stiffer than titanium and lighter than aluminium.
Carbon fibres are obtained by heating regenerated fibres (such as rayon) or synthetic fibres (such as nylon or polyester) in the absence of oxygen. As a result of heating, the fibres decompose to form carbond fibres.
Carbon fibres are produced in a number of ways and from a variety of stating material or precursors such as viscous rayon, polyacrylonitrile, pitch, resins, gases such as methane and benzene.
Carbon fibres may be reinforced in light weight epoxy resin or polyster resin matrix. Such carbon fibres are known as carbon fibre reinforced plastic (CFRP). When carbon fibres are reinforced in a carbon matrix, they are known as carbon fibre reinforced carbon (CFRC).
Carbon fibres, CFRP and CFRC find applications in the following high techno logy fields such as aerospace, military and nuclear.
(i) In the aerospace field, CFRP is used for air craft wings, tail parts, helicopter rotor blades. CRFC is used in breaks in heavy and fast jet air crafts. Due to high thermal conductivity of carbon fibres heat dissipation in components made of carbon fibres takes place quickly. Carbon fibres are used as component of wall material of nuclear reactors.
(ii) General engineering such as transportation, sports and chemical field. In the area of sports – goods, carbon fibres in the form of CFRP are used for making finishing rods, skypoles, tennis and badminton rackets and racing cycle frames.
(iii) Biomedical field: In biomedical field carbon fibres are used as components of bone plates, ligaments and hydraulic motors for artificial heart implants. In India, carbon fibres are mainly used in defence sector and aerospace sector. In defence sector, it is used as nose tips and head shields of missiles and in aerospace sector it is used for making components of rockets.
(ii) Ceramics: The term ceramics comes from the Greek word Keramickos which means burnt stuff, indicating thereby, that desirable properties of these materials are normally achieved through a high temperature heat treatment process called firing. Most ceramics materials fall into an application classification scheme which is given below.
(a) Clay products: Porcelain, pottery, table wares, sanitary fittings, building bricks, tiles and sewer pipes.
(b) Glass ceramics: Kitchenware.
(c) Refractory Materials: Refractory bricks used as furnace linings.
(d) Abrasive ceramics : Cutting and grinding tools. e.g., silicon and tungsten carbides.
Super conducting ceramics:
Recently a family of ceramics have been found to be superconductors with high critical temperatures. One such materials is yttrium barium copper oxide, which has a critical temperature of about 92 K. The technological potential of these materials is extremely promising as their critical temperatures are above 77K. Numerous applications of super conducting materials exist some of these are:
(i) High speed magnetically leviated trains.
(ii) Electrical power transmission.
(iii) Magnets for high energy particle accelerators.
(iv) High speed switching and signal transmission for computers.
Rocket propellants:
A number of satellites have been launched by different countries. In our, country, the interest of the Indian space research organisation (ISRO) has been to launched two classes of satellites – remote sensing satellites and communication satellites. The polar satellite launch vehicles (PSLV) is a remote sensing satellite.
India launched its first successful satellite launch vehicles SLV-3 from Sriharikota, which heralded the beginning of India’s space programme.
In order to push these rocket satellites into the space the lift off these space vehicles is achieved by having a propulsion system in these space vehicles. The propulsion system in most space vehicles consists of rocket engines powered by what are known as chemical propellants.
A propellant is a combination of an oxidizer and a fuel which when ignited undergoes combustion to release great quantities of hot gases. The passage of gases through the nozzle of the rocket motor provides the necessary thrust for the rocket to more forward i.e., uplift.
The function of rocket propellant is similar to that of petrol in a motor car except that in later case, the oxygen needed for burning the fuel (petrol) is taken from the atmospheric air.
Characteristics of a rocket propellants:
A rocket propellant must possess the following characteristics.
(i) It should produce a very large volume of gases for every gram of fuel burnt.
(ii) It should burnt at very fast rate.
(iii) It should burn completely & leaving behind no ash.
(iv) The calorific value of the fuel should be high so that it produces large amount of heat per gram of the fuel.
Classification of Rocket Propellants:
Depending upon their physical state, propellants are classified into the following types.
1. Solid
2. Liquid
3. Hybrid propellants
1. Solid Propellants: These include the use of solid fuel and solid oxidiser. These are of two types.
a) Composite propellants: It is the most common and widely used solid propellant which is a blend of a polymeric binder such as polyurethane or polybutadiene used as fuel and ammonium perchlorate as oxidiser along with some additions like finally divided aluminium or magnesium to modify the performance of the propellant!
b) Double – Base Propellants: These include the use of nitroglycerine (l) and nitrocellulose (s). In fact nitrocellulose gels in nitroglycerine set in as semisolid mass. Solid propellants, once, ignited, will burnt with a pre-determine rate and do not have start and stop capability.
2. Liquid Propellants: Consist of a combination of an oxidiser such as liquid oxygen, nitrogen tetraoxide or nitric acid and a fuel such as kerosene, alcohol, hydrazine or liquid hydrogen. The main advantage of these biliquid propellants is that, they give a better thrust than solid propellants and their thrust can be controlled by regulating the flow of propellants.
The propellants involving use of single liquid are called monopropellants. A number of chemicals like hydrazine, methyl nitrate, nitromethane and hydrogen peroxide are used as monopropellant.
Among these, except hydrazine all other compounds mentioned contain both the oxidiser and the fuel elements in the same molecule.
The other common liquid propellants are unsymmetrical dimethyl hydrazine (UDMH) and monomethyl hydrazine (MMH).
Note: The cryogenic systems generally show high performance.
3. Hybrid propellants: Usually consist of a solid fuel and a liquid oxidizer. e.g., a mixture of a acrylic rubber and liquid dinitrogen tetraoxide (N2O4).
Propellants used in different space vehicle:
(i) Saturn booster rocket of American space programme used a combination of liquid oxygen and kerosene as the propellant for initial stage where as liquid hydrogen and liquid oxygen were used for the upper stage.
(ii) Russian rockets such as PROTON used liquid oxygen and kerosene as liquid propellants.
(iii) Indian satellite launch vehicles such as SL V – 3 and ASLV used composite solid propellants.
(iv) PSLV – C4 September, 2002 has been given the name METSAT MISSION. It is a 4-stage vehicle.
a) The first stage is one of the largest solid propellant boosters in the world and carries 138 tonne of Hydroxyl Terminated Polybutadiene (HTPB) based propellant.
b) The second stage employs indigenously built VIKAS engine and carries 40 tonnes of liquid propellant unsymmetrical dimethyl hydrazine (UDMH) as fuel and nitrogen tetraoxide as oxidizer.
c) The third stage again used 7.6 tonnes of HTPB based solid propellant.
d) The fourth and terminal stage of PSLV-C4 has a twin engine configuration using liquid propellant. Each engine uses 2.5 tonnes of mon-methyl hydrazine as fuel and mixed oxide of nitrogen as oxidizer.
(v) Space Shuttle used liquid oxygen and liquid hydrogen along with solid boosters in the lower stages.
(vi) The Titan Ballistic Missile – used a mixture of hydrazine (fuel) and N2O4 (oxidizer).
Illustration 6: Which of the following represents a double base propellant?
(A) Nitromethane
(B) Nitrocellulose + Nitroglycerine
(C) N2O4 + Monomethylhydrazine
(D) Liquid H2 + Liquid O2
Solution: (B) The mixture of Nitrocellulose and Nitroglycerine represents a double base propellant.
FORMULAE AND CONCEPTS AT A GLANCE
1. The chemicals used for treatment of diseases are called medicines or drugs.
2. A drug is a chemical agent which affects human metabolism and provides cure from ailment.
3. Analgesics are used to relieve pain e.g., aspirin, novalgin.
4. Antiseptics are used to kill or prevent the growth of micro-organism and can be applied on living tissues e.g., dettol (a mixture of chloroxylenol and terpineol), bithional (added to soap), iodine etc.
5. Disinfectants are used to kill micro-organisms but cannot be applied on living tissues e.g., 1% solution of phenol, chlorine (0.2 – 0.4 ppm).
6. Tranquilizers are used to release mental tensions and anxieties e.g., Equanil, derivatives of barbituric acid (veronal, Amytal, membutal, luminal, Seconal), valium, serotonin.
7. Antimicrobials are used to cure infections due to micro-organisms e.g., derivatives of sulphanilamides (sulphadiazine, sulphadimidine).
8. Antifertility drugs are used to control the pregnancy e.g., norethindrone, ethynylestradiol.
9. Antihistamines are used to diminshi or abolish the main action of histamines released in the body and, hence, prevent allergic reactions e.g., diphenylhydramine, chlorpheniramine.
10. Anatacids are used to remove excess acid and raise the pH to appropriate level in stomach e.g., magnesium hydroxide, magnesium carbonate, magnesium trisilicate, aluminium hydroxide, omeprazole.
11. Antibiotics are produced by micro-organisms that inhibit the growth or even destroy the other micro – organisms e.g., penicillin, ampicillin, amoxicillin, chloramphenicol streptomycin.
12. Dyeing involves imparting adequate and fast colour to fabric.
13. On the basis of chemical constitution, the dyes have been classified as
(i) Azo dyes (containing azo grouo) e.g., Organge – I
(ii) Phthalein dyes (containing phthalein group) e.g., indigo.
(iii) Indigoid dyes (containing indigoid group) e.g., indigo.
(iv) Triphenylmethane dyes (containing triphenyl methane group) e.g., malachite green.
(v) Anthraquinone dyes (containing anthraquinone group) e.g., alizarin.
14. Cosmetics are the substances used for decorating, beautifying or improving complexion of skin.
15. Perfumes are the substances used to provide fragrance.
16. Talcum power is used to reduce irritation of skin.
17. Antioxidants retard action of oxygen on the food and help in its preservation e.g., BHT and BHA.
18. Edible colours used for food are essentially dyes e.g., natural dye like carotene.
19. Detergents are substances which are used for cleaning. Synthetic detergents are sodium salts of long chain sulphonates or sulphates.
20. Ceramics are materials which have undergone heat treatment process called firing. Superconducting ceramics are compounds of certain metals with oxygen.
SOLVED PROBLEMS-1
Prob 1: Why do azo dyes not impart fast colours to the fabrics?
Sol: Azo dyes are adsorbed only on the surface of the fabric and do not get linked through ionic or covalent bonds. Therefore, they do not impart fast colours when applied on the fabric.
Prob 2: Write the name of the class of dyes having the following characteristic structural unit
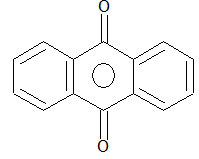
Sol: Anthraquinone dyes.
Prob 3: What is the characteristic group present in azo dyes?
Sol: Group present in azo dyes is – N = N -.
Prob 4: Sulpha drugs work like, antibiotics but they are not antibiotics. Is this a valid statement and why?
Sol: This is a valid statement. Sulpha drugs act against micro-organism like antibiotics. However, these are not obtained from micro-organism like antibiotics.
Prob 5: Pick out the odd one from amongst the following compounds on the basis of their medicinal properties mentioning the reasons.
Luminal, Seconal, phenacetin, Equanil.
Sol: Phenacetin.
Prob 6: What is the role of dimethyl and monomethyl hydrazines in rockets?
Sol: Both these compounds act as fuels in rockets.
Prob 7: What propellant is planned to be used in PLSV rocket?
Sol: The rocket PLSV will use solid propellant in the first and third stage and liquid propellant in second and fourth stage. The liquid propellant will consist of N2O4 and unsymmetrical dimethyl hydrazine (UDMH) and N2O4 and monomethyl hydrazine (MMH) respectively.
Prob 8: Name one synthetic sweetening agent.
Sol: Saccharin
Prob 9: What are micro alloys?
Sol: Micro alloys are materials obtained by adding small amounts of alloying materials to steel to improve its mechanical properties. The common micro alloying elements are vanadiu, titanium, tellurium, boron etc.
Prob 10: Give on example of a non-ionic detergent.
Sol:
SOLVED PROBLEMS-2
Prob 1: An example of anthraquinone dye is
(A) Alizarin (B) Methyl orange (C) Methylene blue (D) Phenolphthalein
Sol: (A) An example of anthraquinone dye is Alizarin
Prob 2: The substance used in the preparation of malachite green is
(A) formaldehyde (B) acetaldehyde (C) benzaldehyde (D) acetone
Sol: (C) The substance used in the preparation of malachite green is benzaldehyde.
Prob 3: Which of the following can possibly be used as analgesic without causing addition and modification?
(A) Morphine (B) N – Acetyl – para aminophenol (C) Diazepam (D) Tetrahydrocatenol
Sol: (B) N – Acetyl – para aminophenol
Prob 4: The following compound is used as
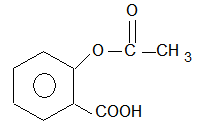
(A) an anti-inflammatory compound
(B) analgesic
(C) hyponotic
(D) antiseptic
Sol: (B) Used as analgesic.
Prob 5: Amoxillin is semi synthetic modification of
(A) Penicillin (B) Streptomycin (C) Tetracyacline (D) Chloramphenicol
Sol: (A) Amoxillin is semi synthetic modification of Penicillin.
Prob 6: SLV-3 uses propellants
(A) Solid (B) Liquid (C) Solid – liquid (D) Biliquid
Sol: (A) Solid propellants are used in SLV – 3.
Prob 7: Which of the following is not a surfactant?
(A)
(B)
(C)
(D)
Sol: (A) is not surfactant.
Prob 8: The compound
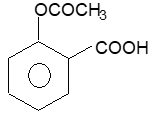
is used as
(A) antiseptic (B) antibiotic (C) analgesic (D) pesticide
Sol: (C) as analgesic.
Prob 9: Which of the following could act as a propellant for rockets?
(A) Liquid nitrogen + liquid oxygen
(B) Liquid hydrogen + liquid nitrogen
(C) Liquid oxygen + liquid argon
(D) Liquid hydrogen + liquid oxygen
Sol: (D) Liquid hydrogen + liquid oxygen is used as a propellant for rockets.
Prob 10: Antipyretics are the compounds which
(A) lower the body temperature
(B) relieve pain
(C) control malaria
(D) None of these
Sol: (A) Antipyretics lower the body temperature in fever.








