Introduction:
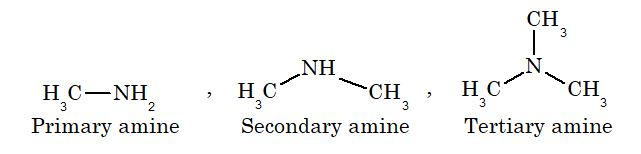
Amines are nitrogen containing organic compounds, which may be considered as derivatives of ammonia in which hydrogen atoms are replaced by alkyl or aryl groups. Amines are classifieds as primary, secondary or tertiary depending on the number of alkyl groups attached to nitrogen atom.
METHODS OF PREPARATION OF AMINES:
1. Reduction:
Amines can be prepared by the reduction of alkyl cyanide, nitroalkane and oxime.
(a) The reduction of alkyl cyanides with the reductant (Na + C2H5OH), is known as Mendius reaction.
(b) Reduction of nitroalkanes by Sn/HCl, Zn/HCl, H2/Ni or LiAlH4 gives primary amines.
(c) Reduction of oximes with LiAlH4 or H2/Ni gives primary amine.
2. Hydrolysis:
(a) Hydrolysis of isocyanides
(b) Hydrolysis of isocyanates
3. Hofmann’s bromamide reaction:
Amide is treated with bromine and KOH solution to give primary amine containing one carbon atom less than the amide.
4. Ammonolysis of alkyl halide:
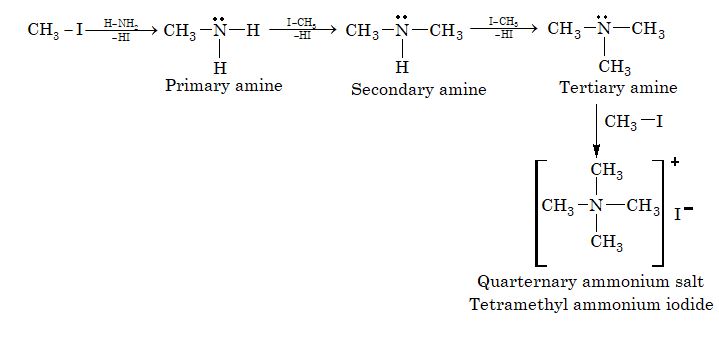
5. From acyl chloride (Curtius reaction):
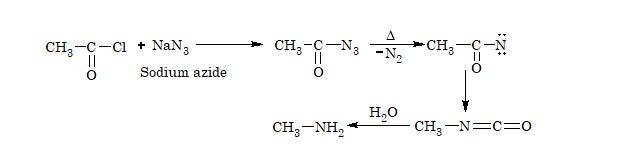
6. From carboxylic acid (Schmidt reaction):
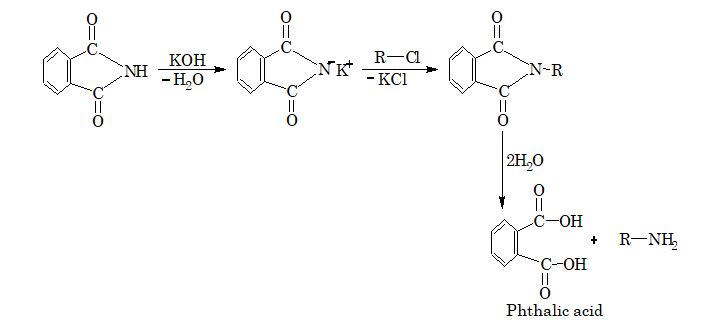
7. Gabriel phthalimide reaction:
8. From reductive amination of aldehydes and ketones:
(a) 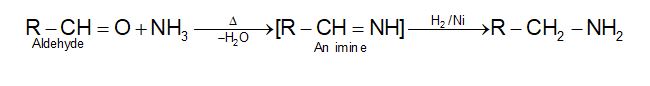
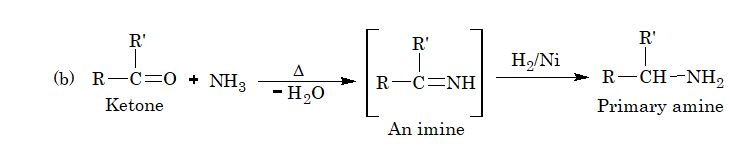
9. Lossen rearrangement:

10. Leuckart Reaction:
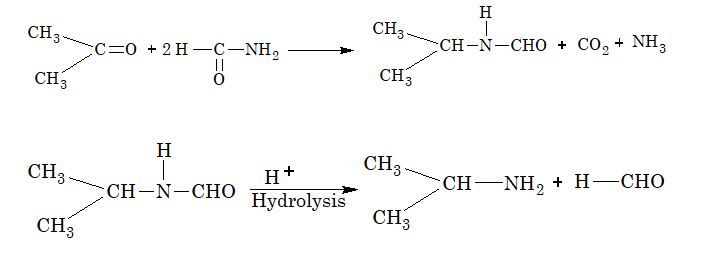
Illustration 1: The products (A) and (B) formed in the given reaction is
(A) RCON and RCN
(B) RCN and RNC
(C) RCON3 and RCN
(D) RCON3 and RNCO
Solution: (C)

Illustration 2: Alkanamide which on Hofmann’s reaction gives 1-phenyl ethyl amine is
(A) 2-phenylpropanamide
(B) 3-phenylpropanamide
(C) 2-phenylethanamide
(D) N-phenylethanamide
Solution: (A)
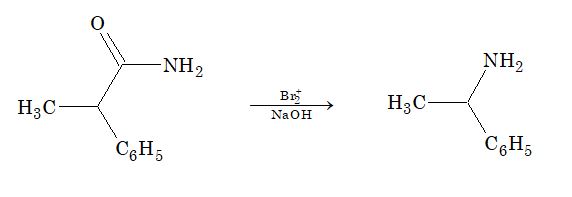
Illustration 3:
Aqueous solution of A
(A) turns blue litmus to red
(B) turns red litmus to blue
(C) does not affect the litmus
(D) decolourize the litmus
Solution: (B) A is ethylamine. It turns red litmus to blue.
PHYSICAL PROPERTIES OF AMINES
1. Boiling points: Primary amines and secondary amines form hydrogen bonding. There are small intermolecular association in primary and secondary amines. Thus, the boiling point is in following order:
Primary amine > Secondary amine > Tertiary amine
2. Order of volatility:
Tertiary amine > Secondary amine > Primary amine
3. Solubility: Low molecular weight amines are easily soluble in water. The solubility in water decreases with increasing size of alkyl groups. Amines are capable of forming H–bonding with water. More H–bonding, more solubility in water.
CHEMICAL PROPERTIES OF AMINES:
I) Basic nature of amines: All amines behave as a base because of the presence of a lone pair of electrons on the nitrogen atom.
However, it has been found that in aqueous solution the order of basicity is different.
Order of basicity of amines in aqueous solution:
(a)
(b)
(c)
(d)
Reasons of deviation of basicity of amines:
a) + I effect: Aliphatic amines are stronger bases than ammonia. This is due the reason that alkyl groups are electron donating groups. As a result, the electron density on the nitrogen atom increases and thus they can donate the lone pair of electron more easily than ammonia.
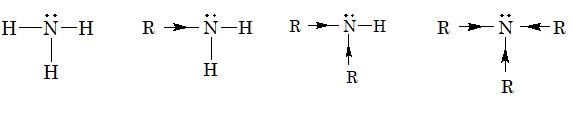
Thus, the basicity of amines follows the order:
Tertiary amine > Secondary amine > Primary amine > NH3
b) Steric–hindrance: In tertiary amines, R3N, three alkyl groups attached to nitrogen atom are bulkier and as such exert steric hindrance and do not allow the approaching proton to reach near the nitrogen atom.
(c) Decrease in hydration of protonated amine: The stability of the ammonium cation is due to H–bonding depends upon the number of hydrogen atoms present on nitrogen atom, more the number of hydrogen atoms more stable is the ammonium cation.
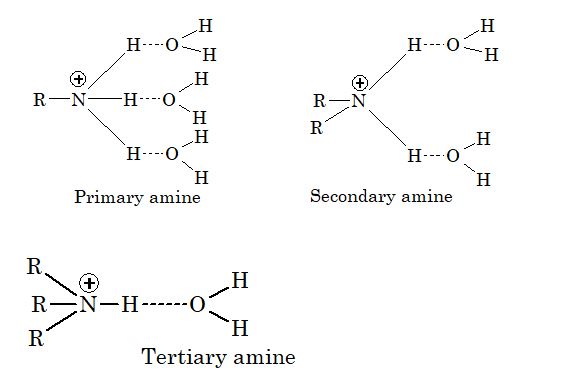
Thus, the ammonium cation derived from primary amine is the most stable as it has three hydrogen atoms which can form hydrogen bonds with water molecule.
The ammonium cation derived from secondary amine is less stable since it has two hydrogen atoms while tertiary amine is the least stable since it has only one hydrogen atom which can form hydrogen bond with water molecule.
[Note: Aniline is less basic than aliphatic amines as the lone pair of electrons present on nitrogen atom interact with the delocalized p–orbital of benzene ring, hence it is less available on nitrogen atom.]
The basic character for aniline, pyridine and pyrrole follows the order, (Kb given the parenthesis).
Pyridine > Aniline > Pyrrole
[2.3×10–9] [4.2×10–10] [2.5×10–14]
Illustration 4: Ethylamine is ………… basic than ammonia while aniline is …………….. basic than NH3.
(A) more, less
(B) less, more
(C) Both of the above
(D) None of the above
Solution: (A). Ethylamine is more basic than ammonia while aniline is less basic.
2. Alkylation:
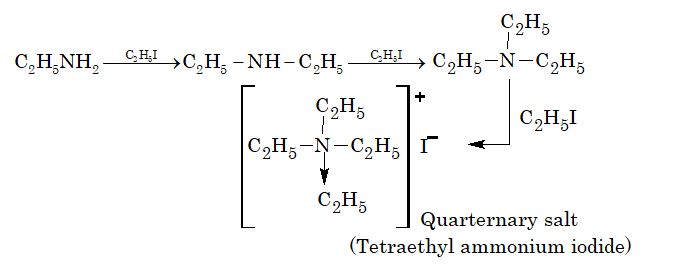
Aniline also gives similar reaction.
3. Acetylation:
Aniline also gives similar reaction.
The reactivity order of acetylating agents is
4. Benzoylation (Schotten–Bauman reaction):
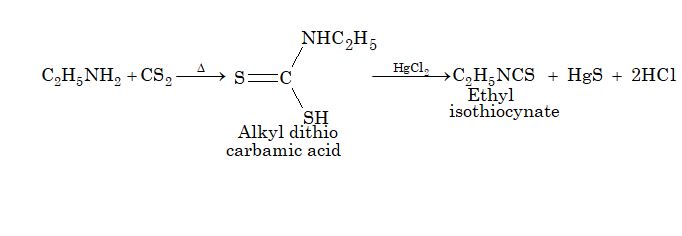
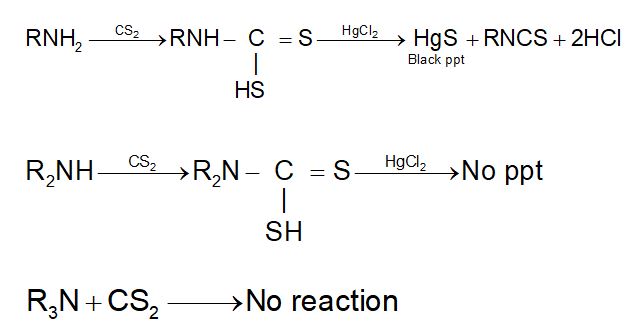
5. Hofmann mustard oil reaction:
Only primary amine give pungent mustard oil smell, while secondary amines are not decomposed by HgCl2 and tertiary amines do not react with CS2.
6. With CHCl3 / KOH (Isocynide test):
This reaction is also known as Carbylamine test.
R2N + CHCl3 + 3KOH → No reaction.
R3N + CHCl3 + 3KOH → No reaction.
7. Formation of Schiff’s base:
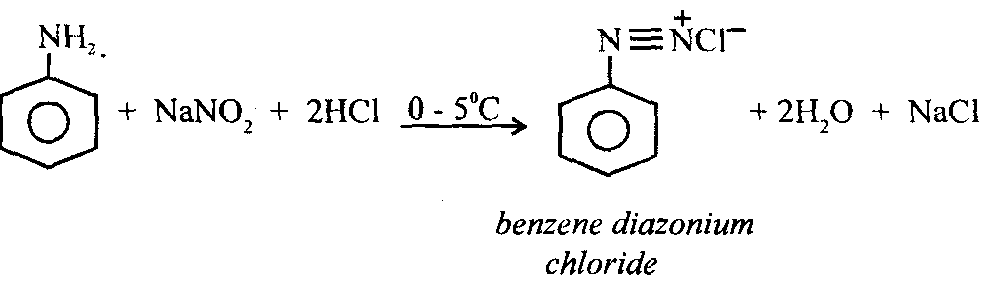
8. Diazotisation: In this reaction aniline reacts with nitrous acid at 0 to 5°C to form benzene diazonium chloride. As nitrous acid (HNO is unstable it is generated in situ from a mineral acid (HO) and sodium nitrite.
9. Substitution reactions of aniline.
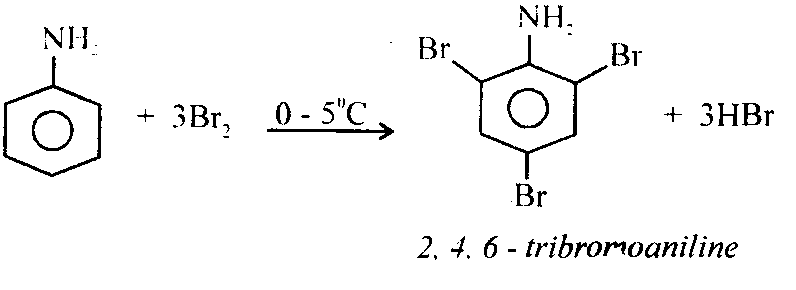
i) Bromination: Aniline reacts with bromine instantaneously to form a white precipitate of 2,4,6 – tribromoaniline.
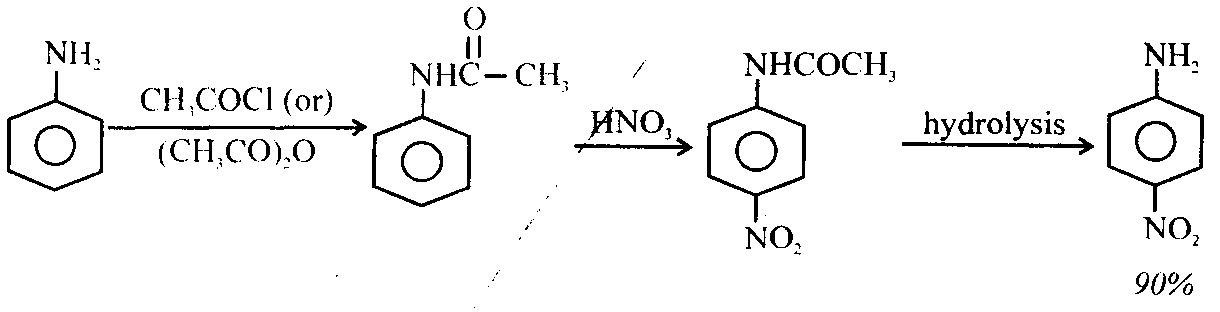
ii) Nitration: Aniline when treated with acid mixture of concentrated nitric acid and concentrated sulphuric acid form nitroanilines.
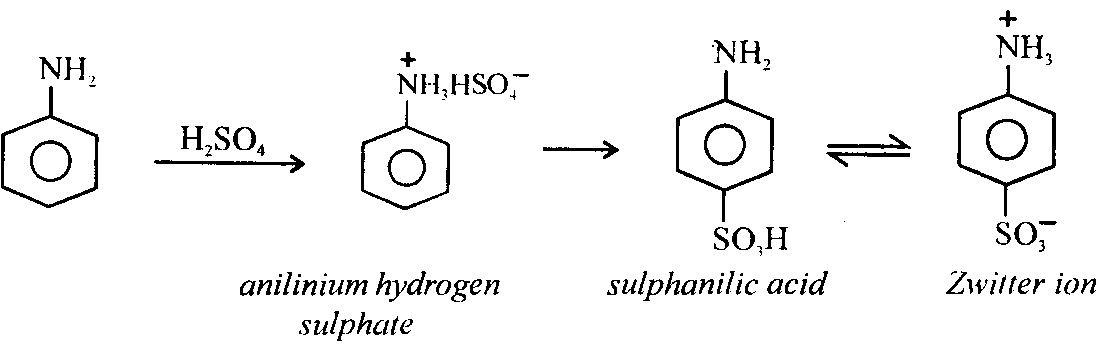
iii) Sulphonation: Aniline is sulphonated by heating anilinium hydrogen sulphate formed from aniline and sulphuric acid at 180 – 2000C, p – amino benzene sulphonic acid called sulphanilic acid is formed.
Uses of aniline:
a) in the manufacture of benzene diazonium chloride, a starting material in the preparation of several organic compounds.
b) in making Schiff’s bases that act as antioxidants in rubber industry.
c) in the preparation of many compounds like acetanilide, sulphanilic acid, sulpha drugs and azodyes.
Distinction (Identification) between 1o, 2o and 3o amines:
1. Reaction with HNO2:
(a) Primary amines react with nitrous acid to produce nitrogen gas.
CH3NH2 is an exception to this reaction.
(b) Secondary amines react with nitrous acid to produce a yellow oily layer.
(c) Tertiary amines react with nitrous acid to form soluble nitrite.
2. Reaction with benzenesulphonyl chloride(Hins Bergs’ Test):
(a) Primary amine reacts with benzenesulphonyl chloride to form a precipitate, which is soluble in NaOH solution.
(b) Secondary amines react with benzenesulphonyl chloride to give a precipitate, which is insoluble in NaOH solution.
(c) Tertiary amines do not react with benzenesulphonyl chloride as they do not possess replaceable hydrogen atoms.
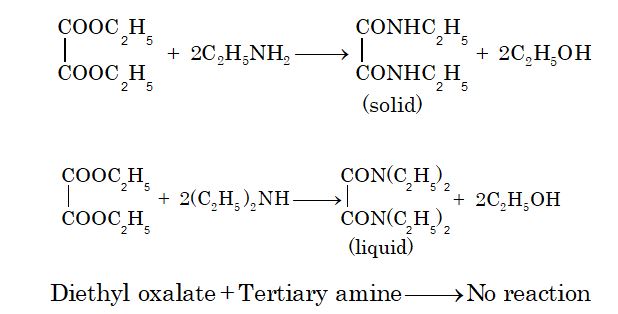
3. Reaction with diethyloxalate (Hofmann’s method)
Primary amines react to form solid crystalline oxamide and secondary amines react to give oily dialkyl oxamic ester. Tertiary amines do not react as they do not contain a replaceable H atom.
4. Hofmann mustard oil reaction
5. Oxidation by KMnO4:
BENZENE DIAZONIUM CHLORIDE:
Methods of Preparation
Aromatic diazonium chlorides are generally prepared by adding a cold aqueous solution of sodium nitrite to the solution of a primary aromatic amine in an acid at 273–278 K.
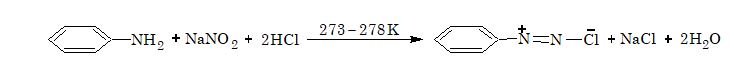
The process of conversion of a primary aromatic amine into its diazonium salt is called diazotization.
Benzene diazonium chloride is a colourless crystalline solid highly soluble in water. It is stable at 00C but on warming it reacts with water. In dry state it easily, decomposes, but in the form of benzene diazonium fluoro borate it is water insoluble and stable at room temperature.
Some Important Chemical Reactions of Benzene Diazonium Chloride (Importance in synthetic organic Chemistry):
1. Sandmeyer reaction:
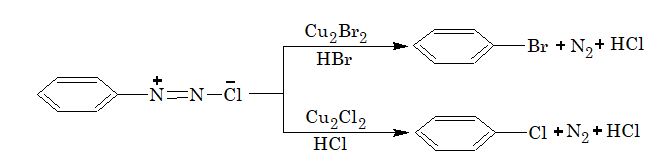
[Note: In the above reaction, if copper powder is used in place of cuprous salt, the reaction is known as Gattermann reaction.]
2. Balz–Schiemann reaction:
 3. Coupling reaction:
3. Coupling reaction:
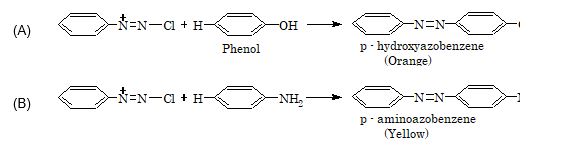
Illustration 5: The product obtained when phenol reacts with benzene diazoniumchloride is
(A) Phenylhydroxylamine
(B) para–aminoazobenzene
(C) Phenylhydrazine
(D) para–hydroxyazobenzene
Solution: (D)
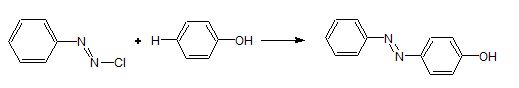
Uses of diazonium salt:
It is used in the preparation of azodye. The azoproducts have extended conjugate system with aromatic rings and – N = N – bond. These compounds are coloured and used as dyes.
NITRO COMPOUNDS:
The class of organic compounds containing nitro group as a substituent are called nitro compounds. They may be aliphatic or aromatic depending upon whether the nitro group is attached to an alkyl or an aryl group.
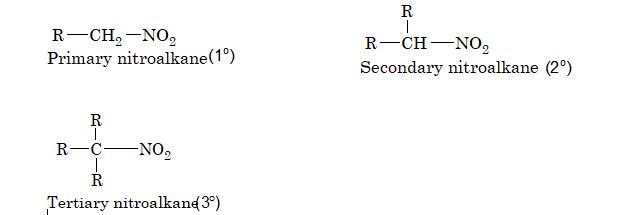
Nitroalkanes are further classified as primary, secondary and tertiary depending upon whether the nitro group is attached to a primary, secondary or a tertiary carbon atom.
METHODS OF PREPARATION OF NITROCOMPOUNDS:
1. From alkyl halides:
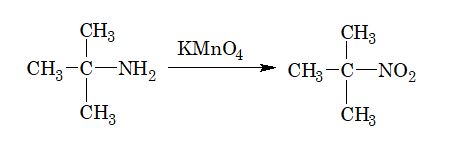
2. From tertiary alkyl amines:
3. From nitration of alkanes: Alkanes do not undergo nitration easily. However, with fuming HNO3 in the vapour phase at 693 – 793 K under pressure, alkanes do undergo nitration to give a mixture of nitroalkanes.
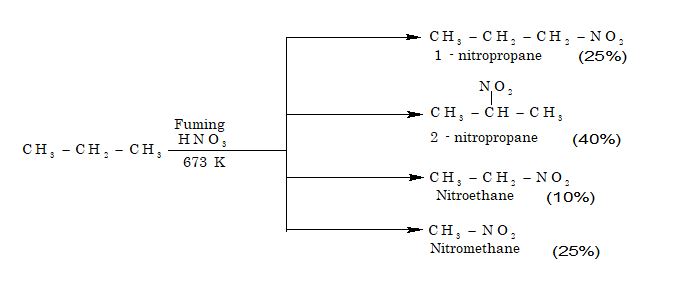
4. From benzene:
Nitrobenzene is a pale yellow coloured liquid. It boils at 1130C. It possesses strong odour of bitter almonds. It is almost insoluble in water but soluble in organic solvents like benzene. It is steam volatile.
CHEMICAL PROPERTIES OF NITRO COMPOUNDS
1. Reduction:
[Note: The final product, however, depends upon the pH of the reaction medium and nature of the reducing agent.]
(a) Reduction in the acidic medium:
(b) Reaction the alkaline medium:
(c) Reduction in the neutral medium:
(d) Reduction with metal hydrides:
(e) Catalytic reduction:
2. Hydrolysis:
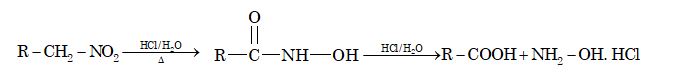
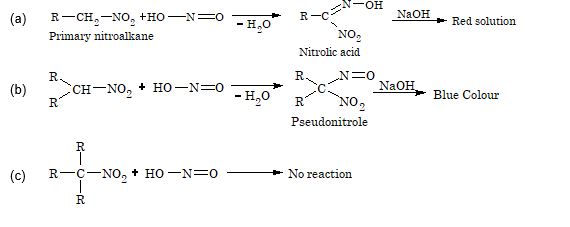
3. Reaction with nitrous acid: Primary, secondary and tertiary nitroalkanes behave differently towards nitrous acid.
4. Halogenation: In this reaction, all the a–hydrogen atoms of nitroalkanes are successively replaced by the halogen atoms.
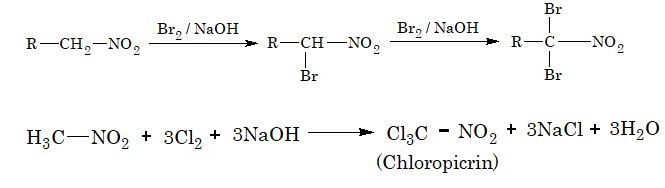
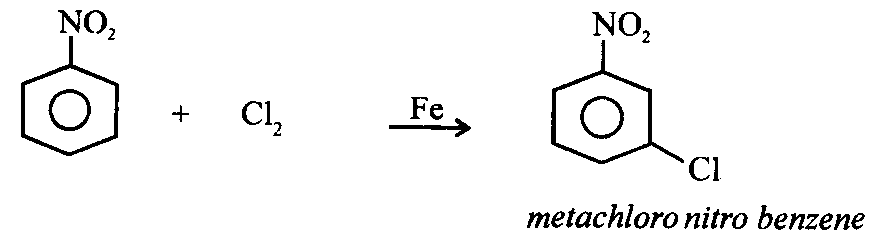
5. Nitration :
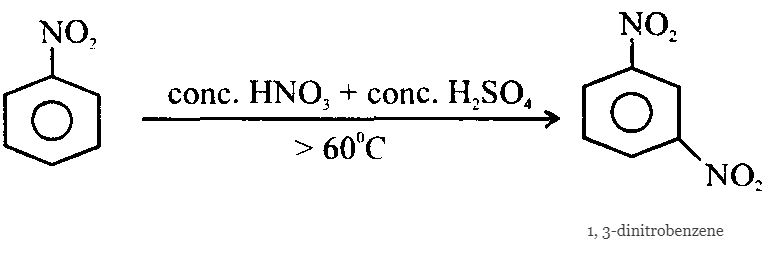 Uses of Nitrobenzene (Oil of mirbane):
Uses of Nitrobenzene (Oil of mirbane):
i) in the preparation of floor polishes.
ii) in the preparation of cheap perfumes under the name of oil of mirbane.
iii) in the preparation of dyes.
iv) as an oxidising agent and as solvent.
Illustration 6: In which of the following sequence of reactions the end product does not exhibit tautomerism?
(A)
(B)
(C)
(D)
Solution: (C). In reaction sequence (C), the end product is a 3o nitro compound.
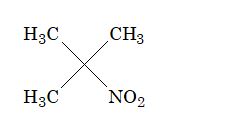
It does not have hydrogen, hence tautomerism is not possible.
CYANIDE AND ISOCYANIDES
The class of organic compounds obtained by replacement of the H–atom of hydrogen cyanide by an alkyl or aryl group is called cyanides or nitriles. The general formula is, , (where R is an alkyl group and Ar is an aryl group).
When H–atom of hydrogen isocyanide is replaced by alkyl or the aryl group, is called isocyanides or isonitriles. It is also called carbylamines. The general formula is,
[Note: group can be attached to the alkyl or the aryl group either through the carbon atom or through the nitrogen atom. Such a group which can be linked through two different sites, is called an ambidient group.]
METHODS OF PREPARATION
1. From alkyl halide:
(a)
(b)
2. From acid amides:
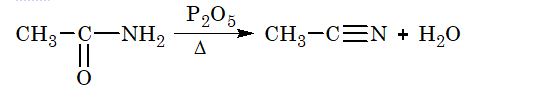
3. From aldoximes:
4. From primary amine:
CHEMICAL PROPERTIES OF CYANIDES AND ISOCYANIDES
1. Hydrolysis:
(i) Complete hydrolysis:
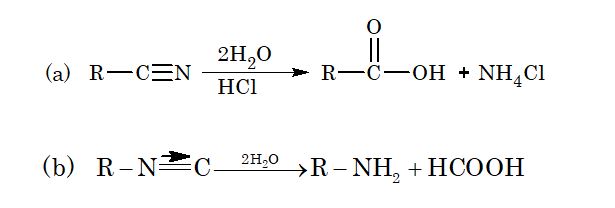
(ii) Partial hydrolysis:
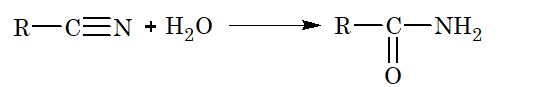
2. Reduction:
(a)
(b)
3. Stephen’s reduction:
4. Reaction with Grignard reagent:
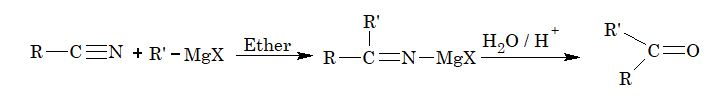 5. Addition reaction of isocyanides:
5. Addition reaction of isocyanides:
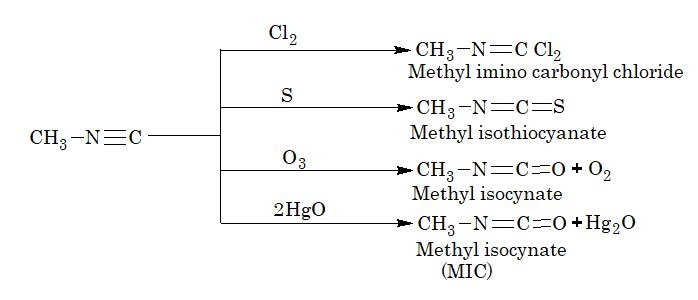
[Note: The methylisocyanate (MIC) gas was responsible for the Bhopal gas tragedy in December, 1984.]
FORMULAE AND CONCEPTS AT A GLANCE
1. Nitroalkanes (R – NO2) and alkyl nitrites (R – ONO) are functional isomers of each other.
2. Treatment of alkylhalides (R – X) with KNO2 gives R – ONO as major product while its treatment with AgNO2 gives R – NO2 as major product.
3. Nitroarenes can be prepared by reacting arenes (Ar – H) with nitrating mixture (conc. HNO3 + conc. H2SO4 ).
4. Nitration of Arene is an electrophilic substitution process, the attacking electrophile being NO2+ (nitronium ion) which is generated during the course of the reaction from nitrating mixture.
5. Amines are alkyl or aryl derivatives of ammonia (NH3).
6. Amines have pyramidal structure with bond angle around N depending upon size and number of alkyl groups linked to N atom.
7. Functional groups of 10, 20 and 30 amines are respectively .
8. Amines can be prepared by (i) Ammolysis of alkyl halides, (ii) Reduction of into compounds, (iii) Rediaction of cyanides, amides and oximes, (iv) Reduction amination of aldehyde ketones, (v) Gabrul’, Phthalimide synthesis.
9. Aniline reacts with HNO2 (NaNO2/HCl) at 273 – 280 K to form benzene diazonium chloride (Diazotisation reaction).
10. Aliphatic amines donot form diazonium salts because to their unstable nature.
11. RCN and RNC are metamers of each other.
12. Alkyl cyanides can prepared from : (i) alkylhalides (treatment with alcoholic KCN), (ii) 10 amides (dehydration with P2O6), (iii) aldoxime (dehydration with (CH3CO)2O).
13. Alkyl isocyanides are prepared (i) from 10 amines (carbylamine reaction), (ii) from alkyl halide, (using alcoholic AgCN).
14. Lower cyanides are soluble in water while isocyanides are insoluble.
SOLVED PROBLEMS-1
Prob 1. Organic compound
What is the compound A
Sol: Product of hydrolysis of B suggests that the N-substituted amide is Hence, must be anti to -OH group of oxime (A). That is, the lighter alkyl group is on the side of -OH group. Hence, compound (A) is anti-ethyl methyl ketoxime.
Prob 2. Identify the end product (C) in the following sequence of reactions
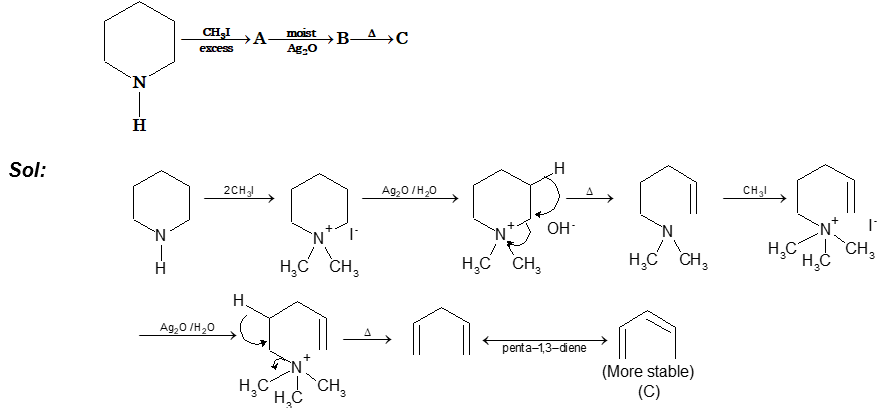
Prob 3. Identify C in the following reaction series
Sol:
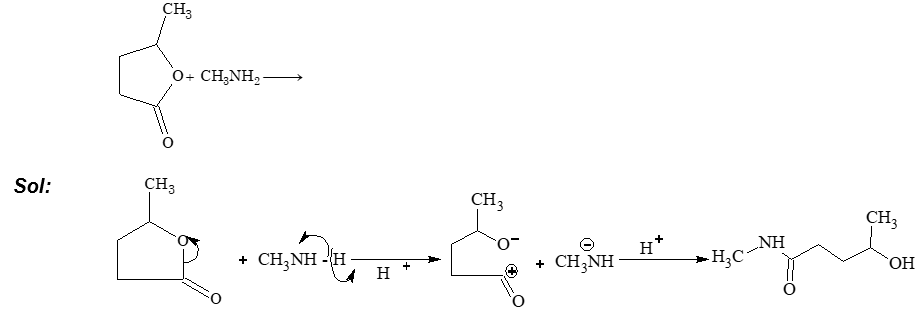
Prob 4. What is the product in the following reaction?
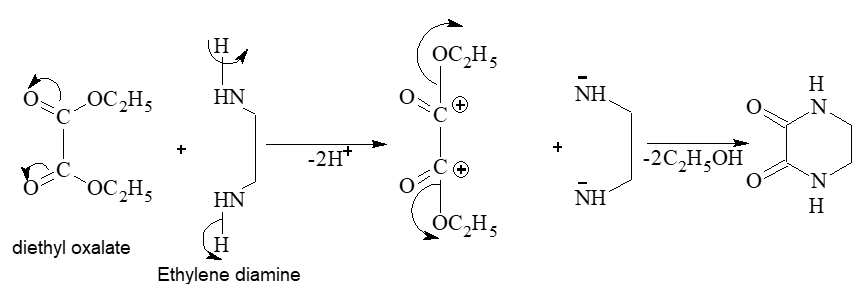
Prob 5. What will be the product when ethylene diamine react with diethyl oxalate?
Sol:
Prob 6. What are (A) and (B) in the following reaction?
Sol: Tertiary amines react with cyanogen bromide (CNBr) to form dialkyl cyanamide, which on hydrolysis gives secondary amine. This is van Braun cyanogen bromide reaction.
Prob 7. p-Cresol reacts with chloroform in alkaline medium to give compound (A) which adds hydrogen cyanide to form compound (B). The latter on acidic hydrolysis gives chiral carboxylic acid. What is the structure of this acid?
Sol: 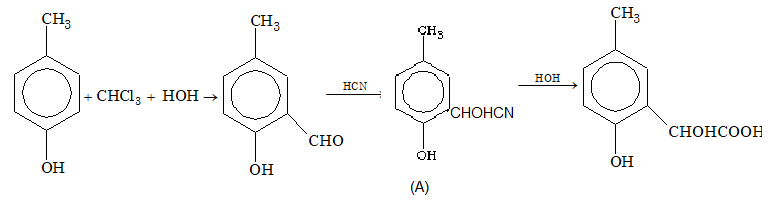
Prob 8. Write the names and structures of four isomeric amines having the molecular formula C3H9N.
Sol:
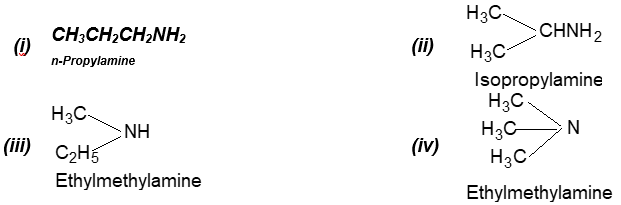
Prob 9. Trimethylamine is less basic than dimethylamine or methylamine. Explain why it so?
Sol: Methyl groups are electron releasing groups. Although in trimethylamine, the electron density on nitrogen is more in comparison to dimethylamine or methylamine yet it is less basic because crowding of three methyl groups makes it difficult for a proton to approach the nitrogen in order to form a bond. Thus, the electrons are there in trimethylamine but the path is blocked.
Prob 10. Compare the basicities of (a) HO(CH2)2NH2, (b) HO(CH2)3NH2, (c) CH3CH2NH2
Sol: The electron withdrawing capacity of OH decreases electron density on nitrogen atom thereby decreasing basicity. This effect decreases as the distance between OH group and N increases in the chain. Thus, decreasing order of basicity is (c) > (b) > (a).
SOLVED PROBLEMS-2
Prob 1. Treatment of ammonia with excess of ethyl chloride will yield
(A) diethylamine (B) ethane (C) tetraethyl ammonium chloride (D) methylamine
Sol: (C)
Prob 2. Identify X in the sequence
(A) CH3CH2NHCH3
(B) CH3CH2CH2NH2
(C) (CH3)3N
(D) CH3CHNH2 CH3
Sol: (B)
Prob 3. The action of nitrous acid on ethylamine gives
(A) ethane (B) ethyl nitrite (C) ethyl alcohol (D) nitroethane
Sol: (C)
Prob 4. What is the end product in the following sequence of reactions?
(A) Ethyl cyanide (B) Ethylamine (C) Methylamine (D) Acetamide
Sol: (B)
Prob 5. Aniline on treatment with conc. HNO3 + conc. H2SO4 mixture yields
(A) o– and p–nitro anilines
(B) m–nitroaniline
(C) a black tarry matter
(D) no reaction
Sol: (B) Aniline with forms anilinium ion , which is deactivating group.
Prob 6. What are the names of the following reactions?
(1)
(2)
(3)
(4)
(A) Ritter, Hofmann, Mendius, Grignard
(B) Grignard, Ritter, Hofmann, Mendius
(C) Hofmann, Ritter, Grignard, Mendius
(D) None of the above
Sol: (B) This is the correct set of names of the respective reactions.
Prob 7.
The name of the reaction and the end product would be
(A) Curtius degradation, 2o amine
(B) Curtius degradation, 1o amine
(C) Schmidt reaction, primary amine
(D) Schmidt reaction, secondary amine
Sol: (B) It is Curtius degradation and the product is a primary amine.
Prob 8. Ethylamine on oxidation with acidified KMnO4 gives
(A) acetaldehyde (B) ethylamine oxide (C) ethanol (D) acetamide
Sol: (A) Oxidation of ethylamine gives acetaldehyde.
Prob 9. Which of the following compound does not liberate nitrogen with HNO2?
(A) Carbamide
(B) Primary amine
(C) Secondary amine
(D) Alkanamide
Sol: (C)
Prob 10. . True statement for the compound A is
(A) it does not undergo alkaline hydrolysis
(B) it can also be produced by RBr and KCN
(C) it gives primary amine on reduction
(D) it gives secondary amine on acidic hydrolysis
Sol: (A) Compound A is an isocyanide. It’s alkaline hydrolysis does not take place.








