1. ALKALI METALS
Introduction:
The elements of group 1 of periodic table are called alkalimetals because the oxides of these elements dissolve in water and produce strong alkalies (water soluble). Group 1 consists of lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr). These elements are s – block elements because the valence electron enters into ‘s’ orbital of balance shell. These elements have ‘one’ electron in their valency shell and thus placed in the group 1 or I-A of periodic table. Lithium is known as a bridge element and was discovered by Arfwedson. Francium is a radio active element. Sodium and potassium were discovered by Davy, rubidium and cesium by Bunsen and Kirchhoff and francium by Perey.
General characteristics of Alkali Metals:
A. Electronic configuration: ns1
| Element | Atomic Number | Electronic configuration |
| Li | 3 | [He] 2s1 |
| Na | 11 | [Ne] 3s1 |
| K | 19 | [Ar] 4s1 |
| Rb | 37 | [Kr] 5s1 |
| Cs | 55 | [Xe] 6s1 |
| Fr | 87 | [Rn] 7s1 |
B. Atomic and physical properties:
1. Physical state (Appearance): All alkali metals are silvery white metals with metallic lustre.
2. Softness: These are soft, malleable and ductile metals which can be cut with knife. They posses metallic lustre when freshly cut due to oscillation of electrons.
3. Atomic and ionic radii: The alkali metals have the largest atomic as well as ionic radii in their respective period because each period in the periodic table starts with an alkali metals (except 1st period). Atomic radii as well as ionic radii increases as we move down the group from Li to Cs due to addition of new shell at each step and increases in shielding effect. But increasing nuclear charge tend to decrease the atomic as well as ionic radii.
4. Density: These are quite light metals having low densities. Lithium is the lightest known metal. On moving down the group, density increases from Li to Cs.
Illustration 1: The density of potassium is lesser than that of sodium. Why? Solution: It is because of the abnormal increase in size on moving from Na to K.
5. Melting and Boiling points: The melting and boiling points of alkali metals are quite low and decrease down the group.
| Li | Na | K | Rb | Cs | |
| MP (00C) | 181 | 97.0 | 63.0 | 39.0 | 28.50 |
| BP (00C) | 1347 | 881 | 766 | 688 | 705 |
Reason: Alaklimetals have only one electron in their valence shell. So, in these metals, the inter atomic forces are weak. This is why, the melting and boiling points of alkali metals are low. In addition to this weaking of metallic bond is also responsible for low mps and bps. On moving down the group melting and boiling points of alkali metals are decreased. Reason: As the size of atom increases, the repulsion of the non-bonding electrons also increases. This increased repulsion between the non-bonding electrons causes lowering of the mps & bps down the group. Francium is liquid at room temperature.
6. Ionization energy: Alkalimetals have relatively lowest first ionization energies amongst the elements in their respective periods.
Reason: The s – electron in valence shell of alkali metals is far away from the nucleus. So, the outer most s-electron is weakly held by the nucleus. Therefore, to remove it very little amount of energy is required. This is why ionization energies of alkali metals are low.
Table–2 First ionization energies of alkali metals
| Element | Li | Na | K | Rb | Cs |
| IE (kJmol-1) | 520 | 496 | 419 | 403 | 376 |
On going down the group, ionization energy decreases. Reason: The distance of valence electron from the nucleus increases as we go down the group. As a result, the ionization energy decreases from Li to Cs.
7. Electropositive character (metallic character): Alkalimetals are strongly electropositive or metallic in nature and this character increases from Li to Cs.
Reason: It is because of low ionization energies of alkali metals. Cesium (Cs) is the most highly electropositive or metallic element due to its low I.E.
8. Electrode potentials: Alkali metals are highly electropositive and tend to undergo oxidation, whenever placed in water liberating hydrogen gas.
Table – 3 The standard electrode potentials for M+ / M half cell
| M+ / M half cell | Li+/Li | Na+/Na | K+/K | Rb+/ Rb | Cs+ / Cs |
| E0 (volts) : | – 3.05 | – 2.71 | -2.92 | – 2.92 | – 2.93 |
Negative values of the electrode potential (E0) for alkali metals indicate that all these metals have strong tendency to undergo oxidation. Thus, Li+ / Li electrode has most negative electron potential. Therefore, Li has the highest tendency to lose electrone. As a result, lithium (Li) is the strongest reducing agent.
9. Reducing character: All the alkalimetals are good reducing agent due to their low ionization, energies. Their reducing character, follows the order Na < Cs < Rb < K < Li (in aqueous solution)
Among the alkali metals ‘Li’ has the highest negative electrode potential (E0 = – 3.05V), which depends upon its (Li) heat of vaporization, ionization energy and its heat of hydration. Hence, Lithium is the strongest reducing agent.
10. Electronegativity: Alkali metals have low electronegativity values and decrease down the group.
| Li > | Na > | K | Rb > | Cs | |
| EN | 1 | 0.90 | 0.80 | 0.80 | 0.70 |
Reason: Because of large size and low nuclear charge, the alkalimetals are not able to attract electrons towards them. As a result, these elements show very low electronegativity, since, the atomic size increases from top to bottom in a group, hence, the electronegativity decreases from Li to Cs.
11. Oxidation states: All alkali metals exhibit only + 1 oxidation state in their compounds.
Reason: Because alkali metals have only one electron in their valence shell and therefore, can lose this valence electron (ns1) to form unipositive cations. These cations have stable gas configuration in the valence shell i.e., ns2np6.
So, all alkali metals in their compounds exhibit + 1 oxidation state. Since, alkali metal ions (M+) have noble gas configuration (ns2np6) with no unpaired electrons, they are diamagnetic in nature (while alkali metals are paramagnetic) and colourless. However, all the compounds of alkali metals are colourless. But their permanganates and dichromate’s are coloured because their anions, MnO4– & Cr2O7– are coloured.
12. Hydration of ions (cations): All the alkali metal salts are ionic (except lithium) and soluble in water, due to the fact that the alkali metal cations (ions) exhibit strong tendencies toward hydration. i.e.,
M+ (g) + H2O (excess) M+ (aq)
Thus, the reaction is exothermic. Since, the hydration tendency depends upon charge to radius ratio (i.e., q/r), of cation, hence it decreases down the group from Li+ to Cs+. Thus, smaller the size of cation, greater is its hydration energy.
Relative ionic radii is Li+ < Na+ < K+ < Rb+ < Cs+
Thus, relative degree of hydration is Li+ > Na+ > K+ > Rb+ > Cs+
13. Flame colouration: All alkalimetals and their salts impart characteristic colour to a non-luminous flames.
Table 4: Flame colouration of Alkalimetals
| Li | Na | K | Rb | Cs |
| Crimson red | Golden Yellow | Paleviolet (purple) | Violet (purple) | Sky blue (Blueish violet) |
Reason: When an alkali metal or its salt is placed in a flame its electrons get excited to the higher energy levels due to the absorption of energy. The excited state is an unstable state. So, the electrons tend to come back to the ground state, the light of particular colour is emitted out. It depends upon the difference of energy between ground and excited states of the electrons in the atom or ion. On moving down the group, the ionization energy goes on decreasing and hence, the frequency (energy) of emitted light increases from Li to Cs.
14. Photoelectric effect: Due to low ionization energy and work function, alkali metals specially K and Cs show photoelectric effect and hence are used in photoelectric cells.
15. Electrical conductivity: All the alkali metals are good conductor of heat and electricity due to the preserve of loosely held valence electrons which are free to move through out the crystal structure. On going down the group, electrical conductivity increases from Li+ to Cs+, i.e.,
Li+ < Na+ < k+ < Rb < Cs+
Exercise 1:
(i) Sodium ordinarily does not show an oxidation state of +2 because of its
(A) high first ionization energy
(B) high second ionization energy
(C) large ionic radius
(D) high electronegativity
(ii) Which one of the following statements about alkali metals and their compounds is correct?
(A) Caesium is used in photo electric cells
(B) Molten NaCl on electrolysis give Na at anode and Cl2 at the cathode
(C) Alkali metal atom has the smallest size in its period
(D) Alkali metals do not react with water
(iii) Caesium forms chiefly ionic compounds because
(A) the valence electrons are poorly bound to the nucleus
(B) its electronegativity is very high
(C) its ionization energy is very low
(D) the polarizability of the cation is high
Answer to Exercise 1:
(i) B
(ii) A
(iii) C
C) Chemical properties:
1. Reaction with moist air: On exposure to moist air, their surface, get tarnished due to the formation of their oxides, hydroxides, and carbonates at the surface.
e.g.,
Thus, alkalimetals are very reactive towards moisture air hence, they are preserved (kept) under kerosene oil or paraffin oil but lithium is kept wrapped in paraffin wax because it floats on the surface of kerosene oil due to its very low density.
Note: Lithium also reacts with nitrogen in the air to form
2. Reaction with oxygen: All the alkali metals, when heated with oxygen form different types of oxides.
e.g. Lithium forms, lithium oxide, sodium forms sodium peroxide, while K, Rb and Cs form their respective superoxides.
(white normal oxide).
lithium oxide
(it also forms normal oxide).
(these also form normal and peroxides).
Illustration 2: Na2O2 (or) K2O2 is used as air purifier in submarines etc. What is the principle involved in it?
Solution: Na2O2 absorbs CO2 and releases O2. So it is used in the purification of air.
2Na2O2 + 2CO2 2Na2CO3 + O2
Here K2O2 is better than Na2O2 in the purification of air in rooms and in submarines.
The principle involved here is Na2O2 acts as reducing agent.
Illustration 3: Super oxides of alkali metals are coloured. Why?
Solution: Superoxides of alkali metals are coloured. It is due to the presence of unpaired electron in the ion .
Illustration 4: What is caustic soda? Why is it given that name?
Solution: When NaOH falls on skin, it decomposes the muscle proteins and makes a pulp. Therefore, it is called as “caustic soda”.
Illustration 5: Sodium pieces catch fire when they are put in water. Why?
Solution: Sodium reacts vigorously with water and water and more amount of heat is evolved. Due to the heat of the reaction, molten sodium formed catches fire.
3. Reaction with water:
All alkalimetals readily react with water evolving hydrogen:
2M (Li, Na, K, Rb, Cs) + 2H2O 2MOH + H2 (g)
(alkalimetal) (Metal hydroxide)
The reaction becomes more and more vigorous in going from Li to Cs i.e., the reactivity with water increases from Li to Cs. The hydroxides are colourless, strong alkaline and corrosive compounds. These are soluble in water and dissolve with the evolution of heat. The hydroxides are thermodynamically stable except Li OH.
e.g., 2LiOH Li2O + H2O
The relative strength of the hydroxides increases from LiOH to CsOH
LiOH < NaOH < KOH < RbOH < CsOH
4. Reaction with hydrogen: The alkali metals combine directly with hydrogen to form ionic hydrides, i.e., crystalline hydrides.
2M + H2 2MH (M = Li, Na, K etc)
Metal hydride.
The reactivity of alkali metals towards hydrogen decreases from Li Cs. i.e., Li > Na > K > Rb > Cs, due to decreasing lattice energy of these hydrides with the increasing size of the metal cation.
Electrolysis of fused hydride (i.e., LiH) yield hydrogen at anode, but other hydrides decompose before melting.
These hydrides react with water, liberating hydrogen, MH + H2O MOH + H2.
The ionic character of bond in these hydrides increases from LiH to CsH.
Thus, the stability of hydrides follows the same order as LiH > NaH > KH > RbH > CsH.
They are powerful reducing agents especially at high temperatures.
SiCl4 + 4NaH SiH4 + 4NaCl
Illustration 6: LiAlH4 is used as
(A) an oxidizing agent
(B) reducing agent
(C) a water softner
(D) moderant.
Solution: (B) LiAlH4 is used as a reducing agent. It is prepared by the following reaction.
5. Reaction with halogens:
- The alkali metals directly react with halogens to form ionic halides, , except certain Li X.
- The reactivity of alkalimetals towards a particular halogen increases from Li to Cs, while that of halogen towards a particular alkali metal decreases from F to I. e., F > Cl > Br > I.
- The halides are crystalline and have high melting and boiling points.
- The fused halides are good conductors of electricity and used for the preparation of alkali metals.
- All halides, except LiF, dissolve in water.
- These are colourless but on heating they turn yellow, blue, etc, owing to non-stoichometry and crystal defects.
- Halides of K, Rb and Cs have a property of combining with extra halogen atom to form polyhalides. KI + I2 KI3
Illustration 7: Why Li F is insoluble in water, whereas other alkali fluorides are soluble? Explain.
Solution: Li F is insoluble due to its very high lattice energy. Lattice energy varies inversely with size of ions i.e., cation and anion. E.g. For a given anion, it decreases with increase in the size of cation. Li+ ion being the smallest has the highest lattice energy in comparison of hydration energy. As the size of cation increases from Li to Cs, lattice energy of the fluorides decreases from Li to Cs, and they become soluble.
7. Reaction with liquid ammonia: All alkali metals dissolve in liquid ammonia without evolution of H2 and give blue solution.
M M+ + e–
- The blue colour of dilute solution is due to the presence of ammoniated electron. But at very high concentration colour changes to metallic copper or on heating, the blue colour of the solution, changes to bronze.
- The solutions are good conductors of electricity due to the presence of ammoniated electrons and ammoniated cations. On cooling the conductivity increases further.
- The solutions are paramagnetic in nature due to the presence of unpaired electrons and ammoniated cations. However, the paramagnetism decreases with increasing concentration (> 3M) due to the association of ammoniated electrons to yield diamagnetic species containing electron pairs. e.,
diamagnetic species.
8) Nature of carbonates and bicarbonates:
- All alkalimetals forms carbonates of the type M2CO3. g.,
- Li2CO3 is unstable towards heat and decomposed on heating to give Li2O and CO2.
The Li+ ion exerts a strong polarizing action and distorts the electron cloud of the nearby oxygen atom of the large ion. This results in the weakening of C – O bond and strengthening of the Li – O bond. This ultimately facilitates and favour the decomposition of Li2CO3 into Li2O and CO2 because the lattice energy of Li2O is higher than the lattice energy of Li2CO3.
- The stability of the carbonates increases from Li CO3 to CsCO3, as the basic strength of corresponding hydroxides increases form LiOH to CsOH. Thus the order of stability is
9. Nature of sulphates:
- Sulphates of the type M2SO4 are known.
- Li2SO4 is insoluble in water where as the other sulphate are soluble in water.
- Li2SO4 does not form alums and is also not amorphous with other sulphates.
- These sulphates, on fusion with carbon form sulphides.
M2SO4 + 4C M2S + 4CO
- These sulphates form double salts with the sulphates of trivalent metals such as Fe, Al, Cr.
- The double sulphate crystallize with number of water molecules. e.g., Potash alum – K2SO4. Al2 (SO4)3. 24H2O, contain 24 water molecules.
- Li2SO4 is not known to form alum.
10. Complex formation:
- Alkalimetals have a very little tendency to form complexes. Lithium being smaller in size forms. Certain complexes i.e., Lithium aluminhydride, (LiAlH4) but polydentate legends such as crown ether and crypt ands form highly stable complexes.
- This tendency of complex formation decreases from Li to Cs.
Anomalous behaviour of lithium:
Reason:
i) smaller size
ii) very high ionization energy
iii) higher electronegativity
iv) absence of d – orbital in valence shell.
Lithium shows following anomalous properties.
i. It is harder than other alkalimetals due to strong metallic bond.
ii. Lithium combines with O2 to form lithium monoxide, Li2O whereas other alkali metals form perioxides (M2O2) and super oxides, MO2.
iii. LiH is the stablest of all the alkali hydrides.
iv. Lithium unlike the other alkalimetals reacts with the nitrogen toform the nitrides.
6Li + N2 2 Li3N
v. LiOH is a weak base and decomposes to give the corresponding oxide while the hydroxides of alkali metals are stable to heat and sublime as such.
vi. LiF, Li2CO3 and lithium phosphates are insoluble in water while the corresponding salts of other alkali metals are soluble in water.
vii. LiCO3 decomposes on heating to evolve CO2 whereas other alkali metal carbonates do not.
Diagonal Relation ship:
- Lithium shows diagonal relationship with magnesium since they have almost same polarizing power, i.e., charge / size, ratio.
- Lithium resembles magnesium in the following respects.
i. The atomic radius of lithium is 1.31Å while that of magnesium is 1.36 Å.
ii. The ionic radius of Li+ is (0.60 Å) while that of Mg2+ ion is (0.65 Å).
iii. Li (1.0) and Mg (1.2) have almost similar electronegativities.
iv. Both Li and Mg are hard.
v. Both decompose water only on heating.
vi. Both combine with oxygen to form monoxides i.e., Li2O and MgO.
vii. LiF is insoluble in water like MgF2.
viii. Both LiOH and Mg (OH)2 are weak bases.
ix. Both Li and Mg combine with nitrogen to form their respective nitrides, Li3N and Mg3N2.
Metallurgy of alkali metals:
Extraction of Alkalimetals: Alkali metals can not be obtained
i) By reduction of their oxides because these are the strongest reducing agent (known).
ii) By the electrolysis of their aqueous solutions because the metal liberated at the cathode at once reacts with H2O to form metal hydroxide and H2.
iii) By metal displacement method from their aqueous solutions because no metal is more electropositive than alkali metals (known).
Therefore, alkalimetals are extracted by electrolysis of their fused chlorides in presence of small amount of other metal chlorides (to lower the melting point) or fused hydroxides.
Metallurgy of lithium:
1. Important mineral of lithium:
i) Spodumene – Li Al (Si O3 )2 :
ii) Lepidolite, – Li2 Al2 (SiO3)3 (FOH)2
iii) Amblygonite – Lial (PO4). F
Extraction of Lithium: Lithium metal can be extracted from its ore in two steps:
i) Preparation of lithium chloride from the ore
ii) Electrolysis of molten lithium chloride
i) Preparation of lithium chloride from the ore:
a) By leaching method. The ore is heated to about 11000C and then washed with sulphuric acid at 2520
The filtrate containing Li2SO4 is then treated with sodium carbonate to precipitate Li2CO3
On treatment with HCl, LiCl is obtained.
b) By fusion method: The washed ore is fused with limestone (CaCO3).
- Li2CO3 so formed is leached with water to form LiOH, which is converted to LiCl by reacting the solution with HCl.
- From the LiCl so obtained, anhydrous LiCl is obtained by heating it in a current of HCl(g)
ii) Electrolysis of molten lithium chloride: A mixture of LiCl and KCl (55% + 45% by mass) is melted at 4500C , and electrolysed in a cell made of steel, lined with a refractory material. A steel cathode and a graphite anode are used for electrolysis.
LiCl Li+ + Cl–
At cathode: Li+ + e–
Li At anode Cl– Cl + e–
2Cl Cl2
Lithium metal containing about 1% potassium is collected. Chlorine gas is obtained as a by-product.
Properties of Lithium:
i) Lithium is a silvery white metal. It is harder than sodium but softer than lead.
ii) It is a good conductor of heat and electricity
iii) Lithium gives all the typical reactions of alkali metals.
Metallurgy of Sodium:
1. Occurrence: Sodium does not occur in free state due to its high reactivity.
2. Important minerals of sodium:
- Sodium is widely distributed in combined state. Sea water contains 2.0 to 2.9% NaCl. Some lakes also contain sodium salts. The important mineral of sodium are __
i) Rocksalt or common salt – NaCl
ii) Chile salt petre –
iii) Sajiimitti or sodium carbonate – Na2CO3 (Occurs in some northern part of India).
iv) Sodium sulphate or Glauber’s salt – Na2SO4. 10 H2O (Mirabilite Na2SO4).
v) Cryolite – Na3AlF6
vi) Borax – Na2B4O7.10H2O (known as Tincal in India).
Extraction of sodium: Two methods are employed the extraction of sodium metal.
i. Castner process:
Devised by Sir Humphry Davy (1807) and improved by Castner (1890). In this process, anhydrous, fused NaOH is electrolysed to get ‘Na’ metal at cathode.
Reaction:
At cathode (Iron rod):
At anode (hollow Ni cylinder surround the cathode):
Moreover, Sodium reacts with small amount of water produced at anode to give hydrogen.
During the electrolysis, the impurities settle to the bottom of the tank the temperature should be maintained around the tank. The temperature should be maintained around 3300C to prevent any sodium metal from going into fused hydroxide which, it happens, reduces the yield.
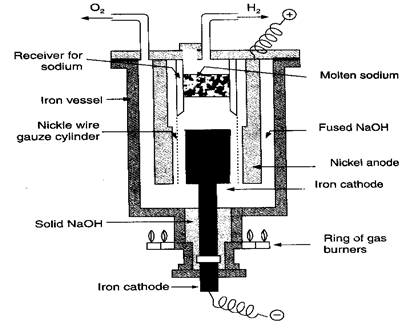
Fig: Castner process (extraction of sodium)
Method:
- In this process, molten sodium hydroxide (caustic soda, NaOH) is electrolysed using an iron cathode and nickel anode.
- The electrodes are prevented from touching each other by a nickel wire gauze cylinder.
- On passing electric current through molten caustic soda, sodium is liberated at the cathode.
- Being lighter than fused caustic soda, liberated sodium metal floats over the surface and collects inside the receiving vessel where hydrogen prevents sodium from oxidation.
- Excess of the gas escapes out from the outlet. Sodium is removed from time to time with the help of perforated spoons.
- Oxygen is liberated at the anode and leaves the iron vessel.
- Sodium so collected to kept under kerosene.
ii. Down’s process:
Sodium is extracted by electrolysis of fused NaCl containing a little CaCl2 or KCl and KF at 6000C. Reaction:
At cathode (iron): Na+ + e– Na (very pure)
At anode (graphite): Cl– Cl + e–
2Cl– Cl2 + 2e–
Inspite of the possibility of ‘Ca’ impurity in this method, the metal obtained is very pure.
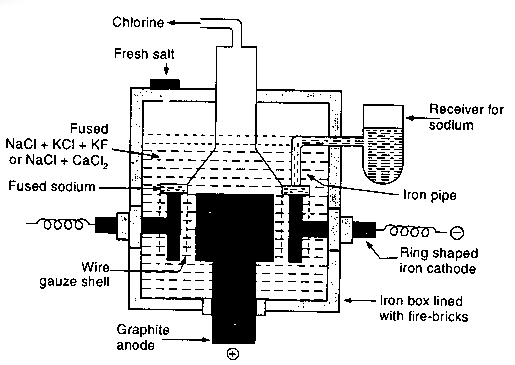
Fig: Down’s processes (extraction of sodium)
Description:
- In this process, sodium metal is obtained by electrolyzing molten sodium chloride (common salt) using a graphite anode and a ring shaped iron cathode.
- The two electrodes are separated by a wire gauge partition.
- On passing electric current, chlorine is liberated at the carbon anode and escapes through the outlet.
- Sodium rises from the cathode and remains in the wire gauze shell.
- The level of molten sodium rises and is forced into the receiver.
- The process is continuous and the fresh salts is introduced to maintain the level of molten electrolyte high enough to allow sodium to rise into the iron pipe.
Note: Melting point of pure NaCl is 8030C, at this temperature extraction of Na from NaCl is difficult. So a mixture of CaCl2 or KCl & KF is added to reduce the m.p. of NaCl upto 6000C.
Physical properties of sodium:
i) It is a soft metal.
ii) Freshly cut sodium is a silvery white metal.
iii) It get tarnished when placed in air.
iv) It is highly reactive hence, it is stored in inert solvents like kerosene.
v) It gives the characteristic D1 (5890Å) and D2 (5896 Å) lines in the yellow region of the visible spectrum.
Chemical properties of sodium:
i) Reaction with air: Sodium loses its lustre when exposed to moist air and turns into a white power due to the slow formation of its oxide, hydroxide and finally carbonate.
ii) Reaction with water: Sodium reacts vigorously with water and liberates hydrogen. Due to heat of reaction, molten sodium dances on water and catches fire finally.
iii) Reaction with non-metals: (Binary compounds are formed):
iv) Reaction with ammonia:
Na / NH3 (liq) are used as strong reducing agent in organic chemistry.
v) Reduction reaction.
4Na + CO2 2Na2CO3 + C
Si O2 + 4 Na 2Na2O + Si
BeCl2 + 2Na + 2NaCl + Be
Al2O3 + 6Na 3Na2O + 2Al
2HCl + 2Na 2NaCl + H2
C2H2 + 2Na Na2C2 + H2
C2H2 + 2Na Na2C2 + H2
(2C2H2 + 2Na 2NaHC2 + H2)
Compounds of sodium:
1. Sodium chloride, common salt or table salt (NaCl):
Manufacture:
i) By evaporation of sea water
ii) By passing HCl gas through saturated solution of crude NaCl, pure NaCl is obtained (due to common ion effect).
Note: 1) 28% NaCl solution is called Brine.
2) Common salt contains CaSO4, CaCl2 and MgCl2 as an impurities is deliquescent in nature.
2. Sodium hydroxide, caustic soda (NaOH):
Manufacture:
i) By Causticizing process (Gossage process): A, 10 – 20% solution of Na2CO3 is treated with milk of lime in an iron tank.
ii) By Electrolytic processes:
- In these process, aqueous solution of NaCl (Brine) is electrolysed to get a pure NaOH. For the electrolysis of NaCl three type of electrolytic cell are used.
- a) Nelson Cell (Process): In this process, a perforated stell ‘U’ tube lined inside with asbestos, is used as cathode.
- The ‘U’ tube is suspended in an outer iron tank and brine solution is taken inside the ‘U’ tube.
- A carbon rod used as anode is sub pended into the brine solution from the top of the tank.
- The cathode and the anode are separated by the asbestos lining present inside the ‘U’ tube.
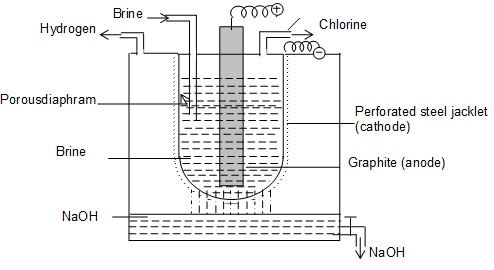
Fig. Nelson Cell
- When brine solution is electrolysed, NaOH as well as H2 are obtained at cathode and Cl2 at anode, the following reactions take place.
At anode (graphite) : Cl– Cl + e–
2Cl Cl2 (byproduct).
At cathode (steet jacket):
Na+ + 2OH– NaOH
Cl2 and H2 are obtained as byproducts.
- The discharge potential of Na+ and OH– ions is higher than those of H+ and Cl–
- Thus 50% of NaCl (approx) is converted into NaOH. The resulting solution with 1% NaCl and 1% NaClO3, is obtained. On further evaporation fused NaOH is obtained, which is cast into different shapes.
Note: Now a days in manufacture of NaOH, the cathode and anode compartments are separated by a Nafion membrane. Nafion membrane is a copolymer of tetra fluoromethylene and pentafluoro-sulphonyl ethoxy ether. The copolymer is supported on a Teflon mesh.
Illustration 8: What happens when the products (NaOH) come into the contact of byproduct i.e., Cl2 in electrolytic cell?
Solution: When product (NaOH) come into the contact of by product (Cl2) chloride and hypochloride are formed. Reaction is
2NaOH + Cl2 NaCl + NaOCl + H2O
b. Caster – Kellner cell (Mercury cathode process):
- This process is used to avoid reaction between NaOH and Cl2.
- NaOH is obtained by the electrolysis of aqueous solution of NaCl (brine).
- The cell has three compartments and involves following reactions.
In outer compartment:
Anode – Graphite rod; Cathode – Mercury (Intermediate), Electrolyte – Brine solution. Reaction: NaCl Na+ + Cl–
At anode: Cl– Cl + e
2Cl Cl2 (g)
At cathode: Na+ + e– Na
Na + Hg Na(Hg) (sodium amalgam)
In central compartment:
Anode – Mercury, Cathode – Iron rods; electrolyte – dilute solution of NaOH.
Reactions:
At cathode:
At anode: NaHg – 2e Na+ + Hg (Hg is intermedial anode).
Na+ + OH– NaOH. (sodium hydroxide)
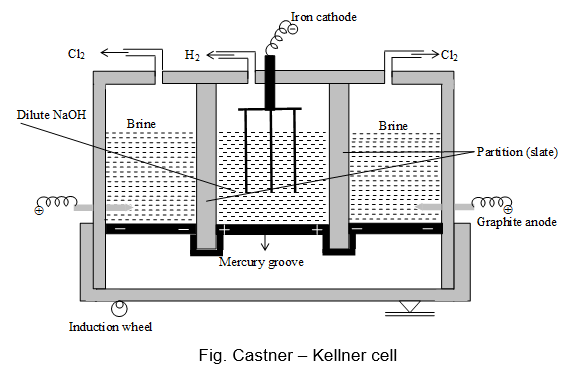
Description:
- Castner – Kellner cell is the most widely used mercury –cathode type cell.
- It consists of a large rectangular iron tank divided into three compartments by means of non-porous slate partitions. These partitions do not touch the bottom of the cell.
- A layer of mercury at the bottom of the cell separates the three compartments from one another.
- The one end of the cell is pivoted, while the other rests on an eccentric (induction) wheel which gives a rocking motion to the whole cell keeping the mercury in circulation.
- The two outer compartments of the cell are fitted with fixed graphite anodes and are filled with saturated brine solution.
- The middle compartment is fitted with a series of iron rods acting as cathode and is filled with a dilute solution of caustic soda.
- Mercury layer acts as cathode in the two outer compartments and as anode in the middle compartment due to induction.
- On passing electric current, brine solution is electrolysed in both the outer compartments. Chlorine is evolved at the anodes, while the sodium ions are discharged at the mercury layer and form sodium amalgam. Here, H+ ions are not discharged at the cathode because of the high overpotential of hydrogen over mercury.
- The sodium amalgam so produced flows to the middle compartment due to the rocking motion of the cell and reacts with water to form sodium hydroxide.
- When the concentration of sodium hydroxide solution in the middle compartment is about 20%, it is drawn out, evaporated, fused and cast into sticks.
c) Kellner – Solvay Cell:
- The cell has replaced Castner – Kellner cell to some extent. Kellner – Solvay cell consists of a rectangular trough, at the base of which there is a thin layer of mercury which flows from one end to the other acting as cathode.
- A concentrated brine solution flows slowly through the cell in the same direction as the mercury and is maintained at a constant level.
- The graphite anodes are suspended into the brine. During electrolysis, Cl– ions are oxidized at the anode and chlorine gas goes out of the cell, while sodium ions are reduced at the mercury cathode forming sodium amalgam.
- The sodium amalgam leaves the cell on the left and flows into an iron tank where it is decomposed by calculated amount of water giving caustic soda, mercury and hydrogen.
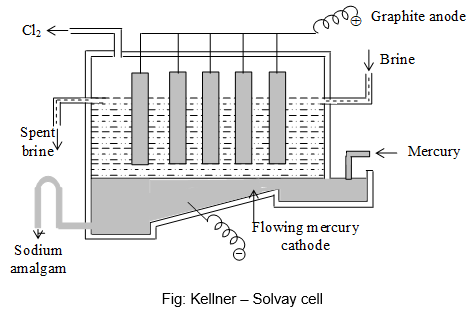
- Mercury so produced is sent back to the cell. The spent brine solution leaving the cell is saturated with more common salt and returned to the cell.
Physical properties of NaOH:
i) It is a hygroscopic, deliquescent white solid which absorbs CO2 and moisture from the atmosphere.
2NaOH + CO2 Na2CO3 + H2O
ii) P = 3180C
iii) It is highly soluble in water and considerable amount of heat is evolved due to the formation of a number of hydrates. g., NaOH. H2O (mp 6400C), NaOH 2H2O (mp 12.70C), NaOH, 7H2O (mp=?)
iv) It is soapy to touch, when it falls on skin, it decomposes the muscle proteins and makes a pulp. (Therefore it is called caustic soda).
Chemical properties:
i. Reaction with acids: NaOH is a very strong alkali on neutralization with acid it gives salt and water.
NaOH + HCl NaCl + H2O
ii. Reaction with metals: Metals like Zn, Al, Sn etc., give H2 gas with NaOH. Similarly certain metalloids like Be, Ge, etc, also produce hydrogen.
iii. Reaction with non-metals: Formation of products depends on the nature of non-metals, concentration of NaOH, temperature, etc., Forexamples:
a) Reaction with halogens:
Other halogens (Cl, Br, I) gives either hypohalites or halates. e.g.
b) Reaction with sulphur: Sodium thiosulphate sulphide or polysulphide are formed.
c) Reaction with phosphorus: PH3 is obtained
d) Reaction with carbon: Carbon reduces fused NaOH.
e) Reaction with silicon: Sodium silicate is formed
iv) Reaction with CO :
v) Reaction with salts: It reacts with metallic salts to form hydroxides, out of which some are unstable and decompose to insoluble oxides.
a) Formation of insoluble hydroxides:
But hydroxides of Zn, Al Sb,Pb, Sn, As etc., dissolve in excess of NaOH.
b) Formation of unstable hydroxides:
2AgNO3 + 2NaOH 2NaNO3 + 2AgOH
2Ag(OH)2 Ag2O + H2O
(Brown)
Note: NH4Cl + NaOH NaCl + H2O + NH3
5. Miscellaneous reactions: Acidic oxides like SO2, CO2 give salts with NaOH, when absorbed in the solution.
2NaOH + CO2 Na2CO3 + H2O
2NaOH + SO2 Na2SO3 + H2O
(Salts)
Uses of NaOH:
i) In the prepeartion of soap, paper, viscon ray on (artificial silk), organic dyestuffs, Na – metal and many other chemicals like sodalime.
ii) In the refining of petroleum and vegetable oils.
iii) As a cleansing agent and in washing powder, for cleaning machines and metal sheets glassware in laboratory.
iv) In purification of bauxite for the extraction of ‘Aluminium’.
v) It used for mercerizing cotton.
vi) As an important reagent in laboratory.
vii) It used to absorb SO2 from the atmosphere near electrical generators.
viii) In reclaiming rubber.
Illustration 9: What happens when NaOH is kept open to air for long-period? Solution: NaOH absorbs moisture from air and solution is formed, it absorbs CO2 from air and turns into Na2CO3.
3. Sodium carbonate (Na2CO3):
- Hydrated sodium carbonate is called washing soda – Na2CO3. 10H2
- Anhydrous sodium carbonate – Soda or soda ash.
Preparation:
1. Le – Blanc process: Raw materials are NaCl, con H2SO4, CaCO3 and Carbon.
2. Solvay or ammonia – Soda process: (1864)
Raw materials: NaCl (brine), NH3 and CaCO3 (For supplying CO2 and CaO) are taken as raw materials.
Reactions (principle):
NH3 + CO2 + H2O NH4 HCO3
Ammonium bicarbonate
NH4HCO3 + NaCl NaHCO3 + NH4Cl
Sodium bicarbonate
2NaHCO3 Na2CO3 + H2O + CO2
Sodium carbonate
Milk of line is obtained by the following reactions.
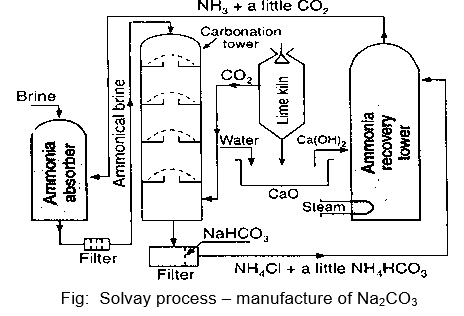
Description:
i) Saturation of brine with ammonia:
- In this step, NH3 absorber tower or saturation tower is filled with brine solution.
- NH3 containing small amount of CO2 is circulated through the brine solution.
- The solution is allowed to settle down and NH3 precipitates calcium, iron and magnesium impurities as their hydroxides or carbonates.
or
2NH3 + H2O + CO2 (NH4)2 CO3
(NH4)2 CO3 + MgCl2 MgCO3 + 2NH4 Cl
- The precipitates are filtered in filter press and the filterate is cooled and sent to carbonation tower.
ii) Carbonation:
- Carbonation process is carried out in carbonation tower which contains perforated plates arranged one above the other.
- Ammonical brine is dropped from upper half of the tower and mixed up with upward steam of CO2 (sent from lime kiln), thoroughly at perforated plates.
- In this step sodium bicarbonate is formed and is taken out from the tower and sent into rotatory vaccum filter.
- NaCl + NH3 + H2O + CO2 NaHCO3 + NH4Cl
- Unmixed NH3 and CO2 is sent back to ammonia absorber tower.
iii) Filtration:
- In this step sodium bicarbonate is filtered off and the filtrate containing ammonium salt is pumped into ammonia recovery tower.
iv) Ammonia recovery:
- The filtrate from the rotary filters is rundown from the top of the ammonia recovery tower.
- It is mixed with ‘milk of lime’ and steam is sent up from bottom of tower.
NH4HCO3 NH3 + H2O + CO2
2NH4Cl + Ca(OH)2 2NH3 + CaCl2 + 2H2O
Ammonia liberated here is sent back to saturation tower.
v) Calcination:
- The NaHCO3 obtained in the rotatory filter is heated in specially designed cylindrical container to get sodium carbonate.
2NaHCO3 Na2CO3 + H2O + CO2
The CO2 produced here is used again.
- The milk of lime (Ca(OH)2) necessary for the regeneration of NH3 is prepared as follows :
CaCO3 CaO + CO2
CaO + H2O Ca (OH)2
Milk of lime
CO2, obtained here, is sent to carbonation tower.
- The soda ash obtained from the pans is dissolved in water and crystals of hydrated sodium carbonate (washing soda), Na2CO3, 10H2O, are recovered from the solution of crystallization.
Merits of the Solvay’s process:
i) This process gives soda of much higher purity (99.5%).
ii) This process is less expensive as the raw materials are cheap, and CO2 and NH3 are recovered to be used again.
iii) No pollution problems because the only waste is the solution of calcium chloride.
iv) Formation of sodium bicarbonate as an intermediate.
3. Electrolytic process:
In this process, CO2 and steam are sent under pressure into NaOH obtained from Nelson cell or castner Kellener cell,
2NaOH + CO2 Na2CO3 + H2O.
Sodium carbonate (separated by crystallization
Physical properties of Na2CO3:
i) Sodium carbonate is a white crystalline solid which can exists as anhydrous salt (Na2CO3), monohydrate salt (Na2CO3.H2O), heptahydrate salt (Na2CO3. 7H2O) and decahydrate (Na2CO3. 10 H2O). Decahydrate salt is commonly called washing soda.
ii) Sodium carbonate is readily soluble in water.
iii) Decahydrate (Na2CO3. 10H2O), effloresces on exposure to dry air forming monohydrate (crystal carbonate) which on heating changes to anhydrous salt called soda ash but does not decompose on further heating even to redness.
Chemical properties of Na2CO3:
i) Reaction with water: Sodium carbonate dissolves in water with the evolution of heat and the aqueous solution is alkaline in nature due to hydrolysis.
ii) Reaction with acids: Sodium carbonate is readily decomposed by acids and carbon dioxide is evolved.
Na2CO3 + 2HCl 2NaCl + H2O + CO2
Na2CO3 + H2SO4 Na2SO4 + CO2 + H2O
iii) Reaction with carbon dioxide: When carbon dioxide gas is bubbled into the aqueous solution of sodium carbonate, sparingly soluble sodium bicarbonate is precipitated.
Na2CO3 + H2O + CO2 2NaHCO3
iv) Reaction with sulphur dioxide: When SO2 gas is passed into the aqueous solution of sodium carbonate, sodium sulphite or bisulphite is produced.
Na2CO3 + SO2 Na2SO3 + CO2
Na2CO3 + 2SO2 + H2O 2NaHSO3 + CO2
v) Reaction with lime: Sodium carbonate is causticised by lime giving caustic soda.
Na2CO3 + Ca (OH)2 2NaOH + CaCO3
vi) Reaction with metal salt solution: With aqueous solutions of certain metal salts, sodium carbonate gives carbonates, basic carbonates or hydroxides.
MgCl2(aq) + Na2CO3 MgCO3 + 2NaCl
ZnSO4(aq) + Na2CO3 ZnCO3 + Na2SO4
2CuSO4(aq) + 2Na2CO3 + H2O Cu(OH)2.CuCO3 + 2Na2SO4 + CO2
basic copper carbonate
vii) Reaction with sand: When sodium carbonate is fused with sand, water glass of sodium silicate is formed.
Uses of Na2CO3:
i) It is used as fusion mixture (Na2CO3 + K2CO3) and black ash (Na2CO3 + CaS).
ii) In manufacture of glass, caustic soda, hypo, borax etc.
iii) In laundries for washing purposes.
iv) Insoftening of water.
v) In paper soap or detergent and petroleum industries.
vi) As household cleansing agent
4. Sodium bicarbonate, baking soda (NaHCO3):
Preparation:
i) It is obtained as an intermediate product in solvay ammonia process.
Properties:
i) It is a white crystallaline solid.
ii) On heating it gives Na2CO3 and CO2.
2NaHCO3 NaOH + H2CO3
iii) Its aqueous solution is alkaline due to hydrolysis.
NaHCO3 + H2O NaOH + H2CO3
It hydrolyse to a lesser extent than Na2CO3.
Note 1:
Na2CO3(ag) or Na HCO3(ag) + Methylorangeindicator yellow colour
Na2CO3(ag) + Phenolphthalien indicator pink colour
NaHCO3 solution + phenolphthalein no colour.
Uses of NaHCO3:
i) It is used as constituent of baking power.
ii) It is used in fire extinguishes (H2SO4 + Na2CO3 + NaHCO3).
iii) In medicine to remove acidity of the stomach (as antacid).
Illustration 10: Potassium bicarbonate cannot be prepared by Solvay processes why?
Solution: Potassium carbonate cannot be prepared by Solvay or Ammonia – soda process because potassium bicarbonate (KHCO3) is much more soluble in water than sodium bicarbonate (NaHCO3).
5. Sodium Peroxide (Na2O2):
Preparation:
Properties:
i) In pure state sodium peroxide is colourless but it becomes yellowish due to the presence of a small quantity of sodium superoxide (NaO2).
ii) When exposed to air, it becomes white due to the formation of sodium hydroxide and sodium carbonate.
iii) Reaction with water:
iv) reaction with acids:
v) oxidizing property: It is a powerful oxidizing agent. It oxidizes SO2 to SO3, manganese (II) salts to manganates, chromium (III) salts to chromates etc.
Uses of Na2O2:
i) as powerful oxidizing agent.
ii) as a bleaching agent for silk, wool, hair, bones etc.
iii) in the respiration apparatus for divers.
iv) for the purification of air in auditorium, halls, submarines etc.
v) for the preparation of dyes and other synthetic organic compounds, such as benzoyl peroxide, sodium perborate etc.
6. Micro cosmic salt (Na (NH4) HPO4, 4H2O):
- It is prepared by dissolving equimolar amount of Na2HPO4 and NH4Cl in water followed by crystallization.
- It is some times used in place of borax for performing bead test for coloured basic radicals.
- On heating, microcosmic salt produces a transparent bead of sodium metaphosphate (NaPO3).
Na(NH4) HPO4. 4H2O NH3 + 5H2O + NaPO3 (transparent glassy bead).
- NaPO3 reacts with certain coloured metallic oxides to form coloured beads of the corresponding orthophosphates.
CuSO4 CuO + SO3
CuO + NaPO3 CuNaPO4
(Blue bead)
Note 2: Baking powder is a mixture of starch, sodium bicarbonate and potassium hydrogen tartarate.
i) Glauber’s Salt – Na2SO4.10 H2O
ii) Salt cake – anhydrous Na2SO4
iii) Nitre cake – NaHSO4
iv) Oxone – Mixture of Na2SO4
v) Kalanamak or Black salt or sulemani namak – when common salt is fused with a little Na2CO3, 5 to 10% Na2SO4 and some sugar, it acquire a dark purple colour, called black salt and has a characteristic saline taste. It is used in medicine and is useful for digestion.
Metallurgy of potassium:
Occurrence: Potassium also does not occur in free state but occurs in combined state as:
i) Sylvine – KCl
ii) Carnallite – MgCl2. 6H2O
iii) Felspar – K2O, Al2O3. 6 SiO2
iv) Kainite – MgSO4. 3H2O
Extraction of potassium:
1. By the electrolysis of fused KOH:
KOH K+ + H–
At cathode: K+ + e– K
At anode: 4OH– 2H2O + O2 + 4e–
2. Modern method:
By the reduction of molten KCl with metallic sodium in stainless steel vessel at 847 – 8770C.
Note:
i) Potassium is not obtained by electrolysis of fused KCl because K has lower boiling point (7660C) than the melting point (7900C) of KCl and hence vaporizes.
ii) K – has three isotopes of mass 39, 40, 41 of which the first one predominates. However due to presence of trace (0.012%) of the K – 40, potassium is radioactive (t ½ = 109 years) and emit, b – rays.
Compounds of potassium:
1. Potassium chloride (KCl):
- Commercially, potassium chloride is called muriate of potash.
- It occurs as sylvine (KCl. NaCl), and as carnallite (KCl.MgCl2.6H2O).
Preparation:
i) From sylvine:
- Sylvine is a mixture of sodium and potassium chlorides. A boiling hot saturated aqueous solution of sylvine is cooled when crystals of potassium chloride (KCl) separate out.
ii) From carnallite:
- Powdered carnallite is extracted with hot 20% solution of magnesium chloride.
- The solution is filtered (to remove insoluble NaCl and MgSO4), and left for crystallization.
- Cubic crystals of potassium chloride (KCl) separate out, leaving behind magnesium chloride (MgCl2) in the mother liquor.
- Potassium chloride (KCl) so obtained is not highly pure, and used as a fertilizer under the name muriate of potash. The mother liquor left behind is recycled to dissolve more carnallite.
Properties of KCl:
i) It is white crystalline solid (m.p. = 7680C; b.p. = 14110C).
ii) It is an ionic compound.
iii) It is fairly soluble in water. In aqueous solution, it gets split into ions (K+ and Cl–) almost completely.
Uses of KCl: It is used
i) as a fertilizer
ii) as a starting material for preparing other potassium salts.
iii) in the pure form, it is used by persons having high blood pressure as a substitute for sodium chloride.
2. Potassium hydroxide, caustic potash (KOH):
Preparation:
i) By electrolysis of aqueous solution of KCl : It is prepared in a cell similar to that used for NaOH.
KCl (aq) K+(aq) + Cl–(aq)
At cathode: K+ + e– K
At anode: Cl– Cl2 (g) + e–
Potassium liberated at mercury cathode forms amalgam with mercury.
K + Hg K(Hg)
Potassium amalgam when treated with a calculated amount of water gives KOH of the desired concentration.
2K(Hg) + 2H2O 2KOH + H2(g) + Hg
ii) From potassium carbonate by reaction of sodalime.
K2CO2 + 2NaOH 2KOH + Na2CO3
Properties of KOH:
i) It is a white highly deliquiescent solid (mp = 3600C).
ii) It is highly soluble in water as well as in alcohol i.e., 12g in 100g water and 33g in 100g alcohol.
iii) It acts has a strong alcohol and absorbs CO2 from here to give potassium carbonate.
Uses of KOH:
i) In manufacture of soft toilet soap.
ii) aqueous solution of KOH is used for absorbing carbon dioxide.
iii) as laboratory regent i.e., alcohol KOH is used in many organic reactions.
3. Potassium carbonate (K2CO3):
Preparation: It is prepared by following two methods.
i) By Precht process process (magnesia process).
ii) By Leblanc process.
4. Potassium cyanide (KCN):
Preparation:
i) By heating potassium ferrocyanide with metallic potassium.
Uses of KCN:
i) It is used in electroplating due to formation of soluble complexes with gold and silver.
ii) It is used in extraction of AC & Ag.
Note:
i) Pure KCl is used by person having high blood pressure as a substitute for NaCl.
5. Potassium chlorate (KClO3):
Preparation:
I) By passing Cl2, through boiling concentrated KOH solution.
6 kOH + 3Cl2 5KCl + KClO3 + 3H2O
ii) By the action of KCl on NaClO3 (obtained by electrolysis of NaCl at 72 – 770e).
NaClO3 + KCl KClO3 + NaCl
Uses of KClO3:
i) It is used as an oxidizing agent.
ii) In preparation of O2 and in laboratory.
iii) In firework and match industries.
Uses of alkali Metals:
1. Uses of Lithium and its compound.
i) Lithium metal is used as an deoxidizer in the refining of nickel and copper.
ii) Lithium is also used in electrochemical cells and thermo nuclear reactions.
iii) It is used in making alloys. Its alloys with aluminium, lead and magnesium, find extensive use.
a) With aluminium, it gives high strength Al – Li alloys for use in making aeroplanes.
b) With magnesium, it (14%, Li) form a tough and corrosion – resistant alloys for use in making armourplates and arospace components.
c) Alloy with lead (called white alloy) is used in making bearings and sheaths.
Uses of Sodium:
i) Sodium is used in the manufacture of sodium peroxide, sodalime, sodium cyanide, tetra ethyl lead (used as anti-knock agent in petrol), etc., (about 50% so ‘Na’ extracted is used in preparation of tetra ethyl lead).
ii) In the preparation of amalgams, which are used as reducing agents.
iii) As a catalyst in the preparation of artificial rubber.
iv) As dioxidediger in the preparation of light alloys.
v) As laboratory reagent.
vi) In illumination engineering and in sodium vapour discharge.
vii) Liquid sodium or its alloys with potassium are used as a coolant in nuclear reactors.
Uses of potassium:
i) In photoelectric cells.
ii) In organic synthesis as a regent.
iii) Sodium – potassium alloy is used in special high temperature thermometers.
iv) In making soft soap with higher fally acids.
Biological Role of Sodium and potassium ions:
i) Na+ and K+ ions help in the maintenance of volumes of various body fluids and cytoplasm, due to the activity of sodium – potassium pump.
ii) Na+ and K+ play a vital role in nerve impulse conduction.
iii) K+ is required for normal activity of myocardium (heart muscles).
iv) K+ efflux (outward movement) in mimosapudice (touch me not) result in drooping movements of leaves.
Exercise 2:
(i) In Solvay ammonia process, sodium bicarbonate is precipitated due to
(A) Presence of NH3
(B) Reaction with CO2
(C) Reaction with brine solution
(D) Reaction with NaOH
(ii) Which of the following properties is not true for an alkali metal?
(A) Low atomic volume
(B) Low ionization energy
(C) Low density
(D) Low electronegativity
(iii) Microcosmic salt is
(A)
(B)
(C)
(D) None of these
(iv) On dissolving moderate amount of sodium metal in liquid ammonia at low temperature, which one of the following does not occur
(A) Blue coloured solution is obtained
(B) Na– ions are formed in the solution
(C) Liquid ammonia becomes good conductor of electricity
(D) Liquid ammonia remains diamagnetic
Answer to Exercise 2:
(i) C
(ii) A
(iii) C
(iv) D
2. ALKALINE EARTH METALS:
- The elements of group II are known as alkaline earth metals because their oxides are alkaline in nature and are found in earths crust.
- Group 2 (or IIA) consists of the elements Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra).
- These elements are called ‘s’ block elements because the valance electron enter into ‘s’ orbital of nth
- These elements have 2-electrons in their valence shell and thus, have placed in group 2 (IIA) of periodic table.
- Ra* is radioactive in nature and discovered, by Madam Curie, in the mineral pitchblende.
- These metals, like alkalimetals, donot occur in free state.
- They are silvery white metals, soft in nature but harder than alkalimetals due to stronger metallic bonding.
General characteristics of alkaline earth metals:
1. Electronic configuration: ns2
| Element | Atomic Number | Electronic configuration |
| Be | 4 | [He]2s2 |
| Mg | 12 | [Ne]3s2 |
| Ca | 20 | [Ar]4s2 |
| Sr | 38 | [Kr]5s2 |
| Ba | 56 | [Xe]6s2 |
| Ra | 88 | [Rn]7s2 |
(Ra* is a radio active element)
2) Atomic and ionic radii:
- The atomic and ionic radii of alkaline earth metals, are quite large, but smaller than those of alkali metals, due to increased nuclear charge of these elements which tends to draw the orbital of electrons, inward.
- On going down the group, the atomic as well as ionic radii increase, due to the gradual addition of new shell at each step and also because of screening effect. Trends are :
Atomic, Ionic radii, alkalimetals > alkaline erathmetals (Å)
Be < Mg < Ca < Sr < Ba < Ra
0.9 1.33 1.74 1.91 1.98 –
Be2+ < Mg2+ < Ca2+ < Sr2+ < Ba2+ < Ra2+
0.31 0.685 0.99 1.13 1.35 –
3. Density:
- These elements are much denser than alkali metals because of their smaller size and greater nuclear charge.
- The density, however, first decreases from Be to Ca and then steadily increases from Ca to Ra due to difference in crystal structure.
Table: Densities of Alkaline earth metals and their crystal structures
| Density | Be | Mg | Ca | Sr | Ba | Ra |
| (g cm–3) | 1.84 | 1.74 | 1.55 | 2.54 | 3.75 | 6.0 |
| hcp | hcp | bcc, ccp | bcc, hcp, ccp | bcc | bcc |
4. Melting and boiling points:
- These elements have higher melting and boiling points than those of alkali metals because the number of bonding electrons in alkaline earth metals, is two and other reason is that they have smaller size and more close packed crystal lattice as compared to those of alkali metals.
- The melting and boiling points do not show a regular trends down the group.
Table: Melting and boiling points of alkaline earth metals
| Element | Be | Mg | Ca | Sr | Ba | Ra |
| M.P.(0C): | 1287 | 647 | 839 | 768 | 727 | 700 |
| B.P. (0C): | 2497 | 1105 | 1494 | 1381 | 1850 | 1527 |
5. Ionization energy:
The first ionization energies of alkaline earth metals are higher than those of the corresponding alkalimetals due to smaller size and higher nuclear charge.
| Element | Be | Mg | Ca | Sr | Ba |
| IE (kJ mol-1) | 899.25 | 737.35 | 589.47 | 548.32 | 502.70 |
- The second IE. Values are higher than their first I.E. values but much lower than the second I.E values of alkali metals.
| Element | Be | Mg | Ca | Sr | Ba |
| IE (kJ mol-1) | 1757.10 | 1450.70 | 1145.40 | 1064.30 | 965.20 |
- On moving down the group due to increase in atomic size the magnitude of I.E values decreases.
- The I.E. of Ra is higher than that of Ba (exception).
7. Electronegativity decrease down the group:
| Element | Be | Mg | Ca | Sr | Ba |
| EN | 1.50 | 1.20 | 1.00 | 1.00 | 0.90 |
8. Conductivity:
The alkaline earth metals are good conductors of heat and electricity due to presence of two loosely held valence electrons (ns2).
9. Electropositive character:
- The alkaline earth metals are strong electropositive elements due to their large size and comparatively low ionization energies, but they are less electropositive than alakalimetals.
- On moving down the group, from Be to Ra, the electropositive character i.e., metallic character increases due to increases in atomic radii and decrease in electronegativity.
10. Reducing character:
- These elements, because of their low electrode potentials, are strong reducing agents but these are weaker reducing agent than the corresponding alkali metals (Li, Na, K, Rb, Cs, Fr).
Table: Electrode potentials of alkaline earth metals.
| Be | Mg | Ca | Sr | Ba | Ra | |
| E0 (V) | -1.70 | -2.37 | 2.27 | -2.29 | -2.91 | -2.92 |
- On going down the group from Be to Ra, the reducing character increases, due to a corresponding decrease in the electrode potential of the metals.
11. Oxidation state:
- Alkaline earth metals uniformly show an oxidation state of +2 despite the fact that the second ionization energy value of these elements is almost double that of their first ionization energy.
Reason:
- In the solid state, the dipositive ions (M2+) form strong lattice due to their small size and high charge i.e., high lallice energy.
- In aqueous, solution, the M2+ cations are strongly hydrated due to their small size and high nuclear charge. Thus the hydration energy released by the M2+ cation is very high and compensates the higher value of second ionization energy.
- The divalent ions are diamagnetic and colourless due to absence of unpaired electron.
Illustration 11: M2+ is readily formed from alkaline earth metal atoms ‘M’ inspite of their high IP values. Why?
Solution: The IP2 values of alkaline earth metals is more than their IP1 values. The hydration energies are more than the IP2 values of alkaline earth metals. So they exist in M2+ state in aqueous solution but not M+ state.
12. Flame colouration:
- Like alkali metal salts, alkaline earth metal salts also impart characteristic flame colouration.
- On going down the group from Ca to Ba, the ionization energy decreases and frequency or energy of emitted light increases. Consequently, the colour imparted to the flame shows a gradual shift from red to violet. Thus
Table: Flame colouration of alkaline earth metals
| Element | Ca | Sr | Ba | Ra |
| Colour | Brick red | Crimson red | Apple green | Crimson |
Illustration 12: Be and Mg do not impart any characteristic colour to the Bunsen flame. Why?
Solution: Be and Mg, because of their high ionization energies, however, donot impart any characteristic colour to the Bunsen flame:
Exercise 3:
(i) Compounds of group 2 elements are less soluble in water than the corresponding alkali metal salts due to
(A) their high ionization energy
(B) their high lattice energy
(C) their less basic character
(D) their low electronegativity values
(ii) The order of increasing lattice energy of the metallic compound is
(A) NaCl < CaO < NaI < BaO
(B) NaI < NaCl < BaO < CaO
(C) NaCl < NaI < BaO < CaO
(D) NaI < NaCl < CaO < BaO
(ii) The hydration energy of Mg2+ ions is larger than that of
(A) Al3+ (B) Na+ (C) Be2+ (D) Li+
Answer to Exercise 3:
(i) B
(ii) B
(iii) B
Chemical properties:
1. Reaction with water:
- Alkaline earth metals are less reactive towards water as compared to alkali metals. These react with H2O, evolving H2 gas
- On going down the group, chemical reactivity of metals with water, however, increases from Mg to Ba.
Illustration 13: What happens when CO2 gas is passed through milk of lime for a long-time? Give equations.
Solution: First white precipitate of CaCO3 is formed, later, it dissolves due to the formation of calcium bicarbonate, Ca(HCO3)2.
2) Reaction with oxygen:
- The affinity for oxygen increases down the group. (∵ E. decreases).
- Thus, Be, Mg, Ca when heated with O2 form monoxides.
- While Sr, Ba and Ra form peroxides
- Order of basic character of oxides is
BeO < MgO < CaO < BaO
Illustration 14: How does the solubility of oxides of Alkaline earth metals vary? How do they react with water?
Solution: Solubility increases from BeO to BaO.
they form Hydroxides when dissolved in water.
MO + H2O M (OH)2 (M = Alkaline earth metal)
3. Reaction with acids:
- Alkaline earth metals displace H2 from acid except ‘Be’.
- Reactivity, however, increases down the group from Mg to Ba. Thus the order of reactivity is Mg < Ca < Sr < Ba.
- Only Mg can displace H2 from a very dilute HNO3.
4. Reaction with hydrogen:
- All the alkaline earth metals (except Be) combine with H2 directly on heating to form metals hydrides (MH2).
- The hydride of beryllium can be prepared indirectly by reducing beryllium chloride with LiAlH4.
2 BeCl2 + LiAlH4 2BeH2 + LiCl + AlCl3
- BeH2 and MgH2 are covalent and polymeric whereas the hydrides of Ca, Sr and Ba are ionic and monomeric in nature.
- MgH2 is partly ionic
- CaH2 is also called hydrolith.
- All the hydrides react with water to evolve H2 and thus behave as strong reducing agents
MH2 + 2H2O M (OH)2 + 2H2
5. Reaction with carbon:
- Alkaline earth metals, when heated with carbon form their respective carbides of the general formula MC2 (except Be) and are called acetylide containing the discrete anion.
(Where M=Mg, Ca, Sr or Ba)
- Under the conditions beryllium, however, forms Be2C called methanide containing the discrete C4–
- All these carbides are ionic in nature and react with water to form acetylene but Be2C gives methane.
- On heating magnesium carbide (MgC2) gives allylide (Mg2C3), which contains the discrete C34– ion and gives allylene (methyl acetylene) on hydrolysis.
6. Reaction with nitrogen:
- Alkaline earth metals burn in nitrogen to form nitrides (M3N2), which are hydrolysed with water to evolve NH3.
2M + N2 M3N2 (where M = Be, Mg, Ca, Sr, Ba).
(Metal nitrides)
M3N2 + 6H2O 3M (OH)2 + 2NH3
- The ease of formation of nitrides, increases from Be to Ba.
- Be3 N2 is volatile in nature.
7. Reaction with halogens:
- Alkaline earth metals directly combine with halogen (X = F, Cl, Br, I) at high temperature, forming their halides (M X2)
- The halides of alkaline earth metals are soluble in water and their solubility decreases in the order.
MgX2 > CaX2 > SrX2 > BaX2 (where x = Cl, Br, I)
- BeF2 is highly soluble in water due to the high solution energy of Be2+ in forming [Be (H2O)4]2+. The fluorides (F–) of other alkaline earth metals have high melting points and are insoluble in water.
8. Solubility in liquid ammonia:
- Like alkali metals, these metals dissolve in liquid ammonia giving bright blue solution (dil) due to presence of solvated electrons but the concentrated solutions are bronze coloured due to the formation of metal clusters.
- These solutions decompose very slowly forming amides and evolving H2.
M + (x + 2y) NH3 M2+ (NH3)x + 2e– (NH3)y
2
- Evaporation of NH3 from these solutions gives hexammoniates which slowly decompose to give amides.
M (NH3)6 M (NH2)2 + 4NH3 + H2
9. Basic strength of oxides and hydroxides:
- BeO and Be (OH2) are amphoteric (due to small size & high ionization energy of Beryllium) while the oxides and hydroxides of other alkaline earth metals are basic.
- The basic strength, however, increases from Be to Ba as the ionization of metal decreases down the group, thus, the order is
BeO < MgO < CaO < SiO < BaO
Be(OH)2 < Mg (OH)2 < Ca (OH)2 < Sr (OH)2 < Ba (OH)2
- The basic character of the hydroxides of alkaline earth metals is lesser then those of alkali metals because the size of alkaline earth metals is smaller than alkali metals.
10. Solubility of hydroxides, carbonates, bicarbonates and sulphates:
i) The solubility of hydroxides of alkaline earth metals increases from Be to Ba because lattice energy decreases more rapidly then the hydration energy from Be to Ba.
Be(OH)2 < Mg (OH)2 < Ca (OH)2 < Sr (OH)2 < Ba (OH)2
ii) The solubility of carbonates in water decreases as we move down the group from Be to Ba due to the decrease in the magnitude of hydration energy. Thus, order of solubility of carbonates is
BeCO3 > Mg CO3 > CaCO3 > Sr CO3 > BaCO3
iii) The bicarbonate of alkaline earth metals do not exist in solid state but are known only is solution. On heating, these bicarbonates decompose forming carbonates with the evolution of CO2.
iv) The solubility of sulphates of alkaline earth metals decreases from Be to Ba due to the reasons that ionic size increases down the group. This is due to the reason that as the size of the cation increases, the heat of hydration decreases while the lattice energy, remains constant because sulphate is so large, so that small change in cationic size doe not make any difference. Thus the order is
BeSO4 > MgSO4 > CaSO4 > SrSO4 > SrSO4 > BaSO4
Illustration 15: What is quick lime? How is it prepared?
Solution: Freshly prepared calcium oxide is called quick lime. Decomposition of calcium carbonate (CaCO3) by heating gives it.
Illustration 16: BaSO4 does not dissolve in water, even though it is ionic compound. Reason out.
Solution: Because, the hydration energy of Ba2+ ion is less than that of its lattice energy.
11. Thermal stabilities of carbonates, bicarbonates and sulphates:
i) Stability of carbonates:
- The carbonates of all alkaline earth metals decompose on heating to form the corresponding metaloxide and CO2,
- The carbonates of the alkaline earth metals can be regarded as salts of weak carbonic acid (H2CO3) and metal hydroxides (M(OH)2).
- Thermal stability of these carbonates, however, increases down the group as the basicity of metal hydroxide increases from Be(OH)2 to Ba (OH)2. (because their temperature of de-composition increase).
Table: Temperature of decomposition of Carbonates of alkaline earthmetals:
| Carbonate | BeCO3 | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
| Temperature of decomposition(0C) | < 100 | 540 | 900 | 1290 | 1360 |
ii) Stability of bicarbonates:
- All the bicarbonates of alkaline earth metal exist only in solution and have not been isolated in the pure state. But the stability of bicarbonates increases down the group.
iii) Stability of sulphates:
- The sulphates of alkaline earth metals, decompose on heating giving the oxides and SO3.
- Like carbonates, thermal stability of sulphates also increases from Be to Ba, as the basic character of metal hydroxide increases. (because their temperature of decomposition increases).
Table: Temperature of decomposition of sulphates of alkaline earth metals.
| Sulphate | BeSO4 | MgSO4 | CaSO4 | SrSO4 |
| Temp. of decomposition (0C) | 500 | 895 | 1149 | 1374 |
12. Oxalates of alkaline earth metals:
- Beryllium oxalate is highly soluble but the oxalates of other metals are sparingly soluble in water.
- The solubility of oxalates of this group increases from calcium to barium.
13. Complex formation:
- Alkaline earth metals have greater tendency of complex formation than the alkalimetals due to smaller size.
- Both Mg2+ and Ca2+ form six coordinate complex with EDTA (ethylene diaminetetra acetic acid) which are used to determine the hardness of water.
- Beryllium, due to small size, forms complexes of type [BeF3]–, [BeF4]2– [(Be(H2O)4]2+.
- Mg exist as natural complex chlorophyll where it complexed with pyrole rings of porphyrin.
Anomalous behaviour of Beryllium:
Beryllium, the first member of alkaline earth metals, differs from the rest of the members of its family, due to the following reasons.
i) Beryllium is harder than other members of its family.
ii) It has higher melting point, boiling point and ionization energy.
iii) Beryllium does not react with water even at high temperatures while other metals do. g.,
iv) Beryllium forms covalent compounds because of high charge density and hence greater polarizing power, whereas other members form ionic compounds. Because of covalent character, salts of beryllium are easily hydrolysed. e.g.,
v) Beryllium oxide and hydroxide are amphoteric whereas oxides of other alkaline metals are basic.
vi) Carbides of Be (i.e., Be2 C and BeC2) are covalent whereas carbides of other members are ionic.
vii) Be forms, nitride (Be3 N2) with nitrogen which is volatile while nitrides of others are non-volatile.
Resemblance between Beryllium and Aluminium (Diagonal relationship):
Beryllium, a member of group 2, show similarities in the properties with its diagonally opposite Aluminium a element of group 13 of the next higher period due to the similar electronegativity (Be = 1.5, Al = 1.5) and the similar polarizing power.
Some points of similarity are given below:
i) Both Mg & Al metals are stable in air.
ii) Both metals have a tendency to form covalent compounds e.g. anhydrous BeCl2 and AlCl3 being covalent are soluble in organic solvents.
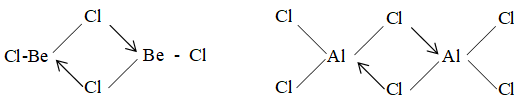
iii) Both BeCl2 and AlCl3 act as strong Lewis acids and have bridge chloride structures in the vapour state.
iv) Both the metals dissolve in strong alkalies to form soluble complexes.
e.g., beryllates: [Be (OH)4]2–, aluminates: [Al (OH4)]–
v) The oxides of both beryllium and aluminium (i.e., BeO, Al2O3) are hard, high melting solids (mp > 25000C).
vi) The oxides and hydroxides of both Be and Al are amphoteric and dissolve in sodium hydroxide solution as well as in HCl.
vii) Salts of both these metals form hydrated ions e.g., [Be (OH2)4]2+ and [Al (OH2)6]3+ in aqueous solution.
viii) Carbides of both the metals reacts with water liberating methane gas.
ix) Due to similar polarizing power both beryllium and aluminium forms complexes. e.g., Be forms tetrahedral complexes such as and and aluminium forms octahedral complexes like and .
Metallurgy of magnesium:
1. Occurrence:
- Magnesium is the eighth most abundant element found in earth crust.
- It does not occur in free state, it occurs in the combined state in nature and it is essential constituent of chlorophyll the, green colouring matter of the plants.
2. Important mineral of magnesium are:
i) Magnesite – MgCO3
ii) Dolomite – MgCO3.CaCO3
iii) Carnallite – MgCl2.6H2O
iv) Epsom salt (epsomite) – MgSO4. 7H2O
v) Asbestos – CaMg3 (SiO3)4
vi) Talc – Mg3H2 (SiO3)4
Sea water contains Mg as MgCl2 and MgSO4. In our country magnesite and dolomite are largely found in the state of Tamilnadu and Karnataka. The major amounts of these minerals are exported.
Extraction of Mg:
Like Na and K, magnesium and calcium Ca can not be isolated by usual methods because of same difficulties, arised during extraction of Na, & K.
i) From Carnallite:
Anhydrous MgCl2 is fused with anhydrous NaCl and CaCl2 and electrolysed at 7000C.
Carnallile anhydrous MgCl2
At cathode: Mg2+ + 2Cl– Mg (99.9%)
At anode: 2Cl– Cl2 + 2e–
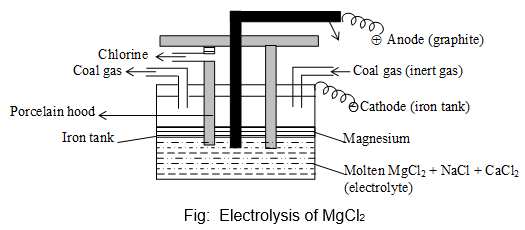
Cathode : Iron tank
Anode : Carbon / graphite rod coated with Pb (surrounded by a porcelain hood).
Electrolyte : Molten MgCl2, NaCl, CaCl2
Function of NaCl and CaCl2: They are added to provide conductivity to the electrolyte and to lower the fusion temperature of anhydrous MgCl2.
ii) From Magnesite:
Magnesium metal can also be extracted from magnesite. Magnesite on heating gives magnesia (MgO).
a) MgO can be reduced to magnesium by electrolysis:
The molten magnesia containing fluorides of barium and sodium on electrolysis produces magnesium metal. The electrolysis is carried out in a steel tank at 900 – 9500
2MgO 2Mg + O2
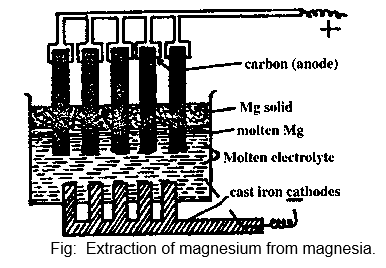
Description:
- Cast iron rods introduced from the bottom of the tank into the electrolyte act as cathode. From the top of the tank, a series a suspended carbon rods acts as anode.
- Magnesium metal rises to the top, gets cooled by the air and forms a scum. This prevents the oxidation of the molten metal by air.
- The metal is removed periodically and fresh amount of magnesia are added simultaneously to the tank.
b) MgO can be reduced to Mg by other methods (Hansging method):
- In this method, the MgO is mixed with carbon and heated up to 20000C in an electric furnace.
- The forward reaction is an endothermic reaction and so proceeds only at high temperature. The metal formed is in vapour state (m.p. of mp = 6500C and b.p = 11100C) mixed with “CO” gas.
- To prevent the back reaction, the vapours are quenched as soon as they come out of the electric furnace. This is done in another chamber maintained at 2000C with hydrogen gas or natural gas.
- Mg only solidifies which is separated and purified by distillation in vacuum.
c) In other methods, MgO is reduced with silicon or ferrosilicon or with calcium carbide at 12000C to 15000C:
iii) From Sea water: Sea water contains MgCl2 and MgSO4 in dissolved state. Extraction of Mg from sea water involves following 3 – steps.
a) Precipitation of Mg (OH)2 from sea water.
b) Conversion of Mg(OH)2 into anhydrous MgCl2 or MgO.
c) Electrolysis of MgCl2 & MgO (as described in (i) and (ii) process):
Physical properties of Mg:
i) It is a soft silvery – white, and light metal (density – 1.75 g/mL)
ii) It melts at 6500C and boils at 11100 It sublimes in vacuum at 5500C.
iii) It is malleable and ductile.
iv) It is good conductor of heat and electricity.
Chemical properties of Mg:
i) Reactivity: It is strongly electropositive because its oxidation potential is high.
Mg Mg2+ + 2e– ; E0 = + 2.37V
Hence, it is a reactive metal and a strong reducing agent.
ii) Reaction with air:
Dry air has no action on magnesium but moist air changes it into oxide or basic carbonate.
Magnesium burns in oxygen with dazzling brilliant light which consists of UV light.
2Mg + O2 (air) 2MgO
Nitrogen of air combine to give a nitride.
3Mg + N2 Mg3N2 + H2
iii) Reaction with water:
Magnesium does not react with cold water, but hot water or steam reacts to give MgO and hydrogen. This reaction is an example for the strong reducing property of magnesium.
Mg + H2O (hot) MgO + H2
iv) Reactions with acids:
Magnesium reacts with dilute mineral acids (HCl, H2SO4) to produce hydrogen.
Mg + 2HCl (dil) MgCl2 + H2(g)
Mg + H2SO4 (dil) MgSO4 + H2
Mg + 2H2SO4 (conc) MgSO4 + SO2 + 2H2O
Nitric acid gives different products depending on the concentration of the acid. e.g., with dilute nitric acid, magnesium gives ammonium nitrate and magnesium nitrate.
10HNO3 + 4Mg 4Mg (NO3)2 + NH4NO3 + 3H2O
(dil)
4HNO3 + Mg ® Mg (NO3)2 + 2NO2 + 2H2O
Conc.
v) Reduction reactions: Mg is a strong reducing agent. It has very high affinity for oxygen. It reduces the oxides of metals and non-metals to the respective element.
vi) Formation of Grignard reagent: Pure Mg reacts with alkyl halides in dry ether to form alkylmagnesium halide, commonly called as the Grignard’s reagent.
Mg + RX R – Mg – X (where X = Cl, Br, I)
vii) Displacement of metals: Mg is a highly electropositive metal. So, it displaces all less electropositive metals from their salt solutions.
2AgNO3 + Mg Mg (NO3)2 + 2Ag
CuSO4 + Mg MgSO4 + Cu
Pb (NO3)2 + Mg Mg (NO3)2 + Pb
Uses of Mg:
i) It is used as a reducing agent in the preparation of elements like B, Si etc.
ii) It is used as ‘deoxidiser’ in metallurgical processes to remove oxygen.
iii) In flash light photography and fire works.
iv) In the preparation of Grignard reagent.
v) To initiate the reaction in aluminothermic process.
vi) In the preparation of alloys e.g.,
a) Magnalium: Magnalium (1 -15% Mg + 25 – 99% Al) used in construction of air craft and light instruments.
b) Elektron: Elektron (95% Mg + 5% Zn) used in construction of air craft.
Compounds of Mg:
1) Magnesium oxide (MgO) or Magnesia:
It is used in the preparation of sorel’s cement or magnesia cement (MgCl2.5MgOxH2O) with MgCl2 and used to fill up the cavities of teeth.
2) Milk of Magnesia (Mg (OH)2:
It’s aqueous suspension is used in medicine as an antacid.
3) Magnesium carbonate or magnesite (MgCO3):
It’s 12% aqueous solution is known as fluid magnesia which is used as antacid, laxative and in tooth paste.
4) Magnesium chloride (Mg Cl2.6H2O): It is a deliquescent white crystalline solid. It crystallizes from aqueous solutions as hexahydrate (MgCl2. 6H2O).
Major sources of magnesium chloride:
a) Sea water
b) Carnallite
Preparation:
a) from sea water:
On industrial scale, magnesium chloride is prepared from sea water.
The sea water on treatment with lime gives precipitate of magnesium hydroxide. The precipitate is separated and dissolved in hydrochloric acid. The solution on crystallization and cooling gives crystals of MgCl2.6H2
b) from carnallite:
- Magnesium chloride is also obtained from carnallite (KCl. MgCl2. 6H2O).
- The ore is powdered and boiled with water for some time. On cooling, KCl crystallizes out, and magnesium chloride being more soluble, remains in the mother liquor.
- The mother liquor is concentrated and on cooling gives crystals of MgCl2.6H2
c) Laboratory method:
- In the laboratory, magnesium chloride is prepared by the action of hydrochloric acid on magnesium oxide or magnesium carbonate.
- The solution on concentration and cooling gives crystals of MgCl2.6H2
Properties of MgCl2:
i) it is deliquescent white crystalline solid.
ii) It decomposes on heating to give magnesium oxide.
But on heating in a current of dry HCl gas it gives anhydrous salt.
iii) A saturated solution of MgCl2 on reaction with MgO gives a hard mass with the formula MgCl2.5MgO.xH2O.
This hard mass is known as sorel cement or magnesia cement.
Uses of MgCl2:
i) In the extraction of magnesium by electrolytic method
ii) In the manufacture of sorel cement which resembles marble and is used for making tiles.
Note: A solution of MgCl2 + NH4Cl in ammonia is known as Magnesia mixture.
5) Magnesium sulphate (MgSO4.7H2O) or Epsom salt:
Magnesium sulphate heptahydrate is commonly known as Epsom salt because it was formerly prepared from water of the Epsom springs in England.
Major sources of magnesium sulphate are:
a) Kieserite – MgSO4. H2O
b) Epsomite – MgSO4.7H2O (in certain gypsum deposits).
Preparation:
i) From dolomite:
Insoluble CaSO4 is removed by filtration and MgSO4.7H2O crystals are obtained from the filtrate.
ii) From magnesite:
iii) Laboratory method:
Properties of MgSO4:
i) It is a colourless efflorescent crystalline substance having a bitter taste.
ii) It is isomorphous with ZnSO4.7H2O, in nature.
iii) Solubility : It is readily soluble in water i.e., 35.5g of the salt dissolves in 100g of water at 200C).
iv) Effect of heat:
When heated to 1500C, MgSO4.7H2O (heptahydrate) changes to Mg SO4. H2O (monohydrate) which at 2000C gives anhydrous magnesium sulphate (MgSO4), which on further heating decomposed into MgO and SO3.
v) Double salt formation:
It readily forms double salts with alkali metal sulphates. e.g., MgSO4.K2SO4.6H2
Uses of MgSO4: It is used
i) As a purgative in medicine
ii) As a stimulant to increase the secretion of bile.
iii) in fire-proofing fabrics.
iv) in the manufacture of soaps and paints.
v) As a mordant in dyeing and in tanning industry.
vi) As a filter for paper.
vii) As a weighting material for cotton and silk in industry.
VI) Magnesium perchlorate Mg (ClO4)2: It is known as anhydrone and used as drying agent.
Metallurgy of calcium:
Occurrence and important minerals:
i) It is an important constituent of teeth and bones (as calcium phosphate), sea shells and corals (as calcium carbonate – CaCO3).
ii) The important mineral of ‘Ca’ are:
a) Limestone, marble, chalk, calcite – CaCO3.
b) Gypsum – CaSO4.2H2O
c) Anhydrite – CaSO4
d) Dolomite – MgCO3.CaCO3
e) Phosphite – Ca3(PO4)2
f) Fluorspar – CaF2
g) Fluorapatite – 3Ca3 (PO4)2.CaF2
h) Hydroxyapatite – 3 Ca3 (PO4)2.Ca(OH)2
Extraction of Calcium:
It is extracted by the electrolysis of a fused mixture of calcium chloride and calcium fluoride (CaF2) lowers the fusion temperature of the electrolyte. The electrolysis is carried out in iron tank lined from inside with graphite.
CaCl2 Ca2+ + 2Cl–
At cathode : A water cooled iron tube acts as cathode
Ca2+ + 2e– Ca(s)
At anode : Graphite rod acts as anode.
2Cl– – 2e– Cl2(g)
Physical properties of Calcium:
i) It is a silvery white lusturous metal. It is softer than most metal and has a very low density, (1.55 g/mL).
ii) It melts at 8500C, boils at 14920 It sublimes at 8000C in vacuum.
iii) It is malleable, ductile and a good conductor of heat and electricity. It possesses fair tensile strength.
iv) It forms amalgam with mercury.
Chemical properties of Calcium:
i) Reaction with air:
Dry air has no effect on calcium. In moist air, a thin layer of oxide gets formed on its surface. When heated in air, it burns with a dazzling light giving its oxide and nitride.
ii) Reaction with water:
It decomposes cold water rapidly to liberate hydrogen.
iii) Reaction with acids:
It react with all dilute mineral acids to produce hydrogen.
iv) Reducing property:
It has a very high affinity for oxygen. It reduces the oxides of metals and non-metals to the respective element.
v) Reaction with non-metals:
It reacts with certain non-metals on heating.
vi) Displacement reactions:
It is highly electropositive metal. It displaces less electropositive metals from their salt solutions.
Uses of Calcium:
i) As a reducing agent in the extraction of such metals from their oxides.
ii) To remove air from vacuum tubes, sulphur from petroleum and oxygen from molten steel and moisture from alcohol.
Compounds of calcium:
1. Calcium oxide, quick lime, lime, Burnt lime (CaO):
Preparation:
(i) By the thermal decomposition of calcium carbonate
Properties:
i) It is a white amorphous solid.
ii) It is a basic oxide.
iii) On heating alone it glows at high temperature, this glow of white dazzling light is called lime light (mp of CaO = 25970C). But on heating with NH4Cl, it gives ammonia.
iv) On exposure to the atmosphere, it absorbs moisture and carbon dioxide to finally give calcium carbonate. Its aqueous suspension is known as slaked like (Ca (OH)2)
v) It reacts with carbon to form calcium carbide
CaO + 3C
vi) Reaction with acidic oxides :
Uses of CaO: It is used
i) As basic flux in metallurgy
ii) as a fertilizer for acidic soil
iii) for removing hardness of water
iv) for preparing mortar (CaO + s and + water) and used as building material.
v) for white washing of buildings and tanning of leather.
vi) for manufacture of bleaching powder, glass, calcium carbide, soda ash etc.,
2. Calcium hydroxide, slaked lime, limewater (Ca (OH)2):
Preparation:
i) By dissolving quick lime in water (slackening of lime).
CaO + H2O Ca (OH)2 ; H = – 63 kJ.
ii) from calcium chloride:
Properties:
i) It is a white amorphous solid.
ii) It is sparingly soluble in water. The solubility decreases with a rise in temperature.
iii) Suspension of Ca(OH)2 in water is known as milk of lime.
iv) Reaction with CO2:
- When CO2 is passed through lime water, it becomes milky due to formation of calcium carbonate.
- The milkiness disappears on passing CO2 gas in excess because calcium carbonate changes to the soluble calcium bicarbonate.
- When slaked lime is exposed to air, it absorbs carbon dioxide to form calcium carbonate.
v) Reaction with Cl2 : It reacts with Cl2 to give bleaching powder (CaOCl2).
vi) Reaction with Acids :
It is soluble in dilute HCl and forms water soluble salt.
However, it is not soluble in dilute sulphuric acid because calcium sulphate formed is water insoluble salt.
Uses of Ca(OH)2: It is used
i) As a building material
ii) As a lime water, in detection of CO2
iii) In tanning industry
iv) For softening of hardwater
v) For white washing the buildings
3. Calcium chloride (CaCl2.6H2O):
Properties:
i) It is a deliquescent solid (by product of solvay’s process).
ii) Fused CaCl2 is used as good drying agent or desiccant, but it can not be used to dry alcohol or ammonia because it forms addition product with them.
iii) CaCl26H2O is widely used for melting ice on roads, particularly in very cold countries, because a 30% eutectic mixture of CaCl2(H2O) freezes at – 55%C as compared with NaCl(H2O) at – 180C.
4. Gypsum or calcium sulphate dihydrate (CaSO4.2H2O):
- It is naturally occurring calcium sulphate and anhydride.
- It is also known as alabaster. Alabaster is finely divided naturally available calcium sulphate.
Preparation:
i) By the action of dil H2SO4 on CaO or CaCO3 or CaCl2
CaCl2 + H2SO4(dil) CaSO4(s) + 2HCl
From the solution, calcium sulphate is obtained as dihydrate (CaSO4.2H2O).
Properties:
i) Calcium sulphate exists as white solid in two forms
a) anhydrous – CaSO4
b) dihydrate – CaSO4. 2H2
ii) It is sparingly soluble in water. Solubility increases upto 400C, beyond which the solubility decreases. Its presence in water causes permanent hardness.
iii) Effect of heat: On heating at 120 – 1300C, it gives plaster of paris. Which on further heating gives an hydrous CaSO4.
iv) It dissolves in solutions like that of ammonium sulphate due to formation of double salt (CaSO4.(NH4)2SO4.H2O).
Uses of CaSO4:
i) dihydrate calcium sulphate is used for making plaster of pairs (POP)
ii) dihydrate calcium sulphate is used as fertilizer as well as in the manufacture of cement.
iii) monohydrate calcium sulphate also used in preparation of ammonium sulphate fertilizer.
5. Plaster of Paris (2 CaSO4.H2O) or calcium sulphate hemihydrate
Preparation:
i) By heating gypsum at 1200C in rotatory kiln, where it gets partially dehydrated.
The temperature should be kept below 1400C, otherwise further dehydration will take place and the setting property of the plaster will be partially reduced.
Properties:
i) It is a white power.
ii) When it is mixed with water (say 1/3 of its mass), it forms first a plastic mass which sets into a solid mass with slight expension due to rehydration and converted into gypsum in two steps. The first step is called the setting stage and is second the hardening stage.
The setting of plaster of paris is catalysed by NaCl, while it is reduced by borax or alum.
Uses of pop:
i) In making black board chalk, crucibles, models, toys and statues.
ii) In surgery for setting broken or fractured bones.
iii) In dentistry – Dentists use it in hospitals, and also used in construction industry.
6. Calcium oxalate (CaC2O4.H2O): It is a main constituent of kidney stone which dissolves in dil strong acids but remains insoluble in bases.
Note:
i) Be & Mg crystallize in hcp, Ca & Sr crystallize in ccp, Ba crystallize in bcc
ii) Finely devided BaSO4 is called blanc fire and used in paints.
Cement: Port land cement:
- It was discovered in 1824 by Joseph Aspdin, a mason of Leeds (u.k.).
- Cement is essentially a finely powdered mixture of calcium silicates and aluminates along with small quantities of gypsum which sets into a hard stone like mass when treated with water.
- Limestone + Clay portland cement
- The chief components of cement are tricalcium silicate (3 CaO. SiO2), di calcium silicate (2 CaO.SiO2) and tricalcium aluminate (3 CaO. Al2O3). Out of these tricalcium silicate is the most important since it has property of setting quickly and acquiring considerable strength within a few days. It is usually constitute 50% of the cement.
Composition of Portland cement:
The average composition of Portland cement is
Lime (CaO) – 50 – 60%
Magnesium oxide (MgO) – 2 – 3%
Silica (MgO) – 20 – 25%
Ferric oxide (Fe2O3) – 1 – 2%
Alumina (Al2O3) – 5 – 10%
Sulphurtrioxide (SO3) – 1 – 2%
For a good quality cement, the ratio of alumina (Al2O3) to silica (SiO2) should lie between 2.5 and 4, while that of lime (CaO) to silica + alumina + ferric oxidize should be as close to 2 as possible.
and
Raw materials:
i) Lime stone : CaCO3
ii) Clay : to provide both silica and alumina
iii) Gypsum : regulates the setting time
iv) MgO, & Fe2O3 : required for imparting suitable colour to cement.
Setting of cement:
- When water is added to cement, an exothermic reaction occurs. During this process, the cement reacts with water to form a gelatinous mass which slowly sets into a hard mass having three – dimensional network structure involving – Si – O – Si – and – Si – O – Al – chains.
- Out of various constituents of cement, tricalcium silicates sets quickly and develops considerable strength within a few days.
- Dicalcium silicates sets slowly and develops appreciable strength after a month.
- Tricalcium aluminate sets instantaneously in presence of water.
- The internal strength acquired by cement is primarily due to the setting of tricalciumaluminate.
- Tetra calcium aluminoferrite also sets rapidly but not quickly as tricalcium aluminate.
The reactions involved in the setting of cement are
i) Hydration of 3CaO.Al2O3 and 3CaO.SiO2 forming colloidal gel.
ii) Hydrolysis of 3 CaO.Al2O3 and 3 CaO.SiO2 forming precipitates of Ca(OH)2 and Al(OH)3.
Cement substitutes:
Fly ash:
- It is a waste product of steel industry possess properties similar to cement, and mainly consists of CaSiO3. This can be added to cement to reduce its costs without affecting the quality.
Rice Husk:
- In many countries, rice husk with high silica content has been used to make cement.
Mortar: Lime Mortar:
- An intimate mixture of 1 part of slaked lime, 3 parts of sand and water is known as lime mortar and is used in building construction.
- Sand makes it porous and harder and prevents the cracks on construction during hardening.
- Finally it sets to a hard mass by loss of water and gradual absorption of CO2 from air.
Ca (OH)2 + SiO2 CaSiO3 + H2O
Ca (OH)2 + CO2 CaCO3 + H2O
Cement Mortar:
- It is a mixture of cement and mortar. This is stronger than mortar.
Hydraulic mortar:
- A mortar obtained from hydraulic lime is called hydraulic mortar.
Lime stone + Clay (10% Aluminium silicate ) hydraulic mortar.
Concrete:
- A mixture of cement, sand, gravel or small pieces of stone and water is known as concrete.
- It sets to an exceedingly hard structure. It is mainly used for construction of floors.
- If the cements concrete is filled in and around wire netting or skeleton of iron rods and allowed to set, the resulting structure is known as reinforced concrete.
Biological importance of Mg and Ca:
i) Ca2+ are concentrated in body fluids out side the cell.
ii) Mg2+ ions form a complex with ATP and are present in chlorophyll – a green colouring pigments in plants which absorbs light and is essential for photosynthesis.
iii) Both Mg2+ and Ca2+ catalyse a number of enzymatic reactions.
iv) Ca2+ are present in bones and teeth as apatite (Ca3(PO4)2 and enamel of teeth as fluoropatite (3Ca3 (PO4)2 CaF2.
v) Ca2+ ions also important in blood clotting and are required to trigger the contraction of muscles and to maintain the regular beating of the heart.
Exercise 4:
(i) Chemical A is used for water softening to remove temporary hardness. A reacts with sodium carbonate to generate caustic soda. When carbon dioxide is bubbled through A, it turns cloudy. What is the chemical formula of A?
(A) CaCO3 (B) CaO (C) Ca(OH)2 (D) Ca(HCO3)2
(ii) A sudden large jump between the values of second and third ionization energies of an element would be associated with the electronic configuration
(A) 2s2 2s2 2p6 3s1
(B) 1s2 2s2 2p6 3s23p1
(C) 1s2 2s22p63s22p2
(D) 1s22s22p63s2
(iii) on heating quick lime with coke in an electric furnace, we get
(A) Ca and CO2 (B) CaCO3 (C) CaO (D) CaC2
(iv) The product obtained on fusion of BaSO4 and Na2CO3 is
(A) BaCO3 (B) BaO (C) Ba(OH)2 (D) BaHSO4
Answes to Exercise 4:
(i) C
(ii) D
(iii) D
(iv) A
FORMULAE AND CONCEPTS AT A GLANCE
1. Minerals of Beryllium: Beryl – Be3Al2Si6O18. or 3BeO.Al2O3. 6SiO2
Phenacite – 2 BeO.SiO2 or Be2SiO4.
2. Minerals of strontium: Celestite – SrSO4, Strontianite – SrCO3
3. Minerals of Barium: Barytes or heavyspar – BaSO4, Whitherite – BaCO3
4. Minerals of Radium: Pitch blende, uranite : Mainly as U3O8
Carnotite : K2O.U2O3. (VO4)2.3H2O
5. Keene’s cement – Mixture of pop and alum. Alum makes pop very hard.
6. Cu – Be alloys and Locky alloy (62% Be + 38% Al) are important alloys of Be.
7. Basic magnesium carbonate is commonly known as magnesia alva, which is used as dental abrasive (in tooth paste) and cosmetics.
8. American baking power: Pure Ca (H2PO4)2 is used as American baking powder.
9. Nitrolim: Mixture of calcium cyan amide and carbon is known as nitrolim, which is used as nitrogenous fertilizers.
10. Norwegian salt petre: It is a mixture of lime and Ca (NO3)2. and has the composition Ca (NO3)2.CaO.
11. Ringer’s solution: It is used in the treatment of wounds and burns contain 4.30g NaCl, 0.150g KCl and 0.165g CaCl2 in 500ml aqueous solution.
12. Free flow salt: It contains a small amount of Ca3(PO4)2 with NaCl.
13. Baryta water: Ba(OH)2 is known as Barytawater.
14. Unlike alkali metals, alkaline earth metals do not show photoelectric effect.
15. Ba (NO3)2 is used in firework to develop green fire.
16. Ba(ClO3)2 is used for producing green fire and for the preparation of chloric acid.
17. Lithopone: It is a mixture of BaSO4 and ZnS. It is used as a white pigment and as a filter in rubber and paper industry.
18. BaO is an excellent drying agent especially for organic bases like pyridine.
19. BaO2 is used in the preparation of H2O2 and ignition mixture, used in thermite process. It is also used as an oxidizing agent.
20. Beryllium does not form peroxide.
21. Only Be9 is a stable isotope while Be6, Be7, Be8 and Be10 areradioactive. Half life of Be8 is 10-6 second. It decay as
22. Beryllium become passive in cold concentrated HNO3 but it dissolves in HCl, dil H2SO4 and dil HNO3.
23. ‘Be’ is highly toxic in elemental, compound and dust forms.
24. In India, limestone is found in Jabalpur, Katini Rewa, Jodhpur etc., white marble is found in Mekrana in Jodhpur, Ajmer, Alwar, Jabalpur and Kishangarh, black marble in Patiala and Bombay, Pink marble in Aravalli belt and Narsingpur (MP) while grey marble in Jodhpur.
SOLVED PROBLEMS
Prob 1. Alkali metal(s) which can not be produced by metal displacement method is/are
(A) Li (B) K (C) Rb (D) Na
Sol: (D) Sodium can not be produced by metal displacement method.
Prob 2. Alkali metals are characterized by
(A) Good conductor of heat and electricity
(B) High oxidation potential
(C) High melting point
(D) Solubility in liquid ammonia
Sol: (A, B, D) They do not have high melting point.
Prob 3. The pair of compounds which cannot exist together in aqueous solution are
(A) NaH2PO4 and Na2HPO4
(B) Na2CO3 and NaHCO3
(C) NaCl and NaH2PO4
(D) NaHCO3 and NaOH
Sol:(D) A base and an acid salt cannot exist together in solution.
Prob 4. Highly pure dilute solution of sodium in liquid ammonia
(A) shows blue colour
(B) exhibits electrical conductivity
(C) produces sodium amide
(D) produces hydrogen gas
Sol: (A, B) A solution of highly pure sodium in liq NH3 is quite stable and no chemical reaction takes place.
Prob 5. A solution of sodium metal in liquid ammonia is strongly reducing due to the presence of
(A) Sodium atoms
(B) Sodium hydride
(C) Sodium amide
(D) Solvated electrons
Sol: (D) A solution of sodium metal in ammonia contains solvated electrons.
Prob 6. Molten sodium chloride conducts electricity due to the presence of
(A) Free electrons
(B) Free ions
(C) Free molecules
(D) Atoms of sodium and chlorine
Sol: (B) Molten NaCl conducts electricity due to free ions.
Prob 7. Nitrogen dioxide cannot be prepared by heating
(A) KNO3 (B) Pb(NO3)2 (C) Cu(NO3)2 (D) AgNO3
Sol: (A) KNO3, on heating does not give NO2.
Prob 8. Molecular formula of Glauber’s salt is
(A) MgSO4.7H2O
(B) CuSO4.5H2O
(C) FeSO4.7H2O
(D) Na2SO4.10H2O
Sol: (D) Glauber’s salt is Na2SO4.10H2O.
Prob 9. The pair of compounds which cannot exist together in solution is
(A) NaHCO3 and NaOH
(B) NaHCO3 and H2O
(C) NaHCO3 and Na2CO3
(D) Na2CO3 and NaOH
Sol: (A) A base (NaOH) and acid salt (NaHCO3) can not exist together in solution.
Prob 10. The metallic lustre exhibited by sodium is explained by
(A) Diffusion of sodium ions
(B) Oscillation of loose electrons
(C) Excitation of free protons
(D) Existence of body centered cubic lattice
Sol: (A) The metallic lustre of sodium is due to oscillation of loose electrons.
Prob 11. An aqueous solution of sodium sulphate is electrolysed using inert electrodes. The products at the cathode and anode are respectively
(A) H2, O2 (B) O2, H2 (C) O2, Na (D) O2, SO2
Sol: (A) Electrolysis of qa. Solution of Na2SO4 using inert electrodes gives H2 (at cathode) and O2 (at anode).
Prob 12. Sodium sulphate is soluble in water but barium sulphate is sparingly soluble because
(A) The hydration energy of Na2SO4 is more than its lattice energy while the lattice energy of BaSO4 is more than its hydration energy
(B) The lattice energy has no role to play in solubility.
(C) The lattice energy of Na2SO4 is more than its hydration energy
(D) None of these
Sol: (A) Na2SO4 is soluble in water because its hydration energy is much more then its lattice energy. BaSO4 is insoluble in water because its lattice energy is much more than its hydration energy.
Prob 13. Which of the following statements is correct for CsBr3?
(A) It is a covalent compound
(B) It contains Cs3+ and Br– ions
(C) It contains Cs+ and Br3– ions
(D) It contains Cs+, Br– and Br2 molecule
Sol: (C) CsBr3 contains Cs+ and Be3– ions.
Prob 14. The pair of compounds which cannot exist together is
(A) Na2CO3 and NaHCO3
(B) Na2CO3 and NaOH
(C) NaHCO3 and NaCl
(D) None of these
Sol: (D) A base and an acid salt can not exist together in solution.
Prob 15. Sodium nitrate decomposes above 8000C to give
(A) N2 (B) O2 (C) NO2 (D) Na2O
Sol:
Prob 16. When zeolite, which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with
(A) H+ ions (B) Ba2+ ions (C) (D) Mg2+ ions
Sol: (D) Zeolite exchanges Na+ ions with the Ca2+ and Mg2+ ions present in hard water.
Prob 17. The compound insoluble in acetic acid is
(A) Calcium oxide
(B) Calcium carbonate
(C) Calcium oxalate
(D) Calcium hydroxide
Sol: (C) Calcium oxalate is insoluble in acetic acid.
Prob 18. An important ore of magnesium is
(A) Malachite (B) Cassiterite (C) Carnallite (D) Galena
Sol: (C) Carnallite (2KCl. MgCl2. 6H2O) is an important ore of Mg.
Prob 19. The decreasing order of second ionization potential of K, Ca and Ba is
(A) K > Ca > Ba (B) Ca > Ba > K (C) Ba > K > Ca (D) K > Ba > Ca
Sol: (A)
Out of three, K has the highest 2nd ionization energy. Calcium has a higher 2nd ionization energy than ‘Ba’.
Prob 20. Which of the following process is used in the extractive metallurgy of magnesium?
(A) Fused salt electrolysis
(B) Self reduction
(C) Aqueous solution electrolysis
(D) Thermite reduction
Sol: (A) Magnesium is obtained by the electrolysis of its fused anhydrous salt.








