HYDROGEN
Introduction:
In 1766, Sir Henry Cavendish prepared hydrogen in pure state by the action of disulphuric acid on zinc. It was named as inflammable air. Lavoisier a French chemist called it hydrogen mean water producer i.e., Greek hydro-water; gene-producer. Hydrogen is the lightest element of periodic table. Atomic form of hydrogen exists only at high temperature. In the normal elemental form, it exists as a diatomic molecule. Hence it is also called dihydrogen.
Occurrence:
Hydrogen is the most abundant element in the universe about 76% eg, Jupiter & Saturn contain mostly hydrogen. It is third most abundant element on the surface of the globe. In our atmosphere it occurs in a minute extent (i.e. H2 is not found) but in solar atmosphere, H2 is the main constituent as nuclear fuel. In the combined state, it constituents 15.4% of the earth crust and the oceans. In free state, it occurs in volcanic gases, some times in natural gases.
Unique position of hydrogen in the periodic table: It is a first element of the periodic table. The position of hydrogen in the periodic table is controversial or anomalous i.e. not fixed due to its resemblance both with alkali metals and halogens.
Resemblance with alkali metals:
(i) Like alkali metals, it has only one electron in the outer shell.
(ii) Like alkali metal, it loses its only electron to form hydrogen ion i.e. H+.
(iii) Like alkali metals, it exhibits an oxidation state of +1 in its compounds.
(iv) Like alkali metal, it has a strong affinity for non-metals.
(v) Like alkali metals, it also acts as strong reducing agent.
Resemblance with halogens:
(i) Like halogens, it has required one electron to gain the stable electronic configuration.
(ii) Like halogen, it can gain an electron and form a negative ion.
(iii) The ionization energy value of hydrogen is comparable to that of halogens i.e. .
(iv) Like halogens, it exhibits oxidation number of -1 in some of its compounds.
(v) Like halogens, it can combine with alkali metals to form salts with similar formulae.
(vi) Like halogens, it also exists as a diatomic molecule.
On the basis of some resemblance it can be placed either in group 1 of periodic table among alkali metals or group 17 of periodic table among halogens.
Isotopes of hydrogen: Hydrogen has three isotopes.
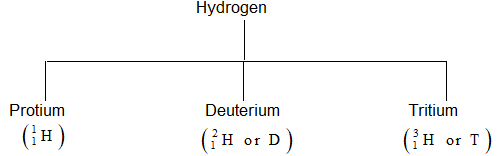
Relative atomic mass 1.007825 2.014102 3.016049
Relative abundance 99.985 0.0156 10–15
Radioactive stability Non radioactive Non radioactive radioactive
(Stable) (Stable) t1/2 = 12.33 yrs
Discovery → Prestley Urey Bleakney Gould
Isotopic effect:
In general, chemical properties of isotopes are same but quantitative differences are noticed amongst them e.g. the reaction between H2 and Cl2 is 13.4 times faster than between D2 and Cl2 under similar conditions. Such differences in chemical properties which are due to difference in atomic masses is called isotopic effect.
Preparation of Hydrogen:
(i) From cold water: By the action of very active metals like Na, K, Ca etc.
Zn-Cu and Al-Hg couple decompose water to give nascent hydrogen.
(ii) From boiling water: Less active metals like Zn, Mg, Al etc decompose boiling water liberating dihydrogen.
(iii) From steams: Less reactive metals like Fe, Sn, Ni etc, decompose steam at high temperature.
(iv) By electrolysis of water: Pure water is bad conductor of electricity.
(v) From alkalies: By the action of Be, Zn, Al, Sn etc.
(vi) From dil. Acids (Lab): By the action of Sn, Zn, Fe, Mg etc.
Preparation of pure hydrogen (>99.95%):
(i)
(ii)
(iii)
Manufacture of H2
(i) From water gas (Bosch process): By passing a mixture of water gas and steam over heated catalyst, Fe3O4 and Cr2O3 or FeCrO4, at high temperature, H2 is obtained.
The CO2 gas is removed by dissolving gaseous mixture in water under high pressure (25 atm)
(ii) By lane’s process: The super heated steam is passed over heated iron at 600-800°C.
Iron is regenerated by passing water gas (H2O + CO). This reaction is called vivifaction and time allotted for this reaction is about 20 minutes.
This iron is again used for decomposition of steam. In order to make the process continuous, the above two reactions are carried out alternatively using two or more furnaces.
(iii) From natural gas: Natural gas containing CH4 is also a good source of hydrogen. Natural gas is mixed with steams and then passed over ‘Ni’ catalyst heated to 900°C.
CO from the mixture is removed as in Bosch’s process. This process of obtaining H2 from natural gas is called steam reforming process.
(iv) Electrolytic method: By the electrolysis of acidified or alkaline water.
At cathode
At anode
(v) As by product:
When NaOH is manufactured by electrolysis of NaCl in Nelson or castner kellner cell, hydrogen is obtained as by product.
Reaction:
At anode:
At cathode:
Properties of Hydrogen:
Physical properties:
It is a colourless, odourless and tasteless gas. It is nonpoisonous but in presence of AsH3 is poisonous. It is slightly soluble in water. Its critical temperature is -234.5°C, which makes its liquification difficult. It is lightest of all element (density = 0.08987 gm L–1). B.P = -252.5 °C (20.5 K)
M.P. = -259.0 °C (14.0 K). Ionization potential = 13.54 eV/atm. Electronegativity = 2.1
Chemical properties:
(i) Combustion: It burns with blue flame in oxygen atmosphere. It is not a supporter of combustion.
Care should be taken with these gases since mixture of H2 and O2 close to a 2:1 ratio is often explosive.
ii) Reaction with non-metals:
iii) Reaction with metals:
A number of metals react with H2 forming hydrides.
The reactions are not violent and usually require a high temperature.
Metals like Fe, Ni and Pd form interstitial or metallic hydrides.
iv) Reducing property: The oxides of less electropositive metals such as copper, tin, iron, lead, etc, are reduced to the metals when heated in hydrogen.
Applications of Hydrogen:
i) Manufacture of methyl alcohol in presence of ZnO, Cr2O3 catalyst.
ii) Manufacture of Ammonia – Haber’s process.
iii) Manufacture of hydrogen chloride (absorbed in water).
iv) Hydrogenation of oils
v) Synthetic petrol – Fisher – Tropsch process
vi) When mixed with helium: It is used for filling balloons.
vii) Oxy – hydrogen flame: It produces temperature of 2800 – 30000C and oxyatomic hydrogen flame produces a temperature of 40000. It is used for welding purposes and for melting platinum and quarts. The atomic hydrogen welding torch is also used for the same purpose.
viii) As cryogenic fluid – To produce low temperature.
ix) As fuel:
a) In rocket fuel: Because of its low mass and high enthalpy of combustion. Saturn V, the rocket that took Neil Armstrong to the moon was powered by liq. hydrogen fuel. Oxygen (need for combustion) as well as hydrogen were carried in rocket in separate tanks. Inspite of its high heat of combustion (242 kJ mol–1) its use as a fuel is limited due to lack of availability of free hydrogen in nature & the difficulty of its safe storage and distribution.
b) In coal gas:
Composition: H2 = 45 – 55%, N2 = 2 – 12%, CH4 = 25 – 35%
CO2 = 0 – 3%, CO = 4 – 11% , O2 = 1 – 1.5%
Ethylene, acetylene, benzene, etc = 2.5 – 5%.
It’s calorific value is about 20,00kJ/m3.
Uses:
a) As illuminant
b) As a fuel
c) To provide a inert atmosphere in metallurgical processes.
c) In water gas: (H2 + CO)
Preparation :
This reaction is endothermic; hence, a blast of steam brings down the temperature of the bed of coke in a few minutes. To maintain the temperature above 10000C alternate blast of air and steam are passed over the coke bed.
Uses of water gases:
i) It burns with blue flame, calorific value is 13000kJ/m3.
ii) As industrial source of hydrogen (Bosch process)
iii) In manufacture of methyl alcohol (Patart Process) and synthetic petrol (Fisher – Tropsch process).
d) In carburetted water gas: It is a enriched mixture of water gas and gaseous hydrocarbons obtained from cracking of petroleum oils.
The gaseous hydrocarbons are used to increase the calorific value of water gas.
e) In semi water gas: It is a mixture of water gas and producer gas.
Composition: CO = 25 – 28% ; H = 10 – 12%; CH4 = 1 – 2%; CO2 = 4 – 5%; N2 = 50 – 55%
Its calorific value is 160 – 180 BTU per cubic foot.
Uses:
i) As fuel in steel industry
ii) For production of power in internal combustion engine.
f) In fuel Cell (H2–O2 fuel cell): Electrical cells that are designed to convert the energy from the combustion of fuels like hydrogen and carbon monoxide or methane directly into electrical energy are called fuel cells. This cell was used to power the Apollo space program and to form the drinking water for the astronauts. In this cell, hydrogen and oxygen are bubbled through a porous carbon electrode into concentrated aqueous sodium hydroxide or potassium hydroxide solution (electrolyte). Hydrogen is fed into the anode compartment where it is oxidized and the oxygen is fed into cathode compartment where it is reduced. Each electrode is made of porous compressed carbon containing a small amount of catalyst like
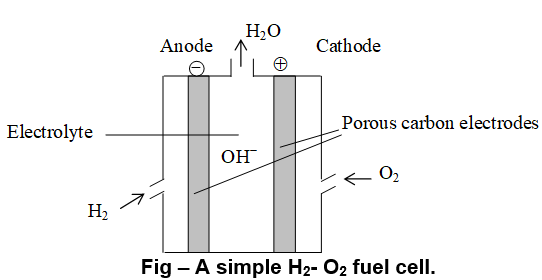
The electrode reactions are given below
Anode:
Cathode :
—————————————————————-
Overall cell reaction:
Advantages:
i. High efficiency (60 – 70%)
ii. Continuous source of energy
iii. Pollution free working.
xii) In metallurgy: Hydrogen is used in metallurgy of molybdenum and tungsten for obtaining pure metals from their oxides at high temperature.
xiii) In nuclear fusion: (Thermo nuclear reaction):
Special forms of Hydrogen:
i) Nascent hydrogen: Freshly prepared hydrogen is known as nascent hydrogen and is more reactive than ordinary hydrogen.
ii) Atomic hydrogen: When hydrogen is passed through an electric arc established between two tungsten filaments, hydrogen is dissociated into atoms. This form of hydrogen is known as atomic hydrogen. Reducing power of atomic hydrogen is more than that of nascent hydrogen.
iii) Active hydrogen: It is obtained by subjecting molecular hydrogen to silent electric discharge at ordinary temperature and 0.5 mm pressure. It has great chemical activity and its half life period is similar to that of atomic hydrogen.
iv) Occluded hydrogen: It is more active than ordinary hydrogen. Hydrogen adsorbed by certain metals e.g., Pd, Pt, Fe, Co, Ni etc., is known as occluded hydrogen. One volume of finely divided metals adsorb the following volumes of hydrogen. Pd black = 870, Pt = 49.5; Au = 3, Fe = 15.7, Cu = – 4.5, Al = 2.7. Colloidal Pd takes up 2050 volumes of H2 than itself. Occlusion decreases with rise in temperature. Order of occlusion Colloidal Pd > Pt > Au > Ni
v) Ortho and para hydrogen (Allotropes): When the spins of the two protons (nuclei) are parallel in the hydrogen molecule, it is known as ortho hydrogen (s = 1). When the spins of the two protons (nuclei) are antiparallel, it is known as para hydrogen (s = 0). Orthoform is more stable than para and para form always tends to revert in orthoform, still orthoform has not been isolated in the pure form. When ordinary H2 is passed over activated charcoal at 20K in quartz vessel. The charcoal adsorb only para H2. The charcoal consists of about 99.8%, para form and may be kept for a week in glass vessel at room temperature without any appreciable change to orthoform. Thus at room temperature ordinary H2 is an equilibrium mixture of 75% ortho and 25% para hydrogen. At low temperature, the percentage of para form increases.
Difference in ortho & para forms:
The two forms are differ in their physical properties but their chemical properties are same
i) Ortho is more stable than para.
ii) Vapour pressure of ortho is more than para.
iii) Specific heat of ortho is more than para.
iv) Conductivity of ortho is less than para.
v) Magnetic moment of para is zero while ortho has twice that of proton.
Transportation of H2:
It is transported in the form of hydrolith (CaH2) or ammonia (NH3). Ammonia is cracked by passing over heated catalysts yielding a mixture of hydrogen (75%) & nitrogen (25%).
Hydrogen economy:
Hydrogen economy is basically a use of liquid hydrogen as an alternate source of energy. The technology involves the production, transportation and storage of energy in the form of liquid hydrogen in bulk quantities. One major advantage of using hydrogen as a fuel is that it is environmentally clean, giving only water as a combustion product i.e., cleaner technology. The second advantage with hydrogen is that the heat of courbustion of hydrogen per gram is higher than any other fuel. e.g.,
Illustration 1: How do isotopes of hydrogen differ in their nuclear composition?
Solution: Isotopes of hydrogen have only one proton but contain nil, 1 and 2 neutrons in the nucleus of protium, deuterium and tritium respectively.
Illustration 2: Name the material which shows a great promise for holding hydrogen during the use of hydrogen in vehicles.
Solution: Carbon nanotubes and metal hydrides (of magnesium, magnesium-nickel alloys and iron- titanium alloys) can hold large quantities of hydrogen. Therefore, these materials are very potential for storing (holding) hydrogen gas.
Illustration 3: In the reaction, Zn(s) + H2SO4(dil) → ZnSO4(aq) + H2(g)
a) Which is more electropositive – hydrogen or zinc?
b) Name another metal that gives similar reaction with dilute H2SO4 but faster.
Solution: a) In this reaction, zinc (Zn) displaces hydrogen (H) from H2SO4. Therefore, zinc is more electropositive than hydrogen.
b) Magnesium, because magnesium (Mg) is more electropositive (reactive) than zinc (Zn).
Hydrides: Binary compounds of hydrogen and other elements are called hydrides. The types of hydrides depend upon electro negativity of an element and hence on the type of bond formed. Hydrides are classified into the following four classes.
1) Ionic or saline or salt like hydrides: These are formed by metals of low electronegativity i.e., elements of IA, IIA (except Be & Mg) and some highly positive lanthanides by heating the metal in hydrogen at high temperature. BeH2 & MgH2 have covalent polymeric structures. These are white colourless crystalline solids having high mp, bp, easily decomposed by water, alcohol, CO2 or SO2. These are used as strong reducing agent. Alkali metal hydrides are used for making etc and for removing last traces of water from organic compounds.
2) Molecular or covalent hydrides:
These are formed by element of group IIIA, IVA, VA, VIA, VIIA by sharing electrons with hydrogen atoms e.g., NH3, HCl, B2H6, AsH3. These are usually gases or liquids having low mp & bp (volatile).
The hydrides of IIIA group elements like B2H6, (AlH3)x act as lewis acid because they are electron deficient molecules. These have been classified as electron precise, electron deficient & electron rich hydrides.
i) Electron precise hydrides: All the valence electrons of the central atom are involved in bond formation. eg., CH4 (Methane); C2H6 (Ethane).
All these compounds have simple covalent bonds between the atoms.
ii) Electron deficient hydrides: The available number of valence electrons is less than the number required for normal covalent bond formation or for writing the Lewis structure for the molecule. e.g., B2H6 ; (AIH3)n;
iii) Electron rich hydrides: The valence electrons on the central atom are more than that are needed for bond formation. i.e., lone pairs are present on the central atom. e.g.,
![]()
Nomenclature of covalent hydrides: The systematic or IUPAC names of these hydrides are deduced from the name of the element and the suffix ‘ – ane’.
Table – 1. Some covalent or molecular hydrides and their names
| S.No. | Element | Group no. in the PT | Formula of the hydride | Name of the hydride | ||
| Short | Long | IUPAC | Common | |||
| 1. | B | III | 13 | B2H6 | Diborane (6) | Diborane |
| 2. | C | IV | 14 | CH4 | Methane | Methane |
| 3. | N | V | 15 | NH3 | Azane | Ammonia |
| 4. | P | V | 15 | PH3 | Phosphane | Phosphine |
| 5. | As | V | 15 | AsH3 | Arsane | Arsine |
| 6. | Sb | V | 15 | SbH3 | Stibane | Stibine |
| 7. | O | VI | 16 | H2O | Oxidane | Water |
| 8. | S | VI | 16 | H2S | Sulfane | Hydrogen sulfide |
| 9. | F | VII | 17 | HF | Hydrogen fluoride | Hydrogen fluoride |
| 10. | Cl | VII | 17 | HCl | Hydrogen chloride | Hydrogen chloride |
3. Metallic or Interstitial hydrides: These are formed by many transition and inner transition elements at elevated temperature, by absorbing hydrogen into the interstices of their lattices. They exhibit metallic properties and are powerful reducing agents. They are non-stoichiometric compounds and their composition varies with temperature and pressure. e.g., TiH1.73, CeH2.7, LaH2.8, PdH0.60, ZrH1.92 . Metals of groups 7, 8, 9 do not form hydrides. This gap is referred as hydride gap.
4. Polymeric hydrides: These are amorphous solids containing molecules linked together in two or three dimensions by hydrogen bridge bonds.
e.g.,.
Illustration 4: Metallic hydrides are called nonstoichiometric hydrides. Why are these so called?
Solution: Many metals form hydrides having nonstoichiometric compositions. That is why these hydrides are called nonstoichiometric hydrides.
Illustration 5: What is the hydride gap?
Solution: The elements in the middle of the d-block do not form hydrides. This absence of
hydrides in this part of the periodic table is called the hydride gap.
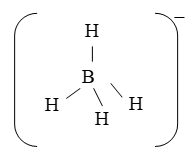
Illustration 6: Draw the structure of borohydride (BH4–) ion.
Solution: B in BH4– shows sp3 hybridisation. Each hybrid orbital forms bonds with the s orbitals of H atoms. Therefore, BH4– has a tetrahedral shape.
Water (H2O):
Water is one of the most abundant available substance or compound of hydrogen in nature.
About three fourth of the earth surface is covered by water. Water is known as universal solvent because.
i) It can dissolve maximum number of compounds.
ii) It is cheaply available
iii) It has high dielectric constant (82D)
Oxygen in H2O molecule is sp3 hybridized and the structure of water molecule is an angular or bent structure.
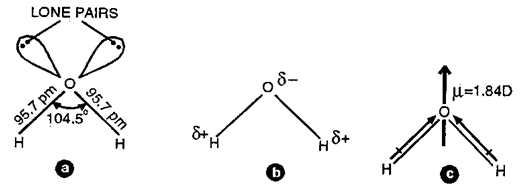
Fig. a) Structure of H2O in the gas phase
b) Polar nature of H2O molecule
c) Resultant dipole moment of water
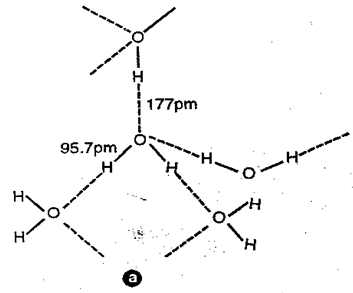
Fig: Hydrogen bonding in liquid water – structure of water molecule in the liquid state
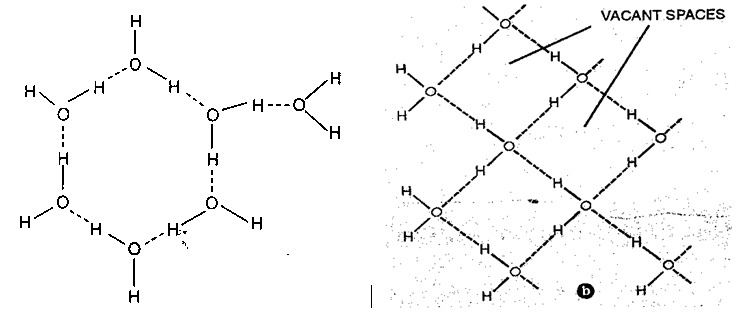
Fig. a)Hexagonal honey comb structure of ice
b) Tetrahedral arrangement of oxygen in ice
Most common physical state of water is liquid, however, it exist as solid (ice) below 00C and gas (steam) above 1000C.
Physical properties: of ordinary water and heavy water:
| Physical property | H2O | D2O |
| Maximum density (g/ml) | 1.00 at 40C | 1.1073 at 11.60C |
| Melting point | 00C | 3.820C |
| Boiling point | 1000C | 101.420C |
| Specific heat at 200C | 1.00 Cal/gK | 1.01 cal/gK |
| Latent heat of fusion | 79.7 cal/g | 75.5 cal/g |
| Latent heat of vaporization | 225.3 J/g | 232.9 J/g |
| Viscosity at 200C | 10.9 millipoise | 12.6 millipoise |
| Dielectric constant at 200C | 82.0D | 80.5D |
| Solubility of NaCl (per lit) at 250C | 359 g | 305 g |
| Molecular mass | 18.016 | 20.03 |
Chemical properties of water:
i) Reaction with metals: Metals of group IA & IIA decompose water to liberate H2 along with evolution of energy (Exothermic reaction). Hot metals like Zn, Mg, Fe etc, decompose water to liberate H2. Pb and Cu decompose water only on heating. Ag, Au, Hg & Pt metals donot decompose water.
ii) Reaction with Non-metals: Fluorine decomposes cold water forming ozonized oxygen. Chlorine decomposes cold water forming HCl & HClO, However, in presence of sunlight HCl and O2 is formed. Red hot coke on reaction with steam produce water gas.
iii) Reaction with non metal oxide: Acidic oxides combine with water to form oxo-acid like carbonic acid, sulphurous acid, sulphuric acid, orthophosphoric acid, nitric acid, perchloric acid etc.,
iv) Reaction with Metal oxides: Basic oxides combine with water to form alkalies.
v) Reaction with hydrides, carbides, nitrides & phosphides: Water decomposes these compounds with liberation of hydrogen, acetylene, ammonia, phosphine respectively.
vi) Hydrolysis: Salt of strong bases with weak acids, weak bases with strong acids and weak bases with weak acids undergo hydrolysis in water. Some slats like BiCl3, SbCl3 on hydrolysis form oxy compounds. Halides of non-metals like PCl3, PCl5, SiCl4 etc are decomposed by water.
vii) Water of crystallization: It forms hydrates like CuSO4. 5H2O, MgSO4. 7H2O, FeSO4.7H2O, BaCl2 . 2H2O etc., on combination with water during crystallization. The water present in the hydrates is called water of crystallization.
These are of 3 – types
(i) cationic (ii) Anion (iii) Lattice.
viii) Water as catalyst: Perfectly dry gases generally do not react but ammonia & HCl gas combine only in presence of moisture.
Soft and Hard water: The water which produces large amount of lather with soap readily is known as softwater e.g., rain water. The water which does not produce lather with soap readily is known as hard water e.g., sea water, river water, lake water, spring water & well water.
Hard water is of two types :
i) Temporary hard water
ii) Permanent hard water.
Cause of hardness of water: Presence of bicarbonates, chlorides and sulphates of calcium and magnesium in it, causes hardness of water.
Types of Hardness of water: Hardness of water is of two types:
i) Temporary hardness: It causes due to presence of soluble bicarbonates of calcium or magnesium or both. It is also called as carbonate hardness. It can be easily removed by simply boiling & filtering the water.
ii) Permanent hardness: It causes due to presence of soluble chlorides and sulphates, nitrates of calcium and magnesium. It is also called as non-carbonate hardness. It can not be removed simply by boiling the water.
Removal of temporary hardness (methods):
i) By boiling: The soluble bicarbonates are decomposed into insoluble carbonates, which are removed by filtration.
ii) By Clark’s process (Commercial scale): By adding lime water or milk of lime, soluble bicarbonates are converted into insoluble carbonates (easily filtered off). e.g.,
Excess of lime causes temporary hardness.
Removal of permanent hardness (methods):
i) By adding washing soda:
Both types of hardness are precipitated as insoluble carbonates which are filtered off
ii) By adding caustic soda:
The temporary and permanent hardness of water can be removed by adding caustic soda (NaOH). e.g.,
iii) By adding sodium phosphate (Na3PO4):
Temporary & permanent hardness of water are precipitated as phosphate of calcium & magnesium.
iv) Calgone process (sequestration) :
Sodium hexameta phosphate [Na2[Na4(PO3)6]] is called calgone (means calcium gone).
It forms soluble complex with Ca2+ or Mg+ present in hard water. e.g.
The complex donot form any precipitate with soap & readily produce lather with soap, Ca2+ & Mg2+ ions are rendered ineffective (sequestration). Water becomes free from Ca2+ and Mg2+ ions.
v) Permutit process: Inorganic ion exchanges: Hydrated sodium aluminium silicate [Na2Al2 Si2O8.x H2O] is called permutit or zeolite (Na2Z). It exchanges its sodium ions with Ca2+ and Mg2+ ions present in permanent hard water.
After some time permutit when fully exhausted, can be regenerated by passing a 10% solution of NaCl through it.
The soluble calcium & magnesium slats thus formed are washed away by water and the regenerated permutit can be used again.
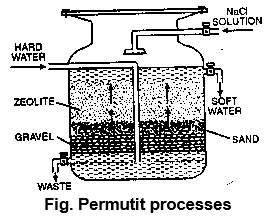
Advantages of the permutit process:
i) It is most efficient method to get water with zero degree hardness.
ii) It can be used to remove both temporary and permanent hardness completely.
iii) This is a very cheap process, since during the process only NaCl is consumed which is quite cheap.
iv) By synthetic Resins: (Done in two connecting tanks). In this process two types of resins are used.
a) Cation exchange resins: (H+ – resin): These are giant molecules containing carboxyl ( – COOH) or sulphonic acid group ( – SO3H) and are capable of exchanging H+ ions with Ca 2+ & Mg2+
The exhausted resins like permutit can be regenerated by passing conc H2SO4 or HCl acid.
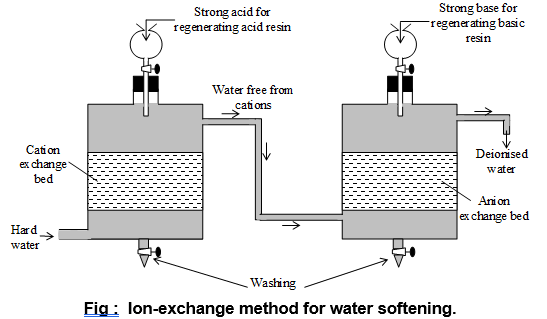
b) Anion exchange resins (OH– – resin):
These are also giant molecules containing basic group (NH3OH) and are capable of exchanging OH– ions with anions such as Cl– & SO42 ions.
Exhausted anion resin can be regenerated by adding moderately strong NaOH solution.
Thus, the water is first passed through cation resins and then through anion resins and pure distilled or deionized water is obtained from second tank i.e.,
Disadvantage of hard water:
Hard water, when used in boilers in industries causes formation of scales in the boiler. This eats away the metal layer and also causes wastage of heat energy. Hence only soft water is used in industries. Besides hard water causes wastage of soap.
Degree of hardness: The hardness of water is expressed in terms of ppm of calcium carbonate.
Illustration 7: A water sample contains 136mg of CaSO4 per litre calculate the hardness in term of CaCO3 equivalent
Solution: CaSO4 = CaCO3
136 100 or
136mg L– of CaSO4 = 100mg L– of CaCO3
Hence, hardness = 100ppm
Illustration 8: A one litre water sample contains 1 mg of CaCl2 and 1mg of MgCl2. Find out the total hardness in terms of CaCO3 in ppm.
Solution:
i)
ii)
Thus, degree of hardness in 1 litre = 0.9 + 1.05 = 1.95 ppm
Illustration 9: Why do lakes freeze from top towards bottom?
Solution: Ice is lighter than water. When the lake water cools, it starts freezing at its surface. The ice top does not sink to the bottom. Being lighter than water, it remains at the top. This top layer of ice acts as a thermal insulation for the lower layers of water and does not allow the lower layers of water to freeze. This phenomenon is of great ecological significance – the aquatic animals and plants can survive in lakes even when the lake from its top side is frozen.
Heavy water (D2O):
Preparation: It is prepared by exhaustive electrolysis of water containing heavy water with Ni – electrode (perforated). The electrolysis is carried out in seven stages.
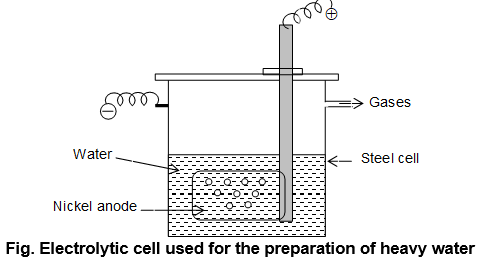
In the first stage, the electrolyte is reduced to of its original volume and the alkali present is partly neutralized by passing carbon dioxide.
It is then distilled and taken to another cell for the second stage of electrolysis. The process is repeated and 99% D2O is obtained after the seventh stage. About 30 litres of ordinary water gives 1 ml of heavy water. In India, heavy water is manufactured at Nangal in Punjab and at Bhabha Atomic Research Centre Trombay, Rourkela & Nayveli by electrolysis of ordinary water. Protium bonds are weaker than deuterium bonds and thus in electolysis of ordinary water, H2 is liberated much faster than D2 and remaining water becomes enriched in D2O.
Properties of heavy water:
i) Physical properties:
It is a colourless, odourless and tasteless mobile liquid.
Nearly all the physical constant for D2O are higher than ordinary water.
ii) Chemical properties:
Heavy water is chemically similar to ordinary water. However D2O reacts more slowly than H2O in chemical reactions.
i) Eelectrolysis : In presence of Na2CO3 or P2O5 (which makes it conductor)
ii) Reaction with metals:
Metals of group IA & IIA liberated deuterium from D2O.
Hot metals like Zn, Fe etc decompose the vapour of heavy water.
iii) Reaction with metal oxides: It form heavy alkalies with oxides like Na2O, CaO etc.
iv) Reaction with non metal oxides:
It forms deutero acids with acidic oxides like N2O5, P2O5, SO3 etc.
v) Reaction with metal carbides, phosphides, nitrides, arsenides.
Like H2O, it forms corresponding deuterocompounds.
v) Deuterolysis : Water brings hydrolysis of certain inorganic salts. It gives similar reactions which are termed as deuterolysis.
vi) Exchange reactions :
It can exchange labile hydrogen of a compound by deuterium partially or completely.
viii) Formation of deutero hydrates: Like ordinary water, it may be associated with salts as water of crystallisation, giving deutero hydrates.
e.g.,
Uses of D2O:
i) As a tracer compound.
ii) As a neutron moderator and coolent in nuclear reactors.
iii) In production of heavy hydrogen.
iv) In deciding the basicity of acids.
Hydrogen peroxide (H2O2):
Structure: H2O2 is best represented as the equilibrium mixture of two tautomeric forms in equilibrium.

In H2O2 the two oxygen atoms are linked to each other by a single covalent bond (i.e., peroxide bond) and each oxygen is further linked to a hydrogen atom by a single covalent bond. The two O – H bonds are, however, in different planes due to repulsions between different bonding and anti bonding orbitals. The dihedral (inter planar) angle between the two planes being 111.50 in gas phase but reduced to 90.20 in the crystalline state due to hydrogen bonding. The other molecular dimensions of hydrogen peroxide in the gas phase and the crystalline state are show in figure.
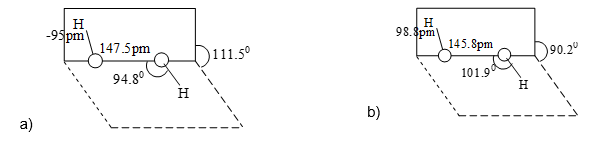
Fig. Open book structure of H2O2 (a) in the gas phase dihedral angle is 111.50 and (b) in the solid phase at 110K, dihedral angle is 90.20.
Preparation of hydrogen Peroxide:
1) Laboratory method:
a) From sodium peroxide: By the action of ice cold dil H2SO4
b) From BaO2:
Anhydrous BaO2 does not react readily with H2SO4 because a coating of insoluble BaSO4 is formed on its surface which stop further action of the acid. Excess of BaO2 should be avoided because it tends to decompose H2O2. H2O2 obtained by this method can not store for long.
The weaker acid such as H2CO3 (CO2) and H3PO4 acid treatment is preferred over sulphuric acid because a coating of insoluble impurities like barium per sulphate tends to decompose H2O2.
H3PO4 also acts as a negative catalyst for H2O2 because H3PO4 removes all heavy metals (catalyst for H2O2 decomposition) in the form of insoluble phosphates.
3BaO2 + 2H3PO4 Ba3 (PO4)2 + 3H2O2
Ba3 (PO4)2 + 3H2SO4 3 BaSO4 + 2H3PO4.
2) Industrial Methods:
i) By electrolysis of 50% ice cold H2SO4:
At anode:
At cathode:
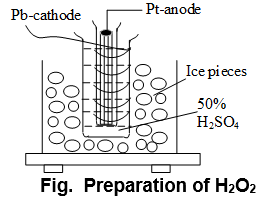
Modification:
In place of 50% H2SO4, an equimolar mixture of H2SO4 and ammonium sulphate is electrolysed.
NH4HSO4 NH4SO4– + H+
At anode: 2NH4SO4– (NH4)2S2O8 + 2e–
Ammonium peroxy disulphate or
Ammonimum per sulphate
At cathode: 2H+ + 2e– H2
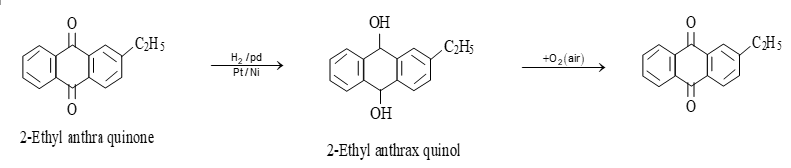
ii) By auto – oxidation: By auto oxidation of 2 – ethyl anthraquinol
3) By oxidation of isopropyl alcohol: A little H2O2 is added to initiate the reaction
Concentration of H2O2 solution: It is very carefully concentrated since it decomposes on heating or on standing.
Decomposition is catalysed by Cu, Ag, Pt, Fe, Co, MnO2 etc. Following methods are employed for concentration of H2O2 :
i) Evaporation on water bath: 50% H2O2 is obtained by careful evaporation of solution on water bath.
ii) Dehydration in vaccum desicator: 50% H2O2 is dehydrated in a vaccum desicator in presence of conc H2SO4, then 90% H2O2 is obtained.
iii) Vaccum distillation: In this step 90% H2O2 is distilled under reduced pressure (10 – 15) to get 99% H2O2.
iv) Cooling: The last traces of water in H2O2 are removed by freezing it in a freezing mixture (dry ice + ether) when crystal of hydrogen peroxide separate out.
Storage of H2O2: It is stored in coloured paraffin wax coated plastic or teflon bottles in presence of trace of alcohol, acetanilide, glycerine or phosphoric acid (i.e., some stabilizer or negative catalyst), which slow down the rate of decomposition of hydrogen peroxide. Impurities like silica, MnO2, Iron, Mn or Al2O3 causes rapid decomposition.
Physical properties:
i) Anhydrous H2O2 is colourless, viscous liquid soluble in ether, alcohol and water (sp. gravity 1.45g/ml at 00C).
ii) It gives blue tinge in thick layers.
iii) It causes blisters on skin.
iv) It is bitter in taste.
v) Pure H2O2 is weak acidic in nature and exist as more associated liquid (due to hydrogen bonding) than water and turns blue litmas red (Ka = 1.57 ´ 10–2 at 200c) & ionize in 2 steps H2O2 H+ + HO2– and HO2– H+ + O2
vi) It boil at 1520C and freeze at – 0.890
vii) It begins to decompose at bp and thus, distilled under reduced pressure.
viii) It is diamagnetic in nature.
ix) In pure state, its dielectric constant is 93.7, which increases with dilution. Due to high dielectric constant, H2O2 and its aqueous solutions are good solvents. However not used commonly on account of its oxidant nature.
Chemical properties:
i) Stability: Pure H2O2 decomposes on long standing or heating into water and O2.
2H2O2 2H2O + O2
It is an example of disproportionation reaction.
ii) Acidic nature: The pure liquid has weak acidic nature but its aqueous solution is neutral towards litmus. It reacts with alkalies and carbonates to give their corresponding peroxides.
H2O2 + 2NaOH Na2O2 + 2H2O
H2O2 + Na2CO3 Na2O2 + CO2+ H2O
3) Oxidising properties (Powerful): It accepts electrons and acts as powerful oxidant in acidic as well as in alkaline (basic) medium.
In acidic solution:
In basic solution:
Thus, H2O2 is more powerful oxidant in acidic medium.
The simple interpretation of H2O2 as oxidant can be shown by the equation.
Some other oxidizing properties of H2O2 are given below
i) It oxidizes black lead sulphide to white lead sulphate.
PbS + 4H2O2 PbSO4 + 4H2O
ii) It oxidizes ferrous sulphate to ferric sulphate in presence of H2SO4.
2FeSO4 + H2SO4 + H2O2 Fe2 (SO4)3 + 2H2O
iii) It oxidizes nitrites to nitrates
NaNO2 + H2O2 NaNO3 + H2O
iv) It oxidizes benzene to phenol in presence of FeSO4
C6H6 + H2O2 C6H5OH + H2O
v) It oxidizes sulphites to sulphates
Na2SO3 + H2O2 Na2SO4 + H2O
vi) It oxidizes ferrocyanide to ferricyanide in acidic medium
2K4 [Fe (CN)6 ]+ H2O2 + H2SO4 2K3 [Fe (CN)6 ]+ K2SO4 + 2H2O
vii) Sulphurous acid to sulphuric acid
H2SO3 + H2O2 H2SO4 + H2O
viii) Chromic hydroxide (ppt) to chromate in alkaline solution
2 Cr (OH)3 + 4OH– + 3H2O2 2CrO42- + 8H2O
Chromate(yellow)
x) In basic medium, formaldehyde to formic acid, in presence of pyrogallol & itself reduced to H2.
2HCHO + H2O2 2HCOOH + H2
xi) Acidified KI to I2
2KI + H2SO4 + H2O2 K2SO4 + 2H2O + I2
xi) Acidified chromic acid or acidified K2Cr2O7 (ether) to blue peroxide of chromium.
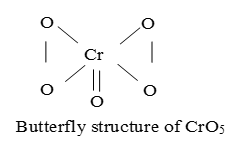
4) Reducing properties: (Poor):
It also acts as a reducing agent towards powerful oxidizing agent in acidic as well as basic medium.
in acidic medium:
in basic medium:
.
From E0op and E0Rp values, it is apparent that H2O2 is powerful oxidizing agent and poor reducing agent.
Reducing property of H2O2 can be expressed as [H2O2 + O H2O + O2]
Some other reducing properties of H2O2 are given below
It reduces:
i) Moist silver oxide to silver.
Ag2O + H2O2 2Ag + H2O + O2.
ii) Ozone to oxygen :
O3 + H2O2 2O2 + H2O
iii) Manganese dioxide to mangnous salt in acidic medium
MnO2 + H2SO4 + H2O2 MnSO4 + 2H2O + O2
iv) Chlorine & bromine to corresponding hydracids. This property is called antichlor.
Cl2 + H2O2 2HCl + O2
v) Red lead to plumbous acid in presence of HNO3
vi) Decolorises acidified KMnO4
vii) Potassium ferricyanide to potassium ferro cyanide in alkaline medium
viii) KMnO4 to MnO2:
ix) Hypohalites to halides:
5) Addition reaction:
6) Reaction with N2O5:
(Pernitric acid)
7) Reaction with NO2:
2NO2 + H2O2 2HNO3
8) Bleaching properties:
It acts as a bleaching agent.
Coloured material + [O] colourless material.
Strength of H2O2 solution:
i) Percentage strength:
It expresses the amount of H2O2 by weight present in 100 ml of the solution.
For example : 30% aq solution of H2O2 implies that 30gm of H2O2 are present in 10ml of the solution.
ii) Volume strength:
The strength of aq solution of H2O2 is expressed in terms of volume strength. For example 10 volume, 20 volume and so on H2O2 solution. 10 volume H2O2 implies that 1ml H2O2 produces 10ml of O2 at NTP or 1 litre H2O2 produces 10 litre of O2 at NTP and so on. Let us now calculate the % strength of a 10 volume H2O2 solution.
Table: strength of H2O2:
| Sample of H2O2 | % strength (w/v) | Molarity (M) | Normality (N) |
| 10 vol H2O2 | 3.036 | 0.893 | 1.786 |
| 20 vol H2O2 | 6.072 | 1.786 | 3.572 |
| 30 vol H2O2 | 9.108 | 2.679 | 5.358 |
Some important relations:
Concentration or strength of 10 volume H2O2 solution = 30.35 g/L
Volume strength = 5.6 x Normality =
Volume strength = 11.2 x Molarity.
Note:
i) 30% H2O2 is called perhydrol.
ii) During photosynthesis H2O2 is oxidize to O2.
Illustration 10: What is meant by “30 volume H2O2”?
Solution: The statement “30 volume H2O2” mean that 1 volume of this sample of H2O2 gives 30 volumes of oxygen at NTP or STP.
Illustration 11: Hydrogen peroxide is used to restore the colour of old oil-paintings containing lead oxide. Write a balanced equation for the reaction that takes place in this process.
Solution: In old paintings, the original white lead paint over the period of time gets converted to black lead sulphide (PbS) due to the presence of H2S in the atmospheric air. Hydrogen peroxide oxidizes the black PbS into PbSO4.
Illustration 12: Explain why hydrogen peroxide is stored in coloured / plastic bottles?
Solution: Hydrogen peroxide decomposes when exposed to light. It is due to this that it is stored in wax-lined coloured glass or plastic bottles in the presence of a stabilizer such as urea.
Illustration 13: Calculate the percentage strength and normality of 20 volumes H2O2.
Solution: (1) 20 volumes H2O2 solution means 1ml of H2O2 on decomposition gives 20ml of O2 at STP i.e.., 1 litre of H2O2 on decomposition gives 20 lit of O2.
Further, let us consider decomposition of H2O2
Since 68g of H2O2 gives 22.4 lit of O2
∴ 20 lit of O2 is given by = g of H2O2 = 60.17g
∴ strength of H2O2 = 6.071%
Now, Normality of 20 volume H2O2 solution is
Illustration 14: The lable on a bottle of hydrogen peroxide solution reads as 10 volumes. Report the concentration of hydrogen peroxide in percentage.
Solution: We know that hydrogen peroxide decomposes as follows
Since 22400 ml of oxygen is obtained from 68g of H2O2 at STP
10ml of oxygen is obtained from = of H2O2
1 ml of H2O2 solution contains 0.3035 gm
100 ml of H2O2 – 0.3035 ⨯ 100 = 3.035 grams of H2O2
or the solution is 30.35%
Concentration of 10 volumes H2O2 solution = 3.035 ⨯ 10 = 30.35 gm/litre
Illustration 15: A sample of water contains 12g of MgSO4 per 100 kg of water. Calculate its degree of hardness.
Solution: Wt. of of MgSO4 = 120
Mol. wt. of MgSO4 = 120
Weight of water = 100 kg = 100 ⨯ 103 = 105 9
Degree of hardness =
= 100 ppm.
Exercise 1:
(i) The volume strength of 1.6 N H2O2 solution is
(A) 8.96 (B) 7.96 (C) 3.56 (D) 10
(ii) The normality of 20 volume hydrogen peroxide solution is
(A) 4.57N (B) 3.57 N (C) 3.0 N (D) None of these
(iii) The volume strength of 3% solution of H2O2 is
(A) 6.5 volume (B) 7.5 volume (C) 9.88 volume (D) 8.5 volume
Answer to Exercises
Exercise 1:
(i) A
(ii) B
(iii) C
SOLVED PROBLEMS
Prob 1: Ortho and para hydrogen differ in
(A) atomic number
(B) mass number
(C) electron spin in two atoms
(D) nuclear spin in two atoms
Sol: (A) Ortho & para hydrogen differ in nuclear spin in two atom.
Prob 2: The volume strength of 1.5N H2O2 solution is
(A) 4.8 (B) 8.4 (C) 3.0 (D) 8.0
Sol: (B) Volume strength = 5.6 ⨯ normality = 5.6 ⨯ 1.5 = 8.4
Prob 3: The oxide which gives H2O2 on treatment with dilute acid is
(A) PbO2 (B) Na2O2 (C) MnO2 (D) TiO2
Sol: (B) Na2O2 peroxide gives H2O2 on treatment with dilute acid.
Prob 4: One mole of calcium phosphide on reaction with excess of water gives
(A) one mole of phosphine
(B) two moles of phosphoric acid
(C) two moles of phosphine
(D) one mole of phosphorus pentoxide
Sol: (C) Two moles of phosphine.
Prob 5: The structure of H2O2 is
(A) planar (B) non-planar (C) spherical (D) linear
Sol: (B) The structure of H2O2 is non-linear (open book)
Prob 6: Action of water or dilute mineral acids on metals can give
(A) monohydrogen (B) tritium (C) dihydrogen (D) trihydrogen
Sol: (C) H2O + Mg MgO + H2; 2HCl + Zn ZnCl2 + H2
Prob 7: The H– ions is stronger base than hydroxide ion. Which of the following reactions will occur if sodium hydride (NaH) is dissolved in water?
(A)
(B)
(C)
(D) None of these
Sol: (C) H– + H2O No reaction.
Prob 8: Which of the following pairs of substances on reaction will not evolve H2 gas?
(A) Fe and H2SO4 (aqueous)
(B) Copper and HCl (aqueous)
(C) Sodium and ethyl alcohol
(D) Iron and steam
Sol: (B) Cu will not evolve H2 gas on reaction with aqueous HCl.
Prob 9: Heavy water is obtained by
(A) boiling water
(B) fractional distillation of H2O
(C) prolonged electrolysis of H2O
(D) heating H2O2
Sol: (C) Heavy water is prepared by prolonged electrolysis of H2O.
Prob 10: Polyphosphates are used as water softening agents because they
(A) form soluble complexes with anionic species
(B) precipitate anionic species
(C) form soluble complexes with cationic species
(D) precipitate cationic species
Sol: (C) Polyphosphates are used as water softening agent because they form soluble complexes with cationic species.








