Introduction:
“The periodic table of elements is one of the most powerful icon in science a single document that consolidates much of our knowledge of chemistry”.
The periodic table of elements is an important landmark in the history of chemistry. It is considered as one of the great scientific achievements. It is difficult to study individually the chemistry of more than one hundred elements known today and their related compounds. In the 19th century, chemists like Dobereiner, Newlands, Dimitri Mendeleev and Moseley thought of proper classification of elements and did monumental work in analyzing the properties of these elements and classifying them.
Finally we will examine that the physical & chemical properties of elements repeat periodically like the seasons in nature.
HISTORICAL STUDY OF PERIODICITY
Dobereiner’s Triads (Law of triads):
The German chemist, John Dobereiner in 1817-1829 classified the elements in groups of three elements called triads; which showed similar chemical properties.
He further showed that the atomic weight of the middle element of each triad was approximately the arithmetic mean of atomic weight of the other two elements.
Table Dobereiner’s Triads
| Triad | At. wt. of middle element |
| 1. | |
| 2. | |
| 3. |
Draback of Dobereiner’s Classification : Dobereiner’s method of classification could arrange only a limited number of elements out of those known at that time in the form of triads. Therefore, the idea of triads could not be applied to all the elements then known.
Newland’s law of Octaves : The English chemist John Newland’s, in 1865-66 arranged the elements in increasing order of their atomic weight and absorbed that every 8th element has similar properties to those of the first just like the eighth note of musical scale, i.e., sare gama pada nisa – – – – -.
According to this law, sodium, the eighth element from lithium has similar properties to that of lithium, the first element and similar observation have been made for Be & Mg, B & Al and so on.
Table Newland’s Law of Octaves
| 1. | |||||||
| 2. | |||||||
| 3. |
Limitation of Newland’s Classification:
(i) It failed badly while dealing with the heavier elements beyond calcium (Ca).
(ii) When the noble gases were discovered, the idea of octaves because beyond calcium there is a different of 18 instead of 8.
Lothar Meyer’s Arrangement:
Lothar Meyer, a German chemist plotted a graph between the atomic volumes and atomic weights of the elements and absorbed that the elements with similar properties occupied similar positions on the curve.
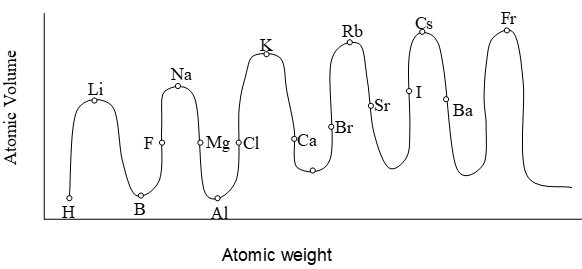
i) Alkali metals occupy the peak position on the curve.Results:
ii) Alkaline earth metals occupy descending positions on the curve.
iii) Metalloids occupy the bottom position on the curve
iv) Halogens occupy ascending positions on the curve.
Thus, it showed certain regularities among the elements. On this basis, Lothar meyer proposed that the physical properties of the elements are a periodic function of their atomic weights i,e,, base of Mandeleev’s periodic table.
Mendeleev’s Periodic Law:
In 1869, Mendeleev, a Russian chemist stated that “The physical and chemical properties of elements are a periodic function of their atomic weights.“ In other words, according to this law, when the elements are arranged in the increasing order of their atomic masses, the elements with similar properties are repeated at regular intervals.
Mendeleev’s Periodic Table:
Mendeleev arranged 63 – 65 elements known at that point of time in the increasing order of their atomic weights in the form of a table, known as Mendeleev’s periodic table and published in 1905. It is noted that at this time noble gases were not discovered.
Mendeleev’s original table consists of eight vertical column (called groups) I-VIII & six horizontal rows (called periods). The group number were indicated by Roman numerals.
He placed elements with similar nature in same group with respect to atomic weight. However
i) At some places the order of atomic weight was changed in order to justify the chemical and physical nature. g. Cobalt (at. Wt. = 58.9) is placed before Ni (At. Wt = 58.7)
ii) Some places were left vacant for new elements which were not discovered at that point of time. e.g., scandium, gallium and germanium etc., were not known at that time. He left vacant space for them and named them as eka-boron, eka-aluminium and eka-silicon believing them to be similar to boron, aluminium and silicon respectively.
iii) The first half of elements were placed in the upper left corner and second half in the lower right corner of each box. e.g., In 4th period of group I, K is written in the top left corner, while Cu is written in the lower right corner.
Merits of Mendeleev’s Periodic Table:
Mendeleev’s classification was considered superior to the others proposed earlier because of the following reasons.
i) Systematic study of the elements: Mendeleev’s classification condensed the study of about 90 elements (only 63 – 65 elements were known at that time but he left a provision for many more) to the study of only 8 groups of elements.
ii) Prediction of new elements and their properties: He left some gaps in his periodic table for the undiscovered elements and could even predict the properties of undiscovered elements.
e.g., eka – boron = scandium
eka – aluminium = gallium
eka – silicon = germanium
iii) Correction of certain atomic masses: By placing elements strictly according to the similarity in their properties, he was also able to correct certain atomic masses. e.g., He corrected the atomic masses of beryllium (Be), gold (Au) and platinum (Pt).
‘Be’ had been assigned atomic mass is 13.5.
Atomic mass = equivalent mass valency factor = 4.5 3 = 13.5
It should be placed between carbon and nitrogen but its properties resembles with magnesium and calcium with valence of 2 and the corrected mass of Be came out to be 9. i.e., 4.5 2 = 9.
Defects in the Mendeleev’s periodic table:
It was a brilliant attempt for the classification of elements but it had certain defects in it. These defects are described below:
i.Position of hydrogen: In Mandeleev’s periodic table, position of hydrogen was not made clear. i.e., It can be placed either in group – I or VII.
ii. Separation of chemically similar elements: Certain elements which appear to be chemically similar like Cu and Hg, Au and Pt etc., have been placed in separate groups.
iii. Grouping of chemically dissimilar elements: Certain chemically dissimilar elements have been grouped together in the Mendeleev’s periodic table. e.g., Cu, Ag and Au have no resemblance with alkali metals (Li, Na, K etc),but these have been grouped together in group I.
iv. Inversion in periodic table: Certain pairs of elements had to be placed in the reverse order of atomic masses in order to confirm the periodic law. e.g., Co (58.9) has been placed before Ni (58.7) and Ar (39.9) has been placed before K (39.1) etc.
v. Position of Isotopes: No separate places were given to isotopes.
vi. Position of lanthanides and actinides: Lanthanide & actinide were not given places in periodic table. From these anomalies, atomic weight does not appear to be a good basis for the periodic classification of elements.
Modern Periodic Law (Based on Atomic Number & Electronic Configuration): Henry G.J.Moseley, a brilliant young British physicist, in 1913 discovered a new property of elements called atomic `number ’, which provides a better basis for the periodic arrangement of the elements.
Moseley found that when element is bombarded with electrons, x-rays are produced. He found that the frequency of x-ray is related to the atomic number of the elements. i.e. constant.
From this frequency he concluded that atomic number is better basis to determine the position of an element in the periodic table and proposed a periodic law, that is know as modern periodic law.
This law states that, “the physical and chemical properties of elements are periodic functions of their atomic numbers or electronic configurations”.
It was observed that the elements with similar properties are repeated according to their electronic configuration at regular intervals of 2,8,8,18,18 or 32. These numbers are called magic numbers and cause of periodicity in properties.
Modern Periodic Table:
i. It was proposed by Moseley
ii. Modern periodic table is based on atomic number
Characteristic of Modern Periodic Table:
i. It consists of 9 vertical columns called groups are families i.e., i to viii + 0 group and 7 horizontal series (rows) are called periods.
ii. Inert gases were introduced by Ramsay
Extended form or Long form of Periodic Table:
i. It is the most widely accepted form of periodic table.
ii. It is based on modern periodic law i.e., atomic number and electronic configuration.
iii. It was constructed by Neils Bohr and proposed by Rang, Werner, Bury and others. It is also known as Bohr’s periodic table (1920).
iv. It consists of 18 groups and 7 periods.
v. In this modified long form of periodic table (based on IUPAC -1984 recommendation) the groups have been numbered from left as group 1 to group 18.
vi. The number of IA, IIA, IIIB – – – 0, in the Bohr’s design has been dropped.
General Characteristics of Long form Periodic Table:
i. The 18 vertical columns of long form of periodic table, are called groups.
ii. Some groups have typical names. g,.
a) Elements of group 1 are called alkali metals.
b) Elements of group 2 are called alkaline earth metals
c) Elements of group 15 are called pnicogens
d) Elements of group 16 are called chalcogens
e) Elements of group 17 are called halogens
f) Elements of group 18 are called noble gases.
g) Elements of group 11 are called coinage metals.
iii) The 7 horizontal rows or columns of long form of periodic table are called periods. i.e.,
a) first period : It contains two elements i.e., H & He. It is called shortest period
b) second period : It contains eight elements e. Li to Ne. It is called short period. The elements of second period are also known as bridge elements.
c) third period :It also contains 8 elements i.e. Na to Ar and known as short period. The elements of this period are also known as typical elements.
d) fourth period : It contains 18 elements, K to Kr and this is called long period.
e) fifth period : It has also 18 elements, Rb to Xe. This is called long period.
f) sixth period : It has 32 elements, Cs to Kr. This period is a longest Period.
g) seventh period : It is an incomplete period which has 29 – elements, Fr to Uuo.
iv) The elements of group 1, 2 & 13 to 17 are called main group elements. These are also called as representative or normal elements.
v) The element of group 3 to 12 are called transition elements.
vi) Elements with atomic number 58 to 71 are called lanthanides whereas the elements with atomic number 90 to 103 are called actinides. These elements are also known as f-block elements or inner-transition elements.
vii) Lanthanides (4f-series) and actinides (5f-series) are placed in two separate rows below the main periodic table to avoid unnecessary side wise expansion of the periodic table.
Table : Period and Energy Levels in Periodic Table
| Periods | Energy Level | No.of elements | Common name |
| 1st | 1s | 2 (H to He) | Shortest Period |
| 2nd | 2s, 2p | 8 (Li to Ne) | Short Period |
| 3rd | 3s, 3p | 8 (Na to Ar) | |
| 4th | 4s 3d 4p | 18 ( K to Kr) |
Long period
|
| 5th | 5s, 4d 5p | 18 (Rb to Xe) | |
| 6th | 6s 4f 5d 6p | 32 (Cs to Rn) | longest period |
| 7th | 7s 5f 6d 7p | 29 | Incomplete, it can accommodate upto 32 elements |
Advantage of Long form of Periodic Table:
- It is based on more fundamental property i.e. atomic number and electronic configuration.
- It completely separates metals and non – metals.
- Due to separation of two sub groups, dissimilar elements do not fall together.
- It correlates the position of elements with their electronic configuration clearly.
- The completion of each period is more logical.
- Periodicity in properties can be easily visualized.
- The greatest advantage of this periodic table is that this can be divided into four blocks namely s,p,d and f-blocks elements.
- This arrangement of elements is easy to remember and reproduce.
- It removes the anomalies of Mandeleev’s periodic table i.e., K39 precides Ar40.
Defects of Long form of Periodic Table:
- Position of Hydrogen is not fixed till now
- Position of helium is not fully justified as its EC justifies it to be included in the s – block.
- Position of lanthanides and actinides has not been given inside the table.
- It does not reflect the exact distribution of electrons among all the orbitals of the atoms of all the element.
Nomenclature of Elements with Atomic Number above 103:
The names are derived by using roots for three digits in the atomic number of the element and adding the ending ium.
Table – 4 : Notation for IUPAC Nomenclature of elements
| Digit | Root Name | Abbreviation |
| 0 | nil | n |
| 1 | un | u |
| 2 | bi | b |
| 3 | tri | t |
| 4 | quad | q |
| 5 | pent | p |
| 6 | hex | h |
| 7 | sept | s |
| 8 | oct | o |
| 9 | enn | e |
Table : Names of elements beyond atomic number 103.
| ATOMIC NUMBER | NAME | SYMBOL | IUPAC OFFICIAL NAME | IUPAC SYMBOL |
| 104 | Unnilquadium | Unq | Ruthor fordium | Rf |
| 105 | Unnilpentium | Unp | Dubnium | Db |
| 106 | Unnilhexium | Unh | Seaborgrium | Sg |
| 107 | Unnil septium | Uns | Bohricem | Bh |
| 108 | Unniloctium | Uno | Hassnium | Hs |
| 109 | Unnilennium | Une | Meitnerium | Mt |
| 110 | Ununnilium | Uun | Darmstadium | * |
| 111 | Unununium | Une | * | * |
| 112 | Ununbium | Uub | * | * |
| 113 | Ununtrium | Uut | – | |
| 114 | Ununquadium | Uuq | * | * |
| 115 | Ununpentium | Uup | ||
| 116 | Ununhexium | Uuh | ||
| 117 | Ununseptium | Uus | ||
| 118 | Ununoctium | Uuo | ||
| 119 | Ununennium | Uue | ||
| 120 | Unbinilium | Ubn |
Element yet to discovered. + Official IUPAC name yet to be announced.
Illustration 1: The element 119 has not been discovered. What would be the IUPAC name and symbol for this element ? On the basis of the periodic table Predict the electronic configuration of this element and also the formula of its most stable Chloride and oxide.
Solution. Z = 119
IUPAC name: Ununennium
Symbol : Uue
E.C. : [Uuo] 8s1
Group no : group 1
Formula of its chloride : Uue Cl and
Formula of its oxide : (Uue)2O
Differentiating electron:
The electron by which the electronic configuration of the given element differs from that of its proceeding element is called the differentiating electron and that electron is the last entering electron of its atom.
Classification of elements on the basis of their electronic configurations into s, p, d, and f- blocks: All the elements of periodic table have been divided into 4-blocks depending upon the subshell to which the last electron enters. (i.e. differentiating electron)
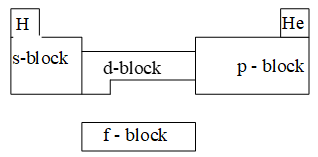
1) s- Block Elements:
i. Elements of group 1 and 2 constitute s-block.
ii. In these elements, differentiating electron enters the s orbital of nth
iii. General electronic configuration of s-block elements =
Table : s – Block Elements
| Group – I (IA) | Group – II (IIA) |
| Li | Be |
| Na | Mg |
| K | Ca |
| Rb | Sr |
| Cs | Ba |
| Fr | Ra |
| GEC ns1 | ns2 |
General Characteristics of s-Block Elements:
i. They are soft metals with low melting and boiling points.
ii. They are highly reactive metals and provide univalent and bivalent ions by losing one or two valence electrons respectively.
iii. They have low ionization energies. They are highly electropositive metals.
iv. They impart (most of them) colours to the flame.
v. They form ionic compounds.
2) p-Block Elements:
i. Element of group 13 to 18 constitute p-block.
ii. In these elements, differentiating electron enters the p-orbital of nth shell.
iii. General electronic configuration:
Table : p – Block Elements
| 13 | 14 | 15 | 16 | 17 | 18 → group |
| He | |||||
| B | C | N | O | F | Ne |
| Al | Si | P | S | Cl | Ar |
| Ga | Ge | As | Se | Br | Kr |
| In | Sn | Sb | Te | I | Xe |
| Tl | Pb | Bi | Po | At | Rn |
General Characteristics of p-Block Elements:
i. These include both metals and non metals.
ii. They form covalent compounds.
iii. They exhibit more than one oxidation states in their compounds.
iv. Their reducing nature increases from top to bottom in a group
v. Their oxidizing nature increases in a period from left to right. s and p block elements are collectively known as normal or representative elements.
3) d- Block Elements (Transition Elements):
i. Elements of group 3-12 constitute d-block.
ii. In these elements differentiating electron enters the d orbital of (n-1) shell.
iii. General electronic configuration: .
iv) The element with atomic number 104 & 105 were earlier known as Kurchatovium (Ku) and Hahanium (Ha) are now accepted by IUPAC as Rutherfordium (Rf) and Dubnium (Db).
v) G12 element Zn, Cd and Hg are not considered as transition elements though they are d-block elements because a transition element is defined as the element which possesses atleast one unpaired electron in its free state or one of its stable oxidation states.
Table : d – Block Elements
| Gp → | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Series ↓ |
| 4th Pd | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | 3d | ||
| 5th Pd | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | 4d | ||
| 6th Pd | Hf | Ta | W | Re | Os | Ir | Pt | Au | 5d | ||
| 7th Pd | Rf | Db | Sg | Bh | Hs | Mt | Ds | Uuu | 112Uub | 6d |
General Characteristics of d-Block Elements:
i. They are hard metals with high melting and boiling point.
ii. They exhibit variable oxidation states. They form both ionic and covalent compounds.
iii. They are good conductors of heat and electricity.
iv. They form coloured ions and complexes.
v. Metals and their ions having unpaired electrons are paramagnetic in nature.
vi. They acts as catalyst eg : V, Mn, Fe, Co, Ni They form alloys.
4) f – Block Elements (Inner Transition Elements):
i. The elements in which the last electron enters the f-orbital of (n-2) shell are called f-block elements.
ii. The f-block has two series (4f & 5f) and each series has 14-elements.
iii. These comprise two horizontal series placed at the bottom of the periodic table to avoid its un-necessary expansion.
iv. f – block elements collectively are also known as rare earth elements.
v. General electronic configuration :
vi. Lanthenides and actinides belong to the sixth and seventh period of 3rd group respectively. These elements present between two transition elements. Therefore, they are called inner transition elements.
Table : f – Block Elements
| 4f | 58Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | 71Lu |
| 5f | 90Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | 103Lr |
General Character of f-Block Element:
i. They are heavy metals. They show high melting points
ii. They exhibit variable oxidation states. They have tendency to form complexes.
iii. Their ions and complexes are generally coloured. Elements of actinide series are radioactive in nature.
iv. These are called rare earth elements.
v. Elements from atomic number 93 to on wards are called transuranic elements. These elements are radioactive in nature.
Classification of Elements Based on Properties: (Bohr’s Classification): On the basis of properties and electronic configuration all the elements are divided into four types.
i) Noble gas elementsg: Elements of `O’ group, these are inert gases & exist as mono atomic molecular gases. GEC:
ii) Representative elementg: Elements of s-block + p-block (except `O’ group) they includes metals, non – metal & metalloids. GEC:
iii) Transition elements e.g: Elements of d-block. (except group 12- elements) GEC:
iv) Inner transition elements: g: elements of f-blocks . They are called inner transition. They lie between two transition elements i.e., between La & Hf in the 6th period Ac & Rf in 7th period. G.E.C:
Prediction of Periods, Groups and Blocks:
Period : number of valence shell
Group : number of valence electrons
Block : type of orbital which receives the differentiate electron (s).
Rules:
(a) for s-block : GP number = number of valence electrons
(b) for p-Block : GP number = number of valence electrons.
(c) for d – Block : GP number = electrons in (n-1) d-sub shell + number of ns
electrons
Illustration 2 : The outer electronic configuration of some elements are given below :
State, to which block of the periodic table each of these elements belong :
Solution. :
Periodicity and Periodic Properties:
In the periodic table, the properties of elements change gradually, with change in atomic number (or electronic configuration) of the elements. This trend repeats itself at regular intervals. It is called ‘periodicity’ and the properties are called ‘periodic properties’.
Cause of Periodicity:
i. When the elements are arranged in the order of increasing atomic numbers, the properties repeat after certain regular intervals.
ii. This is due to the similarity in the arrangement of electrons in the outermost shell of the elements.
iii. In the periodic table, elements with similar properties repeat at certain intervals (i.e. magic number) of 2, 8, 8, 18,18 and 32.
iv. Elements with similar properties are placed in a particular group.
Hence, periodicity is seen when we move from left to right in a period or top to bottom in a group.
Some of the Periodic Properties of Elements are
1. Atomic Size (Atomic Radius): If an atom is considered to be a sphere. It’s size is generally given by its radius (i.e. atomic radius). It may be defined as, the distance from the centre of the atomic nucleus to the outer most shell containing electrons. It is measured in angstrom (Å) or picometer (pm).
Difficulties in determination of atomic radius:
The atomic radius cannot be determined exactly. But the inter-nuclear distance of the bonded atoms can be measured using X ray diffraction of electron techniques and it is taken as a standard.
The atomic radius depends on many factors like the number of bonds formed by the atom, nature of bonding, oxidation state of the atom etc.
However, it is expressed in terms of operational definitions (i.e. based on nature of bonding)
i. covalent radius
ii. van der Waals radius
iii. Metallic radius.(crystal radius)
iv. Ionic radius
i) Covalent radius:
a.) It may be defined as one-half of the distance between the nuclei of two covalently bonded atoms of the same element in a molecule.
b) It is also known as single bond covalent radius.
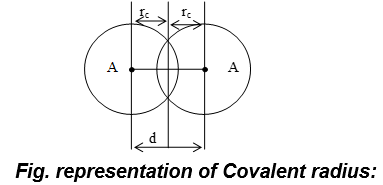
c) Thus, covalent radius, for homonuclear di-atomic molecules (AA),.
where, d = inter nuclear distance (bond length) between two covalently bonded same atoms.
e.g : (i) Covalent radius of hydrogen = 0.37Å
(ii) Covalent radius of chlorine = 0.99 Å
d) For heteronuclear diatomic molecules (AB); Bond length (A – B) = ra + rb
ii) van der Waal’s Radius:
a) It is one-half of the distance between the nuclei of two adjacent atoms belonging to two neighboring molecules of an element in the solid state.
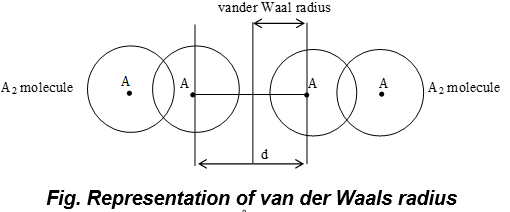
e.g.(i) vander Waal radius of hydrogen = 1.2Å
(ii) vander Waal radius of chlorine = 1.8Å
b) Magnitude of van der Waal’s radius depends upon the packing of the atoms when the element is in the solid state.
c) Atomic radii of inert gases are usually expressed in terms of van der Waal’s radius. Sometimes, therefore, it is also called as inert gas radii.
d) The Covalent radius is always smaller than the corresponding van der Waal’s radius
Note : Atomic radii of Xe & Kr can be expressed in terms of vander Waal’s radii or covalent radii.
iii. Metallic Radius (Crystal Radius):
a) It is the half of the distance between two successive nuclei of two adjacent metal atoms in the metallic close packed crystal lattice. i.e. metallic lattice.
e.g: i) For sodium, r metallic = 1.86Ao, rcovalent = 1.54 Å
ii) For potassium, r metallic = 2.31Ao, rcovalent = 2.03 Å
b) In a metallic lattice, the valence electrons are mobile, therefore, they are only weakly attracted by the metal ions or kernel.
c) In contrast, in a covalent bond, a pair of electrons are strongly attracted by the nuclei of two atoms.
Thus, a metallic radius of an element is always greater than its covalent radius.
Finally, three types of atomic radii can be compared as.
van der Waal’s radius > metallic radius > covalent radius
iv. Ionic radius:
The distance between the nucleus and the point where the nucleus exerts its influence on the electron cloud.
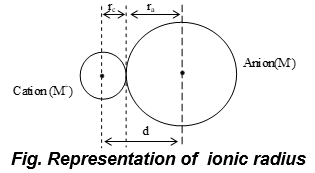
a) According to Pauling,
e.g , In NaCl , .
b) General trend in ionic radii is
c) (Å or pm).
d) Cationic radii < Covalent radii < vander Waal Radii < Anionic radii
e) The ionic radii of isoelectronic ions decrease with the increase in the magnitude of the nuclear charge.
e.g : are isoelectronic species containing 10 electrons.
Therefore, their ionic radii decrease in the following order.
Factors Influencing the Atomic or ionic Radius:
i) Nuclear charge: With increase in nuclear charge, force of attraction between the nucleus and electron cloud increases. Hence, the electron cloud moves closer to the nucleus and atomic size or radius decreases.
The actual charge felt by the valence shell electrons is called effective nuclear charge (Zeff) ; where Z = total nuclear charge, = screening constant.
ii) Number of orbits or shells: With increase in number of orbits or shells, the distance between the nucleus and the last shell increases.
Hence, atomic radius or size increases similarly, with the decrease in number of orbits, the atomic radius or size decreases.
iii) Percentage of ionic character: Covalent radius of H, in HCl, HBr and HI are different due to different degree of covalent nature in HCl, HBr & HI.
iv) Multiplicity of bonds: Covalent radii depend on the multiplicity of bonds
eg : a) for C-C bond, bond length = 1.54Å, and radius of C = 0.77 Å
b) for C= C bond, bond length = 1.34, and radius of C = 0.67 Å.
Variation of Atomic Radii in the Periodic Table:
i) Variation in a Group:
On moving down the group the valence shells become far away from the nucleus and thus the atomic radius increases. It is primarily due to the addition of a new energy shell.
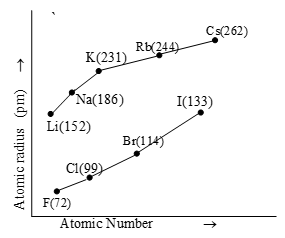
Fig. Variation of atomic radius (metallic) with atomic number for alkali metals and halogens.
ii) Variation in a Period:
On moving along the period, the effective nuclear charge increases and thus the electron cloud is attracted more strongly toward the nucleus resulting in the contraction of atomic radius.
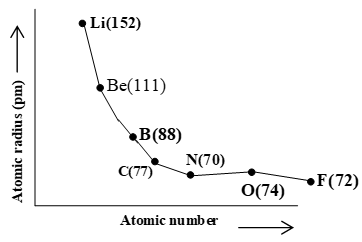
Alkali metals which are at the extreme left of the periodic table have the largest size in a period. The halogens which are present at the extreme right of the periodic table have the smallest size. However, in the second period, the atomic size of nitrogen is the smallest. After nitrogen atomic size increases for oxygen and then decreases for F.
The size of the atoms of inert gases are however, larger than those of the proceeding halogens. (i.e. due to van der Waal radius).
Variation of Atomic or Ionic Radii in Transition Elements:
Left to right (L → R):
The decrease in the atomic radii of transition elements is small since the differentiating electrons enter into inner `d’ levels.
The additional electrons into (n-1) d levels effectively screen much of increased nuclear charge on the outer ns electron and therefore, size i.e., atomic radius remains almost constant.
Top to bottom (T → B):
In vertical columns of transition elements there is an increase in size from first member to second member on moving down the group as expected but from second member to third member, there is very small change in size and some times sizes are same. This is due to lanthanide contraction.
Thus, the increase in size in the second and third transition series is almost compensated by the lanthanide contraction.
Table: Covalent Radius of Transition Elements (in pm)
| 1st series (3d) | Sc Ti V Cr ………………..Cu |
| covalent radius | 144 132 122 117 ……………….117 |
| 2nd series (4d) | Y Zr Nb Mo ………………. Ag |
| covalent radius | 162 145 134 129 …….………. 134 |
| 3rd series (5d) | La Hf Ta W ………………Au |
| covalent radius | 169 145 134 130 ……….……..134 |
4d and 5d elements are most similar in properties when compared with to those of 3d and 4d transition elements due to lanthanide contraction.
Variation of atomic or ionic radius in lanthanides:
In inner transition elements, say lanthanides, the differentiating electron enters 4f sublevel of the anti penultimate shell. With an increase in atomic number in the lanthanide series, the atomic and ionic radii steadily decrease. This may be attributed to the dispersed shape of the f-orbitals and the poor screening effect of the f-orbital electrons.
Lanthanide contraction and its consequence:
The decrease in size of the atoms or ions among the lanthanides is known as ”Lanthanide contraction”. Due to lanthanide contraction, the crystal structure and other properties of the elements become very closely similar. As a consequence it becomes difficult to separate them from a mixture. The effect of lanthanide contraction is also observed in the sizes of the 5d transition series. Therefore i.e., in the elements that follows lanthanides. The 4d and 5d transition elements are more closer in properties than those exhibited by the elements of 3d and 4d transition series.
Table: Covalent Radii of Lanthanides (pm)
| Element | Ce | Pr | Nd | Pm | Sm………….Tb | Lu |
| Covalent radius(pm) | 165 | 165 | 164 | 163 | 162…………159 | 156 |
Variation of atomic or ionic radius in actinides:
The sizes of these elements decrease regularly along the series because the extra charge on the nucleus is poorly shielded by the f-electrons. This results in “Actinide contraction”. This decrease in sizes of the actinide elements is similar to lanthanide contraction.
Illustration 3: Select from each group, the species which has the smallest radius stating appropriate reason?
Solution.
Reason: Effective nuclear charge is greater than the other.
2. Ionization Potential (or Energy):
The minimum amount of energy required to remove the most loosely bound electron from an isolated , neutral gaseous atom is called ionization energy or enthalpy or potential.
It is also called first ionization energy (IE1). It is measured in kJ/mole, kcal/mole or electron Volt/atom (1eV = 96.49 kJ mol–1 or 23.06 kcal mol–1)
For example :
The energy required to remove the second electron (single) from the unipositive cation is called second ionization energy (IE2) i.e.,
Similarly an atom has third, fourth ….. ionization energies or potentials.
(and so on)
Thus, first ionization (IE1) causes ionization of neutral gaseous atom (M), to form M+(g), second ionization energy causes ionization of to form and third ionization energy causes ionization of to form and so on.
The order of successive ionization energy is (where n = no. of electron removed)
Ionization energies are determined from discharged tube experiments or from spectral studies. In discharged tube the particular voltage at which, an atom receives sufficient energy to lose an electron, is called ionization energy or potential.
Factors Affecting Ionization Potential (or Energy):
i) Size of Atom:
Ionization energy decreases as the atomic size increases because force of attraction of its nucleus for the valence electron decrease. ‘Cesium’ has least ionization energy and helium has highest iodization energy in the periodic table.
ii) Nuclear Charge:
Greater is nuclear charge, greater will be the ionization energy i.e.,
IE Nuclear charge Force of attraction of nucleus for valence electron.
Ionization potential increases with increase in nuclear charge. e.g.
| Element | Li | Be | C | N | F |
| IP1( eV) | 5.390 | 9.320 | 11.26 | 14.54 | 17.42 |
iii) Screening effect:
The reduction in force of attraction by the shells present in between the nucleus and valence electrons is called screening effect or shielding effect. Greater the number of shells between the nucleus and valence electron, lesser will be electron nucleus attraction and hence smaller will be the ionization energy. In other words, an increase in the number of electrons in the inner shells tends to decrease the ionization energy.
iv) Penetration effect:
Ionisation energy increases as the penetration effect of sub-shell or electrons increases.
Thus, the order of penetration of different subshell is s > p > d > f (I.E. s > p > d > f).
s- sub shell is more penetrating towards the nucleus than p subshell. Removal of an electron from s-sub shell requires greater energy than p-subshell.
v) Stable electronic configuration:
Atoms with stable configuration i.e. half filled or completely filled sub-shells, have exceptionally high value of first ionization energy because such a stable atom required more energy to loose a single electron i.e.
Thus, more stable is the electronic configuration, greater is the ionization energy.
Variation of Ionization energy in periodic table:
i) Variation in a group
Ionization energy decreases down the group due to the combined effect of the increase in size and shielding, which compensates the effect of increased nuclear charge. Hence, the force of attraction of nucleus for valence electron decreases. Thus, ionization energy decreases down the group.
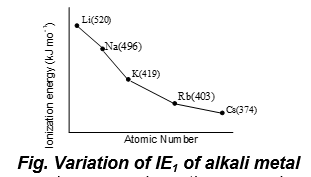
Exception: Exceptionally ionization potentials of some elements (6th period) from atomic number 73(Ta) to atomic number 82 (Pb) are higher than the upper elements (5th period) of the same group, due to lanthanide contraction, as shows in the table.Thus, in a group, ionization energy decreases down the group due to increase of atomic size and decrease of nuclear attraction on the outer most electron i.e. valence electron.
| Group | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| IE1(eV) |
6.8 |
Mo 7.1 |
Tc 7.2 |
Ru 7.3 |
Rh 7.4 |
Pd 8.3 |
Ag 7.5 |
Cd 8.9 |
In 5.7 |
7.3 |
5th pd |
| IE1(eV) |
7.7 |
W 7.8 |
Re 7.8 |
Os 8.7 |
Ir 9.2 |
Pt 9.0 |
Au 9.2 |
Hg 10.4 |
Te 6.1 |
7.4 |
6th pd |
The ionization energies of inert gases are also exceptionally high due to stable electronic configuration. i.e.,
Element He Ne Ar Kr Xe Rn
IE1(ev) 24.5 21.6 15.8 14.0 12.1 10.7
ii) Variation in a Period:
In a period ionization energy increases from left to right due to decrease of atomic size and increase of nuclear attraction on the valence electron i.e. effective nuclear charge increases.
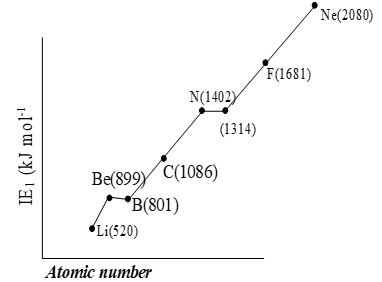
Variation of ionization energy of elements of second period
| Group → | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period 1 |
H 1312 |
He 2372 |
||||||
| Period 2 |
Li 520 |
Be 899 |
B 801 |
C 1086 |
N 1402 |
O 1314 |
F 1681 |
Ne 2080 |
| Period 3 |
Na 496 |
Mg 737.6 |
Al 577 |
Si 786 |
P 1011 |
S 999 |
Cl 1255 |
Ar 1520 |
Table : IE1 of some elements (in kJ mol-1)
Order of Ionization energies.
a) In 2nd period
b) In 3rd period
In each period alkali metals possess least ionization energy while noble gases have highest ionization energies. In periodic table cesium (Cs) has lowest ionization energy while helium (He) has highest ionization energy.
Illustration 4 : The correct order of ionization energy is
(A) C> N < O (B) C < N > O
(C) C > N > O (D) C < N < O
Solution. (B) Nitrogen has highest ionization energy than carbon and oxygen due to half filled 2p subshell.
3. Electron Affinity (Electron Gain Enthalpy):
The amount of energy released when an electron is added to a neutral gaseous atom of an element to form a negative gaseous ion, is called first electron affinity. e.g.
It is measured in kcal/mole or in eV or kJ/mol.
The energy changes accompanying the addition of first, second, ,third , etc, electrons in neutral gaseous atoms are called successive electron affinities and are denoted as , EA3 etc. The successive electron affinities or electron gain enthalpies may be positive or negative.
e.g :
In general,
Electron affinities cannot be determined directly but are obtained indirectly using the Born Haber cycle.
Note : , where egH = electron gain enthalpy, EA = electron affinity, R = gas constant and T = absolute temperature. At 250C
Factors Affecting Electron Affinity:
i) Atomic Size : Smaller the size of atom, stronger is the attraction of its nucleus for the incoming electron, greater is the electron affinity
ii) Nuclear Charge : Greater is the nuclear charge of element, stronger is the attraction of its nucleus for incoming electron, thus greater is the electron affinity.
iii) Electronic Configuration: An atom with stable configuration i.e. fully filled orbitals or exactly half filled orbitals of the same sub shell, has zero or positive electron affinity.
iv) Penetration Power : s > p > d > f
Variation of Electron Affinity in Periodic Table:
i) Variation in a group: In a group electron affinity decreases from top to bottom due to increase in atomic size and decrease in nuclear attraction for incoming electron.
Electron affinity of first member (element) in the group of representative elements, is lower than the second member (element), due to smaller size, as a result of which it has strong electron – electron repulsions.
Table : Electron gain enthalpies of some elements (kJ Mol-1)
|
1 |
2 |
13 |
14 |
15 |
16 |
17 |
18 |
|
H -73 |
He +48 |
||||||
|
Li -60 |
Be +66 |
B -83 |
C -122 |
N +31 |
O -141 |
F -333 |
Ne +48 |
|
Na -53 |
Mg +67 |
Al -50 |
Si -119.6 |
P -74 |
S -200 |
Cl -348 |
Ar +96 |
|
Se -195 |
Br -324 |
||||||
|
Te -190 |
I -295 |
||||||
|
Po -174 |
At -270 |
From the table, it is evident that electron gain enthalpy does not show a perfectly regular trend along a group or a period due to number of exceptions. In halogens, Chlorine has most negative electron gain enthalpy i.e., halogen have the most negative electron gain enthalpies.
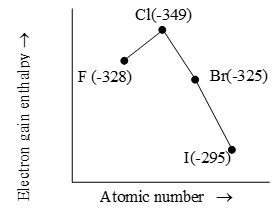
Fig. Variation of electron gain enthalpy of halogen (kJ Mol-1)
Noble gases have positive electron gain enthalpy (i.e. EA = 0). Electron affinities of Be, Mg & N are almost zero.
II) Variation in a period:
In a period electron affinity increases from left to right, due to decrease in atomic size and increase in nuclear attraction for incoming electrons. The electron affinity of exactly half filled configuration is approximately zero and exactly zero for fully filled stable configuration. Noble gases have zero electron affinities or positive electron gain enthalpies due to stable configuration. The elements of 3rd period have some what higher electron affinities than the elements of 2nd period in a same group.
Importance of Electron Affinity (Applications):
i) Electron affinity Capacity of accepting electron by element
tendency of formations of anion
electronegative nature
oxidizing nature (F,Cl, Br, O, S etc are strong oxiding agents)
1/ reducing nature
ii) On the basis of EA and IP some properties can be predicted as
a) Metallic Nature 1/EA 1/IP, in Gp – increases, in Pd – decrease
b) Non – metallic character EA IP, in Gp – decreases, in Pd – increases
c) Reducing nature 1/EA 1/IP ; in Gp – increases, in Pd -decreases
d) Oxidising nature EA IP ; in Gp – decreases , in Pd – increases
e) Stability of metal EA IP, in Gp – decreases , in Pd – increases
f) Activity of metal 1/EA 1/IP, in Gp – increases, in Pd – decreases , only (IA, IIA, IIIA)
g) Basic nature of oxides 1/EA 1/IP in Gp – increases, in Pd – decreases
h) Acidic nature of oxides EA, IP, in Gp – decreases, in Pd – increases
Illustration 5: The electron gain enthalpy of Chlorine is -349 kJ mol-1. How much energy in kJ is released, when 3.55 gm of Chlorine is converted completely into Cl– ion in the gaseous state ?
Solution:
Energy released, when 35.5 g (1 mol) of chlorine atoms change completely into Cl–
(g) = 349kJ
Energy released, when 3.55 g of chlorine atoms change completely into
4. Electronegativity (EN):
i) The power or tendency of an element (atom) in a molecule to attract the shared pair of electrons to wards itself is known as its electronegativity.
ii) The element having higher electronegativity with draws the shared pair of electrons easily towards itself. eg.
iii) The electronegativity concept was introduced by Pauling in 1932.
Scales to measure Electronegativity: There are several electron negativity scales.
a) Pauling Scale:
The electron negativities of elements are calculated by the following formula.
Where, are electronegativities of A & B elements (atoms) in AB type compound.
E = Bond polarity = extra bond energy in kcal mol-1 or k J mol-1
(geometrical mean).
Where, Bond energy of AB compound
Bond energy of AA molecule
Bond energy of BB molecule
In this scale, ( to avoid any negative value)
b) Mulliken Scale: Mulliken regarded, electronegativity as the average value of ionization potential and electron affinity of an atom.
(electronegativity calculated from this formula is not correct).
If the values of I.P and EA are taken in k J mol-1 unit
(544 2 x 2 . 8 x 96.5)
If the values IP & EA are taken in eV (unit)
If the values of IP & EA are taken in k.cal mol-1 unit.
Thus, Mulliken’s values are 2.8 times larger than the Pauling’s values. i.e.
(Relationship between the Pauling & Mulliken’s scale).
c) Allred Rochow’s Electronegativity: According to All red- Rochow, electronegativity is the force exerted by the nucleus of an atom on its valence electrons.
Where, Zeffective = Effective nuclear charge.
r = the covalent radius
Mulliken scale is limited to monovalent atoms and does not cover multivalent atoms.
Factors Affecting Electronegativity:
i) Atomic Size: The electronegativity decreases with increase of the atomic size.
Smaller atoms have more electronegativity than bigger atoms.
ii) Nuclear Charge: The electronegativity increases with increase of the nuclear charge.
iii) Screening Effect: The electronegativity decreases with increases of screening effect.
iv) Oxidation State: The electronegativity increases as the positive oxidation state increases.
v) Hybridization: For the same element, the electron negativity changes with hybridization, with decrease in the s character of the hybrid orbitals, the electronegativity decreases. e.g., sp – has more s-character i.e. more EN.
vi) Electronic Configuration: Atoms with nearly filled shell of electrons, will tend to have higher electronegativity than those with sparsely occupied ones. Inert gas elements ( have zero electronegativity due to completely filled outer shells.
Variation of EN in Periodic Table:
a) Variation in Group: In a group, the electronegativity decreases from top to bottom, due to increase in atomic size i.e. atomic radii and decrease of nuclear attraction on the valence electron(s) i.e; outermost electron(s).
Table 19 : Electronegativity of representative elements (on paulilng scale)
| Group → | 1 | 2 | 13 | 14 | 15 | 16 | 17 |
| I pd |
H 2.1 |
|
|||||
| I pd |
Li 1.0 |
Be 1.5 |
B 2.0 |
C 2.5 |
N 3.0 |
O 3.5 |
F 4.0 |
| III pd |
Na 0.9 |
Mg 1.2 |
Al 1.5 |
Si 1.8 |
P 2.1 |
S 2.5 |
Cl 3.0 |
| IV pd |
K 0.8 |
Ca 1.0 |
Ga 1.6 |
Ge 1.8 |
As 2.0 |
Se 2.4 |
Br 2.8 |
| V pd |
Rb 0.8 |
Sr 1.0 |
In 1.7 |
Sn 1.8 |
Sb 1.9 |
Te 2.1 |
I 2.5 |
| VI pd |
Cs 0.7 |
Ba 0.9 |
Tl 1.8 |
Pb 1.8 |
Bi 1.9 |
Po 2.0 |
At 2.2 |
(EN1 increases across the period)
Halogens, have high electronegativity values whereas alkali metals, have low electronegativity values except `H’. Fluorine has highest electronegativity in periodic table while cesium has least electronegativity in periodic table. First, second & third highest electronegative elements are F,O, N or Cl respectively.
b) Variation in period: In a period, the electronegativity increases from left to right, due to decrease in atomic size and increases of nuclear charge or attraction on outer most electrons i.e. the effective nuclear charge over comes the shielding effect. In each period noble gases have zero electro negativity due to completely filled outer shells. As the electron negativity increases across the period, the non-metallic character increases.
Applications of Electronegativity:
a) Electronegativity difference and partial Ionic Character in ab type Bond:
i) If EN = 7, bond = approximately 50% covalent & 50% Ionic in nature.
ii) If, EN > 1.7, bond = ionic more than 50%
iii) If, EN < 1.7, bond = covalent (more than 50%)
iv) If EN = 0, i.e; bond = purely covalent (non polar)
v) If EN = small, bond = polar covalent % of ionic character = 16
in A –B type bond
| EN | 0.6 | 0.8 | 1.2 | 1.9 | 2.2 | 2.8 | 3.2 |
| Partial ionic character | 10 | 15 | 30 | 50 | 70 | 86 | 92 |
b) Nature of Oxides:
predicts the nature of oxides formed by the element A,
where = electronegativity of oxygen.
= electronegativity of element A.
i) If is high ; Oxides = Basic in nature
ii) If is small ; Oxides = Acidic in nature
Illustration 6: Calculate the electronegativity of fluorine from the following data.
Solution. Applying the pauling equation
In this equation dissociation energies are taken in k cal mol-1
On the other hand electronegativity of fluorine can be calculated by following relation.
Illustration 7: Ionization potential and electron affinity of fluorine are 17.42 and 3.45 eV respectively. Calculate the electronegativity of fluorine.
Solution. According to Mullikens equation
Electronegativity of fluorine = (where IP and EA are taken in eV).
Thus, the electronegativity is 3.7267.
Illustration 8: Calculate the electronegativity of silicon using Allred Rochow method. Covalent radius of silicon is 1.2 Å.
Solution. According to Allred Rochow equation.
Electronic configuration of . According to slater rule
Now,
5. Valency:
i) Valency of an element is the number of electrons gained or lost or shared with other atoms in the formation of compounds.
ii) In other words the number of hydrogen atoms or chlorine atoms or twice the number of oxygen atoms with which one atom of the element combines is known as the valence (or valency of the element).
iii) Valency of group 1 and 2 elements = number of electrons in outer most shell
iv) Valency of group 13 to 14 = group number – 10.
v) Valency of group 15 to 18 = 8 – number of electrons in outermost shell.
Variation of Valency in Periodic Table:
a) Valency in a group:
i) The elements in a same group have same valence or valency and similar properties. e.g : alkali metals have 1-valence electron i.e. 1 valency in their compounds.
ii) alkaline earth metals have 2-valency or valence in their compounds and so on.
iii) Transition elements and inner transition elements have variable valencies.
iv) Noble gases are zerovalent i.e. chemically inert.
b) Valency in a period:
i) In a period the valency i.e. valence increases unit by unit from element to element. Therefore, the elements across a period, differ in their properties.
ii) Valency is useful in writing the formulae of compounds.
6. Oxidation States:
In modern concept valence is almost replaced by the oxidation number or oxidation state. Oxidation number or state is a residual charge possessed by an atom as element in a given species. The charge present on the atom in a molecule or ion is called its oxidation number or state. e.g
i) In s-block Elements ; oxidation states = group number
e.g. Oxidation state of alkalimetals = +1
Oxidation state of alkaline earth metals = +2
ii) In p-block Elements :
Oxidation state changes by two number because they show multi valency.
Common oxidation state of group IIIA & IVA elements = group number
e.g : common oxidation state of group IIIA elements (ns2 np1) = +3
But for heavy metals like thallium +1 state is more common due to Inert pair effect.
Inert pair Effect:
It is the reluctance of the s-electrons of the valence shell to take part in bonding. It arises due to poor shielding of the electrons of valence shell by the intervening d and f-electrons. Inert pair effect increases down the group and thus the elements of p-block present in the bottom of the group show lower oxidation states.
Oxidation states of IVA group elements : = +2, + 4.
Common Oxidations states of IVA → VIIA group elements = group number – 8.
Oxidation states of VA group elements = -3 + 3 +5
e.g.
Oxidation states of VIA group elements = -2, +2, +4, +6
Oxidation states of VII A group elements = -1, +1, +3, +5 + 7
Fluorine always shows -1 oxidation state in its all the compounds.
The oxidation state of noble gases = Zero (except Kr, Xe)
iii) In d & f-block elements
All the Transition and inner transition elements show variable oxidation states.
The most common oxidation state in transition elements is +2
Ruthenium (Ru) and osmium (Os) show maximum oxidation state of +8.
The maximum oxidation state of transition elements = +2 + x.
where x = number of unpaired d-electrons.
The most common oxidation state of inner transition elements = +3
The maximum oxidation state of element = its group number.
The minimum oxidation state of metal = zero
Table : Oxidation states of representative elements
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | ZERO |
|
H – 1,+1 Li +1 |
Be +2
|
B +3 |
C +4,-4 |
N +5 to -3 |
O -1,-2 |
F -1 |
He, Ne O |
|
Na, K, Rb,Cs +1
|
Mg Ca,Sr Ba,Ra +2 |
Al +3 Ga,In,Tl +3,+1 |
Si +4 Ge,Sn,Pb +4,+2 |
P,As +3,+5,-3 Sb,Bi +3,+5 |
S,Se,Te -2,+2 +4,+6
|
Cl,Br,I -1,+1,+3 +5,+7 |
Kr +2,+4 Xe +2,+4 +6,+8 |
Table (a) : Oxidation states of first transition series
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
| +2,+3 |
+2,+3 +4 |
+2,+3 +4,+5 |
(+1) +2, +3,(+4), (+5),+6 |
+2,+3 +4,(+5) (+6),+7 |
+2,+3 +4, +5 +6 |
+2,+3 (+4) |
+2 +3 +4 |
+1 +2 |
+2 |
Table (b) : Oxidation states of second transition series
| Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd |
| +3 | (+3) | (+2) | +2 | +2 | +2 | +2 | +2 | +1 | +2 |
| (+4) | (+3) | +3 | (+4) | +3 | +3 | (+3) | (+2) | ||
| +4 | +4 | (+5) | +4 | +4 | (+4) | (+3) | |||
| +5 | +5 | (+5) | |||||||
| +6 | (+6) | (+6) | |||||||
| (+7), (+8) |
Table (c) : Oxidation state of third transition series
| La | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg |
| +3 | (+3) | (+2) | +2 | (-1) | +2 | +2 | +2 | +1 | +1 |
| +4 | (+3) | (+3) | (+1) | +3 | +3 | (+3) | +3 | +2 | |
| (+4) | +4 | (+2) | +4 | +4 | +4 | ||||
| (+5) | +5 | +3 | +6 | ||||||
| +6 | +4 | +8 | (+6) | (+5) | |||||
| (+5) | (+6) | ||||||||
| (+6) | |||||||||
| +7 |
Table (a) : Oxidation state of 4f – series
| Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| +3 | +3 | +2 | +3 | +2 | +2 | +3 | +3 | +3 | +3 | +3 | +2 | +2 | +3 |
| +4 | +4 | +3 | +3 | +3 | +4 | +4 | +3 | +3 | |||||
| +4 |
Table (b) : Oxidation state of 5f – series
| Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
| +3 | +3 | +3 | +3 | +3 | +3 | +3 | +3 | +2 | +2 | +2 | +2 | +2 | +3 |
| +4 | +4 | +4 | +4 | +4 | +4 | +4 | +4 | +3 | +3 | +3 | +3 | +3 | |
| +5 | +5 | +5 | +5 | +5 | |||||||||
| +6 | +6 | +6 | +6 | ||||||||||
| +7 | +7 |
* The oxidation states in parenthesis are uncommon.
Illustration 9. The valence shell of an element `X’ contained 2 electrons. The valence shell of element `y’ has 7 electrons. The formula of the compound formed between X and Y is
(A) XY (B) XY2
(C) X3Y2 (D) X2Y
Solution. (B) X represents element of group 2 and Y represent element of group 17. Hence, they form XY2 compound.
Illustration10. Though, thallium has a 3 valence electrons, but its stable oxidation state is +1 due to
(A) large size (B) low I.P
(C) diagonal relationship (D) Inert pair effect.
Solution. (D) Thallium exhibit both +3, + 1, oxidation states but due do inert pair effect its +1 oxidation state is more stable than + 3 oxidation state.
7. Electro Positive Character:
i) The tendency of an atom to lose, its valence electron is called electro positive character or electropositivity.
ii) Atoms with low ionization energy have high electropositive character i.e. metallic character.
iii) It increases with increase in atomic size and it decreases with increase in nuclear charge.
In a Group: In a group electro positive character increase from top to bottom due to increase in atomic size and decrease in nuclear attraction on the valence electron(s). Alkali metals are highly electro positive in nature.
In a Period: In a period, electropositive character decreases from left to right, due to decrease in atomic size and increase in nuclear attraction on the outer most electron(s).
Highly electropositive metals are able to form ionic compounds easily.
e.g. They form strong basic oxides which dissolve in water to give hydroxide ions.
The ions of highly electropositive metals do not undergo hydrolysis.
Illustration 11: Which of the following elements represents highly electropositive as well as highly electronegative characters in its period ?
(A) Hydrogen (B) Fluorine
(C) Nitrogen (D) Oxygen
Solution: (A) Hydrogen shows similarities with the elements of group 1 as well as elements of group 17. Hence, it represents highly electropositive as well as highly electronegative characters.
8. Metallic and Non-Metallic Characters:
Nature of elements can be explained with the help of values of electronegativity. Elements of low electronegativity have metallic nature.
Non –Metallic character
a) In a Group : Metallic character increases and non metallic character decreases from top to bottom due to increase in electropositive character and decrease in electronegativity, electroaffinity and ionization energy. Group I and group II elements have strong metallic nature. Thus, metals are good conductors. In metals, mercury (Hg) exist in liquid state at room temperature. Silver is the best Metallic conductor.
b) In a Period : Metallic character decreases and non metallic character increases from left to right, due to decrease in electropositive character and increase in electro negativity, electroaffinity and ionization energy. Aluminum is the most abundant metal in the earth’s crust. Graphite (c) is the best non-metallic conductor.
Illustration 12: Which one of the following configurations represents the most metallic element? a) 2,8,7 b) 2,8,1 c) 2,8,4 d) 2,8,2
Solution: (B) Elements of group 1 are most metallic elements, because they have one valence electron and they can lose it easily, hence, they are most electropositive i.e., most metallic elements.
9. Nature of Oxides:
In general, oxides of metals are basic in nature, and they give alkaline solution with water.
e.g : Oxides of alkali metals and alkaline earth metal (CaO, MgO etc are basic in nature.
Oxides of non-metals are acidic in nature & they give an acidic solution with water.
The oxides of metalloids are amphoteric in nature. e.g.
The Oxides of Al, Zn, Sn are also amphoteric in nature.
In a group: Basic nature of oxides increases or acidic nature of oxides decrease from top to bottom in a group.
In a period: Basic nature of oxides gradually decreases or acidic nature of oxides gradually increases from left to right in a period.
Table : Nature of oxides of elements of third period.
| IA | IIA | IIIA | IVA | VA | VI A | VIIA |
| Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO3 | Cl2O7 |
| more basic | basic | Amphoteric | weak acidic | weak acidic | acidic | more acidic |
Acidic character of the oxides increases, as the electronegativity of element increases. When a single element forms several oxides, their acidic nature increases as the percentage of oxygen increases.
Illustration 13: Which of the following oxides is most acidic?
Solution: (D) SO3 is more acidic because electronegativity of S is more than P, Al, Na.
Illustration 14: Among the following oxides which one is amphoteric oxide?
Solution: (D) The oxides of Al, Zn, Sn are amphoteric in nature.
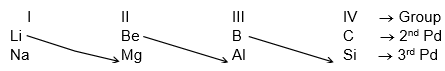
Diagonal Relationship:
In the periodic table, the first element of a group in the second period has similar properties with the second element of the next higher group in the 3rd period. This type of relationship are resemblance is called diagonal relationship.
Reason :
(i) Similar sizes of atoms or ions
(ii) Similar electronegativity
(iii)Similar polarizing power-polarizing powers of & ions are almost the same due to similar sizes and electronegativities.
e.g :
| Atomic radius | Li (152 Pm) | Mg (160 pm) |
| Ionic radius | Li+ (76 pm) | Mg2+ (72 pm) |
| Electronegativity | Li+ (1.0) | Mg (1.0) |
The elements which exhibit diagonal relationship have similar properties. e.g.,
i) Oxides of beryllium and aluminiums are amphoteric in nature.
ii) Carbides of beryllium & Aluminium produce methane gas on hydrolysis.
Illustration 15. Which set contains pair of elements that do not belong to same group but show chemical resemblance?
(A) K, Mg (B) Hf, Zr
(C) Be, Al (D) B, Al
Solution. (C) Be and Al show chemical resemblance due to diagonal relationship.
FORMULAE AND CONCEPTS AT A GLANCE
- Each period starts with an alkali metal and ends with a noble gas.
- Based on the differentiating electrons, the elements are classified into s, p, and f blocks.
- Electronic configuration of s-block elements is ns1 & ns2.
- Electronic configuration of p-block elements is ns2 np1 to ns2np6.
- Electronic configuration of d-block elements or transition elements is (n-1)d1-10 ns1–2.
- Electronic configuration of f-block elements or inner transition elements is (n-2) f1–14 (n-1)0–1 ns2.
- Based on number of incomplete shells the elements are classified into inert gases, representative elements, transition elements.
- Electronic configuration of representative elements is ns1 to ns2np5.
- Electronic configuration of inert gases or noble gases is ns2np6. (except He)
- Atomic size increases from top to bottom in a group and decreases from left to right in a period.
- Cations are smaller and anions are larger than its corresponding atoms of the elements.
- For isoelectronic species (ions), the size decreases with, increase of nuclear charge or atomic number.
- Ionization potential decreases from top to bottom in a group and increases from left to right in a period.
- Elements with half filled and completely filled electronic configuration having high ionization potentials.
- Metals having low ionization potential while non-metals having high ionization potentials.
- Electron affinity decreases from top to bottom in a group and increases from left to right in a period.
- Elements with half filled and completely filled electronic configuration having zero electron affinity.
- Electronegativity decreases from top to bottom in a group and increases from left to right in a period.
- According to Pauling scale, electronegativity is calculated by the following equations.
20. According to Mulliken scale, electronegativity is calculated by the following equations.
(i) If the values of IP & EA are taken in kJ mol –1 ; Electronegativity =
(ii) If the values of IP and EA are taken in eV. ; Electronegativity =
(iii) If the values of IP and EA are taken in k cal mol –1 ; Electronegativity =
- Fluorine and oxygen are the two most electronegative elements.
- Metals having low electronegativities while non-metals having high electro negativities.
- Electropositive character increases from top to bottom in a group and decreases from left to right in a period.
- Metallic character increases from top to bottom in a group and decreases from left to right in a period.
- Nonmetallic character decreases from top to bottom in a group and increases from left to right in a period.
- Basic nature of oxides increases and acidic nature of oxides decreases from top to bottom in a group.
- Basic nature of oxides decreases and acidic nature of oxides increases from left to right in a period.
- Li – Mg, Be – Al, B – Si etc are diagonally related.
- Oxidation states of s-block elements = group number.
- Oxidation states of elements of group IIIA & IVA = group number.
- Oxidation states of the elements from IVA to VII A = group number – 8.
SOLVED PROBLEMS
Prob 1. Which of the following pairs is isoelectronic?
(A) Cl2O3, ICl2– (B) ICl2–, ClO2
(C) IF2+, I3– (D) ClO2–, ClF2+
Sol: (D) are isoelectronic ions.
Number of electrons in ClO2– = 17 + 2 8 + 1 = 34
Number of electrons in ClF2+ = 17 + 2 9 – 1 = 34
Prob 2. The element which forms oxides in all oxidation states from +1 to + 5 is
(A) N (B) P
(C) Sb (D) Ab
Sol: (A) In a VA group only nitrogen shows all the oxidation states from – 3 to + 5. It forms oxides in all oxidation states from +1 to + 5 viz N2O, NO, N2O3, NO2 & N2O5.
Prob 3. For electron affinity of halogens which of the following is correct?
(A) F > I (B) F > Cl
(C) Br > F (D) Br > Cl
Sol: (A) Practically electron affinity of fluorine is greater than that of bromine and iodine but smaller than that of chlorine. The reason is that the size of fluorine atom is very small, therefore it has large electron density due to which it tends to repel the incoming electron(s). Hence, electron affinity of fluorine is comparatively less than that of chlorine.
Prob 4. Which of the following electronic configuration shows noble gas?
(A) (B)
(C) (D)
Sol: (A) Electronic configuration of noble gases is ns2np6 (except He).
Prob 5. The pair having identical ground state configuration is
(A) F+ and Ne (B) Li+ and He
(C) Cl – and Ar (D) Na and K
Sol: (C) The configuration of Cl – and Ar is same as given below
Cl – = 2, 8, 8 ; Ar = 2, 8, 8
Prob 6. Paulings electronegativity values for element are useful in predicting
(A) polarity of bonds in molecules
(B) position of elements in electrochemical series
(C) coordination number
(D) dipole moment of various molecules
Sol: (A) Paulings electronegativity values for elements are useful in predicting polarity of bonds in molecules. e.g.,
i) If EN = 1.7 than the bond will be approximately 50% ionic and 50% covalent.
ii) If EN > 1.7 than the bond will be ionic more than 50%.
iii) If EN < 1.7 than the bond will be covalent.
Prob 7. Which of the following configuration is associated with biggest jump between 2nd and 3rd IE?
(A) (B)
(C) (D)
Sol: (C) configuration is associated with biggest jump between 2nd and 3rd IE.
Prob 8. The element with atomic number 26 will be found in group
(A) 2 (B) 8
(C) 6 (D) 10
Sol: (B) The element with atomic number 26 is Fe.
For a transition elements group number = (n – 1) d es + ns es = 6 + 2 = 8.
Prob 9. Which one of the following arrangements not truly represents the property indicated against it?
(A) : electronegativity (B) : electron affinity
(C) : covalent radii (D) : oxidizing power
Sol: (C) Along the group covalent radii increases from top to bottom as the number of shells increases.
Prob 10. Ionic radii of
(A) (B)
(C) (D)
Sol: (B) P5+ has more effective nuclear charge than P3+ and is smaller in size than P3+.








