Introduction:
- “They (the electrons) serves as the bonds of union between atom and atom”.
- Why do atoms of the same elements or different elements combine to form compounds?
- What are the forces which hold atoms together in molecules in different arrangements?
- Why particular shapes are adopted by molecules? What particular arrangement of ions in ionic compound are preferred?
- These are some of the basic questions to which we will seek answers in this unit.
Electronic theory of valence of chemical bond:
- This theory was proposed by W. Kossel and G.N.Lewis (1916). It was based on electronic configuration of the elements and Bohr’s atomic structure. This theory is also known as chemical bond theory. Kossel explained the formation of ionic bond while Lewis explained the formation of covalent bond.
Important postulates of this theory are given below:
i. Valency of an atom depends mainly on the number of electrons present in the outermost orbit. These electrons are termed as valency electrons.
ii. Electronic configuration of noble gases is stable (ns2 np6) i.e., eight electrons are present in the outer most orbit except helium having two electrons. These gases are chemically inert and do not form compounds.
| Noble gas | Atomic Number | Electronic Configuration |
| Helium (He) | 2 | 2 (duplet) |
| Neon (Ne) | 10 | 2, 8 (octet) |
| Argon (Ar) | 18 | 2, 8, 8 (octet) |
| Krypton (Kr) | 36 | 2, 8, 18, 8 (octet) |
| Xenon (Xe) | 54 | 2, 8, 18, 18, 8 (octet) |
| Radon (Rn) | 86 | 2, 8, 18, 32, 18, 8 (octet) |
iii. Even though He, has two electrons, it is highly stable and chemically inert.
iv. Elements, other than zero group elements are chemically active because they have less than eight electrons in their outer most orbit. eg. Na (2, 8, 1), Cl (2, 8, 7)
The number of electrons which take part in a chemical combination determines the valency of the atoms.
v. ery atom, therefore, tries to acquire the stable electronic configuration in its outermost orbit as in the zero group elements in periodic table (except H, Li, Be).
vi. Therefore, this stable configuration ns2np6 (octet), ns2 (duplet) is attained either by losing, gaining or sharing of electrons during bond formation.
vii. Octet Rule: An atom must possess eight electrons in its outermost orbit for its stability. BeCl2, BF3, AlCl3, PCl5, SF6, XeF2, XeF4, XeF6, etc, the molecules do not obey the octet rule.
Duplet Rule: H, Li, Be, B etc attain stable nearest noble gas configuration i.e., two electron in their outer most orbit like He.
Lewis symbols of elements:
- N. Lewis introduced simple symbol to denote the valence shell electrons in an atom.
- To write Lewis symbol for an element, we write down its symbol surrounded by a number of dots or cross equal to the number of valency electrons.
- These symbols ignore the inner shell electrons.
Table: Lewis symbols for representative elements:
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 |
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | |
| Lewis symbol |
Significance of Lewis symbols:
The number of dots around the symbol gives information of the number of electrons present in the outer most shell. This number of electrons helps to calculate the common valency of the element (X).
- If dots are 4: the common valency of the element = No. of dots in lewis symbol.
- If dots are > 4 : the common valency of the element = 8 – no. of dots.
Chemical bond:
The force of attraction between atoms in a molecule or ion is called chemical bond. The modern concept believes that the electrons are responsible for chemical combination.
Types of chemical bonds:
Depending upon the mode of acquiring the stable electronic configuration the chemical bonds can be classified as :
i. Ionic bond or electrovalent bond.
ii. Covalent bond.
iii. Coordinate bond or dative bond.
Ionic bond & Electrovalency:
- The electrostatic force of attraction between oppositely charged ions is called ionic bond.
- It is formed by the complete transfer of one or more electrons from one atom (metal) to another atom (non-metal).
e.g., formation of ionic bond in NaCl.
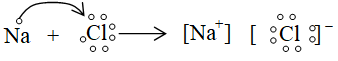
- Ionic bond is also called as electrovalent bond or polar bond.
- The compounds containing ionic bonds are called ionic, electrovalent or polar compounds.
- Ionic bond is a non-directional bond.
- The number of electrons lost or gained by an atom represent the electrovalency of an atom.
Some more examples of formation of Ionic bonds: (Lewis dot structures)
i. Formation of potassium chloride
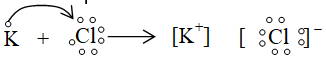
ii. Formation of sodium sulphide
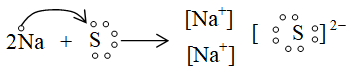
iii. Formation of magnesium oxide:
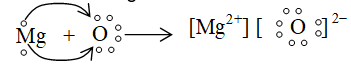
iv. formation of magnesium fluoride :
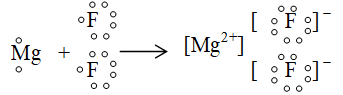
v. Formation of calcium sulphide
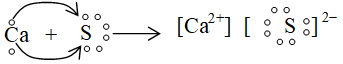
Factors Favourable for the formation of ionic bond:
a. Factor favourable for cation formation:
i. Large atomic size:
Large atoms form cations easily compared to small atom i.e., form ionic compounds easily, due to decrease in force of attraction of the nucleus on the valence electrons.
e.g : compounds of Cs are more ionic than the compounds of other alkali metals due to large size and low IP.
ii. Low ionization potential (IP):
Ionization energy of cation forming atom should be low, for the formation of ionic compounds easily. e.g., potassium forms ionic compounds easily than sodium, because it has low ionization energy.
K, IP = 495.57 kJ mol-1
Na, IP = 519.82 kJ mol-1
iii. Low charge on the ion:
- Ions of lower positive charges are easily produced compared to the ions of higher charges.
- An atom that has to lose more electrons to get stable configuration does not readily form cation or ionic compound or Ionic bond.
e.g., The relative ease of the formation of ionic compounds with Na+, Mg2+ and Al3+ follow the given order Na+ > Mg2+ > Al3+.
iv. Inert gas configuration:
- Those elements, which give cations with noble gas configuration (ns2 np6), form readily ionic compounds.
- The other elements, which give cations with 18 electrons in the outermost orbit known as pseudo inert gas configuration or nickel group configuration also form ionic compound but not as readily as the elements with noble gas configuration.
e.g., (i) out of the two cations Zn2+ (2, 8, 18) and Ca2+ (2, 8, 8), calcium ion (Ca2+) gives ionic compound readily than Zn2+ due to larger size and more stability.
b) Factors favourable for anion formation:
i) Small atomic size:
Small atoms hold the electrons, gained by them strongly and hence form anions as well as ionic compounds easily than the large anions.
e.g., the order of ionic nature in halides is F– > Br‑ > I–
ii) High electronegativity and electron affinity:
Higher the electronegativity and higher electron affinity of the atoms, greater will be the ease of formation of anions as well as ionic compounds.
e.g., The order of ease of formation of anions is F– > O2- > N3-.
In addition to the factors discussed above other influencing factors are also known for the formation of an ionic bond which are given below.
i. Over all decrease in energy:
The summation of three energies should be negative i.e., energy is released.
Ionization potential + electron affinity + lattice energy = negative value.
ii. Difference in electronegativity:
Greater the difference of electronegativity between two atoms, higher will be the possibility of ionic bond formation.
| S.No. | Electronegativities of elements | Mode of combination | Nature of the bonds formed | |
| Element A | Element B | |||
| 1. | Low | High | Transfer of electrons | Ionic bond |
| 2. | High | High | Sharing of electron pairs | Covalent bond |
| 3. | Low | Low | Sea of free electrons | Metallic bond |
• Highly electropositive elements of groups IA and IIA combine with highly electronegative elements of VIA and VIIA groups, to form ionic or electrovalent compounds.
e.g., halides, oxides, sulphides, nitrides and hydrides of alkali metals and alkaline earth metals are generally ionic.
• The atoms of transition metals can also form ionic bond but do not acquire inert gas configuration always.
iii. High Lattice energy:
• Higher the value of lattice energy of the ionic compound, the greater will be the stability of the compound and hence greater will be the ease of its formation.
• The value of the lattice energy increase with increase of charge on the ions due to greater force of attraction between them.
Properties of Ionic compounds:
The important properties of ionic compounds are ____
i. These compounds usually exists in the solid state.
ii. The crystalline ionic compounds have well defined crystal structure or crystal lattice.
iii. Ionic compounds have high melting points and boiling points due to powerful electrostatic force between ions.
e.g., m.pt of NaCl is 8030C.
iv. They are generally soluble in polar solvents, having high value of dielectric constant but insoluble in non-polar solvents due to low dielectric constant. The solubility of ionic compounds decreases with increase in covalent character of ionic compounds. The solubility of these compound is governed by ___
a) Lattice energy: Larger the lattice energy, the lesser is the solubility.
e.g., sulphates and phosphates of Ba & Sr are insoluble in water due to high lattice energy.
b) Heat of hydration: Larger the heat of hydration, the more is the solubility of ionic compound.
e.g., AlCl3, though covalent in nature is soluble in water due to high value of heat of hydration.
v. In solid state they do not conduct electricity since there is no free movement of electrons but in molten state and in solution they are good conductor of electricity due to presence of charged ions in them.
vi. Ionic compounds undergo ionic reactions which have very high reaction rates. i.e., quite fast e.g., white ppt.
vii. They do not exhibit isomerism due to non-directional nature of the polar bonds present in these compounds.
Illustration 1: NaCl is more ionic but CuCl is more covalent. Why?
Solution: In NaCl, Na+ has inert gas configuration, so it is ionic in nature. Whereas CuCl is more covalent because in CuCl, Cu+ has pseudo inert gas configuration.
2. Covalent Bond – Lewis Langmuir concept:
- This concept of covalent bond was given by G.N. Lewis and improved by Langmuir, in 1919, by suggesting that atoms may also combine by mutual, sharing of electrons in their outermost shells and attain noble gas configuration.
- A chemical bond which is formed by mutual sharing of two or more electrons between two atoms of same or different elements, is called covalent bond.
- A covalent bond, on the basis of sharing of two or more electrons can be devided into three types.
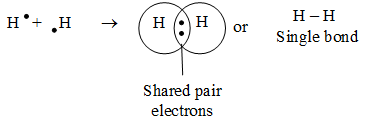
a) Single covalent bond:
A covalent bond which is formed by sharing of two electrons (i.e., 1 electron pair) is called single bond or single covalent bond. It is represented by a single line between two atoms.
Example :
(i) formation of H2 molecule:
(ii) Formation of Cl2 molecule:
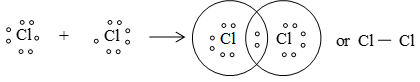
(iii) Formation of HCl :
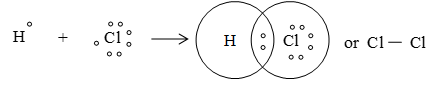
b) Double bond or double covalent bond:
A covalent bond, which is formed by sharing of two electron pairs, between two atoms is called a double bond and it is represented by a double line ( = ) between two atoms.
Example. Formation of O2 molecule :
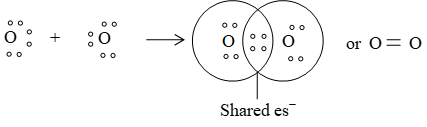
c) Triple bond:
A covalent bond, which is formed by sharing of three electron pairs, is called a triple bond and it is represented by a triple line ( ) between two atoms.
Example. Formation of N2 molecule
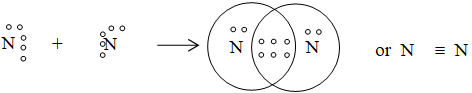
Thus, the number of covalent bonds formed by an atom depend on
i. the number of unpaired electrons in their valency shell.
ii. the number of electrons required to attain inert gas configuration. Generally p – block elements are involved in the covalent bond formation. In the covalent bond formation both atoms contribute equal number of electrons.
Some more examples of formation of covalent Bonds:
1) Formation of BeCl2:
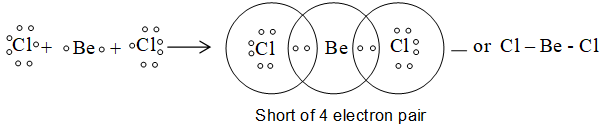
2) Formation of BF3 or BCl3:
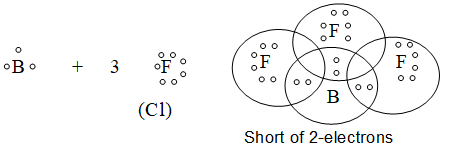
3) Formation of CO2:
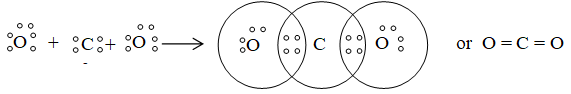
4) Formation of NH3 (Pyramidal):
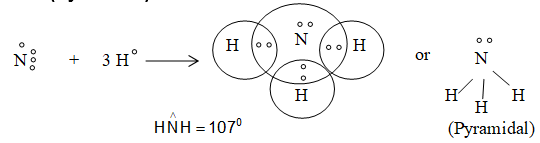
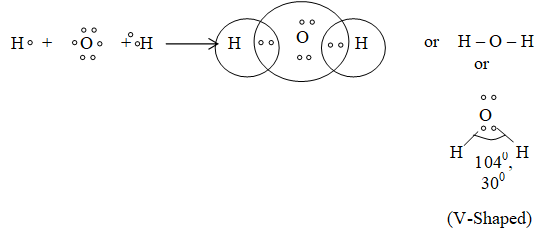
5) Formation of H2O (Angular) :
6) Formation of PCl5:
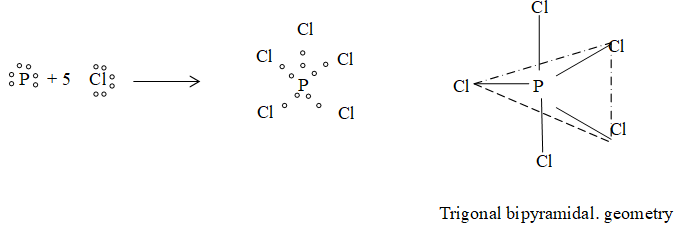
7) Formation of SF6:
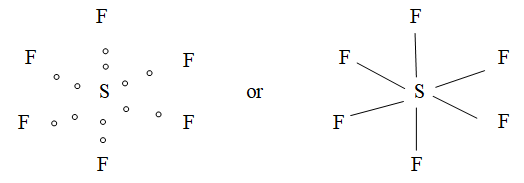
Covalency:
The number of electrons contributed by an atom of the element for sharing to forms a covalent bond is called covalency of the element.
Covalency of IA, IIA, IIIA group elements = group number.
Covalency of IVA, VA, VIA & VIIA group elements = 8 – group no.
e.g.
i. Covalency of hydrogen in water = 1
ii. Covalency of oxygen in water = 2
iii. Covalency of nitrogen in ammonia = 3
iv. Covalency of C in methane = 4
Variable Covalency:
The variable covalency is shown by elements having vacant d orbitals in their valency shell. The unpairing of the s and p electrons is possible by promoting them to higher orbitals.
e.g.,
(i) Phosphorous shows 3 and 5 covalencies.
(ii) S atoms shows 2, 4 and 6 covalencies
(iii) Chlorine shows 1, 3, 5 & 7 covalencies
Note:
Such a shifting is not possible in the case of H, N, O and F because d orbitals are not present in their valency shell.
- HCl, CH4, CO2, AlCl3, BCl3, PCl3, PCl5, SF6, SiO2, H2O, NH3, SO2 etc are covalent compounds.
- All the organic compound and the compound formed by the combination of two different non-metals are covalent in nature.
- NO, NO2, ClO2 etc molecules contain odd electron bond.
Properties of covalent compounds:
i) Under normal conditions of temperature and pressure, these compounds exist as gases or liquids of low boiling points, due to weak van der waal’s forces exist between discrete molecules. But, sulphur, phosphorus, iodine are exist as soft solids.
ii) They have low melting points and boiling points because weak van der waal’s forces of attraction operate between the molecules.
Example. H2, O2, N2, CH4 are gases and H2O, CHCl3, C6H6 are low boiling liquids.
iii) The crystal structure of covalent compounds differs from that of ionic compounds. They usually consist of molecules rather than ions.
iv) In general, they are insoluble in polar solvents like H2O alcohol and soluble in non-polar solvents like CCl4 & benzene, because this is based on the principle “like dissolves like”.
e.g., Polar covalent substances like glucose, fructose etc are soluble in polar solvents and non polar substances like iodine, camphor etc are soluble in non-polar solvents like benzene and carbon tetra chloride etc.
v) In, general, covalent substances are bad conductors of electricity. Substances which have polar character like HCl, in solution, can conduct electricity.
- Covalent solids having giant molecules, are bad conductors since they do not contain charged ions or free electrons. Therefore, they are non-electrolytes.
- The graphite can conduct electricity due to delocalization of – electrons in a layer but not from one layer to another.
vi) Rate of reaction:
Covalent substances undergo molecular reactions, they have low reaction rate than ionic compounds, because they involve, breaking of covalent bonds of reactants and making of new bonds. Hence these reactions are generally slow and rarely proceed to completion.
vii) Isomerism:
The covalent compounds exhibit isomerism due to directional nature of a covalent bond. e.g.
a covalent substance with molecular formula, C2H6O exhibits two isomers i.e., ethyl alcohol (C2H5OH) and ether (CH3OCH3).
Comparison between ionic and covalent bonds.
| Ionic bond | Covalent bond |
| 1. It is formed by complete transfer of one or more electrons from electropositive metal atom to electronegative non-metal atom. | 1. It is formed by mutual sharing of electrons between two non-metal atoms. Both the atoms contribute equal no. of electrons. |
| 2. It is possible in between metal and non metal only. | 2. It is possible in between similar or dissimilar non-metallic atoms. |
| 3. It consists of electrostatic force between ions i.e., | 3. It consists of shared pair of electrons which are attached by both nuclei. |
| 4. It is non-rigid and non directional in nature and does not cause isomerism. | 4. It is rigid and directional in nature and causes structural & space isomerisms. |
| 5. It is a weak bond, since the electrostatic force between the ions can be broken easily. | 5. It is a strong bond, since the paired electrons can not be separated easily. |
| 6. It is polar in nature | 6. It is non polar, if EN is zero or small. |
| 7. It was proposed by Kossel. | 7. It was proposed by Lewis. |
Comparison between ionic and covalent compounds:
| Ionic Compounds | Covalent Compounds |
| 1. These are crystalline solid at room temperature. | 1. These are gases or soft solids under ordinary conditions. |
| 2. They have high melting and boiling points | 2. They have low melting & boiling points except giant covalent molecules. |
| 3. They are hard & brittle | 3. They are soft & waxy, except giant covalent molecules. |
| 4. These are soluble in polar solvents & insoluble in non-polar solvents. | 4. These are usually insoluble in water and polar solvents but soluble in non polar solvents. |
| 5. These are bad conductors of electricity in solid state and good conductors in molten state and in solutions. | 5. These are bad conductors of electricity except graphite. |
| 6. These undergo, ionic reactions. | 6. These undergo molecular reactions. |
| 7. Rates of reactions are very high & these are fast & instantaneous. | 7. Reaction rates are low & reactions are slow. |
| 8. They consist of ions | 8. The usually consist of molecules rather than ions. |
Illustration 2: Which of the following has one lone pair of electrons on the central atom?
(A) NH3 (B) CH4
(C) H2 (D) NH4+
Solution. (A) NH3 has one lone pair electron on central atom.
Illustration 3: Which ion will have more polarizing power?
(A) Mg2+ (B) Al3+
(C) Ca2+ (D) Na+
Solution. (B) Al3+ has more polarsing power due to presence of more nuclear charge.
Coordinate bond or Dative bond:
It may be defined as, “ a covalent bond in which both electrons of the shared pair are contributed by one of the two atoms”. It is represented by an arrow (→) pointing its head towards the acceptor atoms. Atom / ion / molecule donating electron pair is called donor or Lewis base. Atoms / ion / molecule accepting electron pair is called acceptor or Lewis acid. Compounds having this linkage are known as coordinate compounds. Since this bond has some polar character. As the coordinate bond is a combination of one electrovalent bond and one covalent bond, it is also known as dative or semipolar or co-ionic bond.
e.g :(i). combination of ammonia and boron trifluoide :
e.g. ii. Formation of Ammonium ion :
e.g. iii. Formation of SO2
e.g. iv. Formation of SO3
In addition to above examples hydronium ion (H3O+), carbon monoxide (CO), ozone (O3), complex compounds, etc., contain coordinate bonds.
Properties of co-ordinate compounds:
i. These exist as gases, liquids and solids under ordinary conditions.
ii. Their melting; and boiling points are higher than purely covalent compounds and lower than purely ionic compounds.
iii. These are sparingly soluble in polar solvents like water but readily soluble in non polar solvents like benzene carbon tetra chloride etc.,
iv. Like covalent compounds, these are also bad conductors of electricity.
v. The compounds containing coordinate bonds possess high values of dielectric constants.
vi. The bond is rigid and directional so these compounds show isomerism.
vii. These are as stable as covalent compounds.
viii. These undergo molecular reactions and the reactions are slow.
Illustration 4: Coordinate bond is formed
(A) by transfer of one electron from one atom to another
(B) by the loss of one electron of each from both the atoms
(C) by sharing of one electron from each atom
(D) none of these
Solution. (D) A coordinate bond is form by donation of one or more pair of electrons by one atom to the another atom in a molecule.
Illustration 5: BF3 forms an adduct with NH3 because
(A) N – has high EN
(B) B – has high EN
(C) B – has an empty pZ – orbital & N – has lone pair of electrons
(D) B – has lone pair of electrons
Solution. (C) BF3 forms an adduct with NH3 because B – has an empty pZ – orbital & N – has lone pair of electrons.
Fajan’s rules: Fajan’s rules are useful to predict the nature of the bond formed between two atoms. The rules are given below:
i) Increase in cationic size increases the ionic nature of the bonds. g., Among the alkali metal ions, the ionic nature of the bond follows the order
ii) Decrease in anionic size favours the formation of ionic bond. e.g., Among the calcium halides (CaX2), the fluoride is more ionic than iodide. The iodide is more covalent.
iii) Less charged cation or anion or both generally form ionic bonds.
e.g., Na+Cl is more ionic because both ions have low chares, but AlCl3 is more covalent due to the presence of highly charged Al3+.
iv) Cations with inert gas configuration form ionic compounds white those cations with pseudo inert gas configuration favours covalent bond formation.
e.g., Na+ in NaCl has an inert gas configuration. So Na+ Cl – is ionic. But CuCl is more covalent because Cu+ has not acquired inert gas configuration in this compound, but has pseudo inert gas configuration.
Formal charge: Formula charge = Total no. Of valence electrons in the free atom – Total no. of lone pair electrons – total number of bonding electrons.
For example formal charge on ‘S’ in .
Similarly formal charge on ‘Cl’ in HClO4 =
Lattice energy:
Lattice energy (U) of crystal is the energy evolved when one mole of the crystals is formed from the gaseous ions. For example:
The lattice energy thus is the sum of the attractive and the repulsive forces.
where
‘A’ is Madelung constant, ‘B’ is repulsion coefficient, NA is Avogradro’s number. or .
Where r+ is radius of cation and r– is the radius of anion.
- In general Lattice energy decreases with increase in the radius of the ion.
- The order of lattice energy for different metal halides is
Li F > Li Cl > Li Br > LiI & LiF > NaF > KF > RbF > CSF
Calculation of lattice energy for NaCl – Born – Haber cycle:
Let us consider the formation of NaCl.
(Heat of reaction).
For above reaction lattice energy cannot be measured directly but experimentally it is measured by using Born Haber cycle in following steps. (Born Haber cycle is based on Hess law).
i. Sublimation of Na(s) to Na(g):
ii. Formation of cation (Na+):
iii. Dissociation of one chlorine molecule:
v. Formation of anion (Cl–):
v. Combination of Na+ & Cl– ions to form NaCl.
- Born – Haber cycle for the formation of NaCl (s) may be represented as :
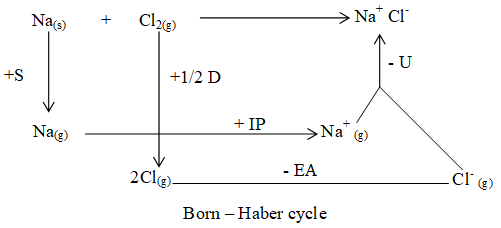
- According to Hess law, the amount of heat liberated ( – Q) in the overall reaction is the heat of formation of NaCl.
Crystal structure of NaCl and Cs Cl:
I.Crystal structure NaCl:
- The structure of NaCl is considered as face centred cubic (FCC) structure. In this structure Cl– ions have cubic close packed arrangement.
- Each Na+ ion is surrounded by six Cl– ions situated at the six corners of a cube or regular octahedron.
- Similarly each Cl– is surrounded by six Na+ ions
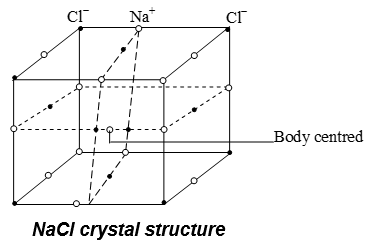
Coordination no. of Na+ = 6
Coordination no. of Cl– = 6
Radius ratio
Effective no. of Na+
Effective no. of Cl– =
So, 4 Na+ ions and 4 Cl– ions are present per unit cell of NaCl.
II. Crystal structure of Cs Cl:
- The structure of CsCl is considered as body centered cubic type lattice structure.
- In this structure each Cl– ions have cubic close packed arrangement, in which each Cs+ ion is surrounded by eight Cl– ions and each Cl– is surrounded by eight Cs+ ions
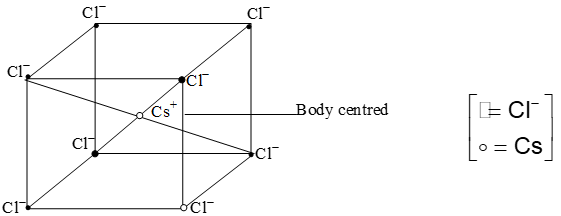
Coordination no. of Cs+ = 8
Coordination no. of Cl– = 8
Radius ratio =
Effective no. of Cs+ = 1 x 1 = 1
Effective no. of Cl– =
So, one Cs+ and one Cl– ions are present per unit cell of CsCl. CsCl is considered as a cubic structure.
Radius ratio and co-ordination number:
Limiting radius ratio (rc/ra) determines the coordination number and coordination number determines the shape of crystal. (The number of nearest oppositely charged ions surrounding any particular ion in an ionic crystal is called the co-ordination number of that ion).
Limiting radius ratio and the structure
|
Limiting radius ratio rc/ra |
Coordination number | Shape of the crystal (structure) | Examples |
| Upto 0.155 | 2 | Linear | – |
| 0.155 – 0.225 | 3 | Planar triangular | B2O3 |
| 0.225 – 0.414 | 4 | Tetrahedral | Zns |
| 0.414 – 0.732 | 6 | FCC (Octahedral) | NaCl |
| 0.732 – 0.999 | 8 | BCCl | CsCl |
Position of an ion or particle in cubic unit cell and its contribution to the unit cell:
1) The particle or ion at corner of cube will be shared by 8 unit cell and the contribution of the ion or particle to one unit cell is 1/8.
2) The ion (particle) at edge of a cube will be shared by 4- unit cells and the contribution of the particle or ion to one unit cell is ¼.
3) The ion or particle at the face of a cube will be shared by 2-unit cells and the contribution of particle to one unit cell is 1/2.
4) The particle or ion at the body centre of a cube will be shared by only one unit cell and the contribution of the particle to one unit cell is 1.
In ionic solid crystals, each constituent ion has a definite position and orientation relative to its neighbours.
Rules for calculation of number of particles or ions in a unit cell:
i. In simple cubic cell : No. of particles present in each unit cell =
ii. In body centered cell : No. of particles =
iii. In face centered cubic cell : No. of particle =
Illustration 6: Define the crystal lattice.
Solution: The orderly three dimension pattern in which ions are arranged in the crystal is termed as crystal lattice or space lattice.
Illustration 7: What is unit cell?
Solution: A unit cell is a regular three dimensional group of lattice points that generates the whole lattice by stacking or repeating through out the crystal.
Illustration 8: What is the number of nearest neighbours for a given lattice point in a face centered cubic packing?
Solution. In a face centered close packed cubic structure, the number of nearest neighbours for a given lattice point is 12.
Illustration 9: What is the coordination number in a closed packed structure?
Solution: Twelve
Illustration 10: What structural changes may occur?
a) If NaCl crystal is subjected to high pressure?
b) If CsCl crystal is heated to 760K.
Solution. a) When NaCl is subjected to high pressure, it transforms into body centered cubic structure of CsCl. i.e., Coordination number increases.
If we heat CsCl to a high temperature of 760K, it converts to a structure of NaCl. i.e., coordination no. decreases.
Illustration 11: Why NaCl is a bad conductor of electricity in the solid state?
Solution: In the solid state, Na+ and Cl– ions are strongly held together and are not free to move.
Modern concept of covalent bonds:
a. Valence bond theory:
- This theory was put forward by Heitler and London in 1927, to explain how a covalent bond is formed, and further developed by Pauling and Slater in 1931. (According to this theory, a covalent bond is formed by overlapping of atomic orbitals].
- The important points of this theory are
- A covalent bond is formed by overlapping of atomic orbitals of valence shell of the atoms.
- Orbitals undergoing overlapping should be half filled.
- Half filled orbitals should contain the electron with parallel spin.
- Overlapping of orbitals causes delocalization of electrons, which in turn, lowers the energy and increases the stability.
- Strength of covalent bond depends upon the extent of overlapping, for example, axial or lateral overlapping.
- If the overlapping of orbitals is higher, the bond formed is stronger.
- The bond formed by an axial overlapping of orbitals is called sigma ( ) bond.
- The bond formed by sidewise overlapping of orbitals is called pi ( ) bond.
- A sigma bond is always stronger than a pi bond due to greater overlapping of orbitals.
- In a sigma bond the electrons are oriented along the internuclear axis but in pi – bond, the electrons are oriented above and below the internuclear axis.
- Between two orbitals of the same energy, one more directionally concentrated would form stronger bond. Dumb-bell shaped p – orbitals will forms stronger bond as compared to spherically symmetrical s – orbital.
- Increasing order of strength of covalent bonds is
- A single bond is always a sigma bond. A double bond consists of one bond and one bond. A triple bond consists of one sigma bond & two bonds.
- s orbitals are non-directional in nature and therefore always overlap along the inter nuclear axes; so s orbitals form bond ( s – s).
To understand the concept of VBT (more easily), let us consider the following examples.
i) Formation of Hydrogen Molecule:
In terms of orbital overlap concept, when two hydrogen atoms having electrons with opposite spin come close to each other, their s orbitals overlap with each other resulting in the union of two atoms to form a molecule.
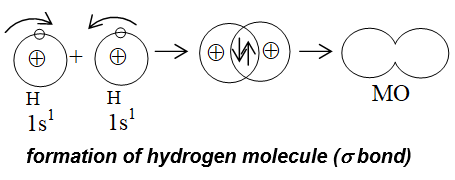
- In term of energy a stable bond is formed only when, the energy of the system becomes minimum. In this situation, the two hydrogen atoms are said to be bonded together to form a stable molecule.
- If we try to bring these atoms still closer (< 74pm) then repulsive forces start overlapping the attractive forces.
Consequently, energy of the system (i.e., molecule) increases, causing instability of the system.
ii) Formation of Nitrogen molecule:
In nitrogen three unpaired electrons are present in orbitals. Giving the same reasons that were given to the formation of an oxygen molecule, one can show that nitrogen molecule has one and two bonds.
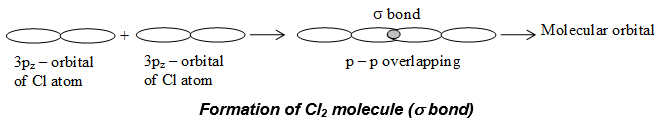
iii) Formation of chlorine molecule:
The electronic configuration of chlorine is – 1s2, 2s2 sp6, 3s2, 3px2 3py2 3pZ1. It has a half filled 2pZ1 orbital. When a pZ – orbital of one atom overlaps with the pZ orbital of the another atom, a chlorine molecule is formed as
iv) Formation of H2O molecule:
Water has two O – H bonds. The two bonds are bonds. They are the result of overlap of orbitals of oxygen containing unpaired electrons separately with the 1s orbitals of two hydrogen atoms. The angle between these bonds should be 900 (because 2py and 2pz orbitals are perpendicular to each other and they are involved in overlapping). But the experimental results show it as 104030′.
v) Formation of hydrogen chloride molecule:
The 1s orbital of hydrogen atom and 3pz orbital of chlorine atom has unpaired electrons. These orbitals overlap to form bond.
Comparison of sigma and pi bond
| Sigma bond | Pi bond |
| i) This bond is formed by overlap of orbitals along their inter nuclear axis i.e., end to end over lap. | i) This bond is formed by sidewise overlapping of orbitals i.e., lateral overlapping. |
| ii) It involves, s – s, s – p or p – p overlapping of orbitals. | ii) It involves p – p overlapping of orbitals only. |
| iii) It has maximum overlapping. | iii) It has minimum overlapping. |
| iv) It is a strong bond. | iv) It is a weak bond. |
| v) Electron cloud is symmetrical about the line joining the two nuclei. | v) Electron cloud is unsymmtrical. |
| vi) Free rotation about a bond is possible. | vi) Free rotation about a bond is not possible. |
| vii) It can have independent existence. | vii) It always exist along with a bond. |
| viii) It consists of single electron cloud symmetrical about the internuclear axis. | viii) It consists of two electron clouds, one above the plane of atomic nuclei and the other below it. |
| Ix) These bonds help in deciding the shape of molecule. | ix) – electrons are referred as mobile electrons. |
| x) These bonds help in deciding the shape of molecule. | x) These bonds do not affect the shape of the molecule. |
| xi) These are less reactive. | xi) These are more reactive. |
b. Molecular orbital theory:
- This theory was proposed by Hund and R.S. Mulliken in 1932 to explain the nature of bonding in covalent compounds.
- This theory of chemical bonding is more rational and more useful in comparison to valence bond theory.
- Mulliken was awarded noble prize for chemistry in 1966.
- Salient features of this theory are :
- The wave function of an electron in a molecule is called molecular orbital (MO).
- The atomic orbitals (AO’s) of nearly equal energy, and appropriate symmetry combine to give equal number of MO’s. The MO’s are constructed by the linear combination of the atomic orbitals (LCAO method).
- ‘MO’ of lower energy is called bonding ‘MO’ () while that of higher energy known as antibonding ‘MO’ ().
- The bonding molecular orbitals are represented by etc, whereas the corresponding anti bonding molecular orbitals are represented by
- The shapes of molecular orbitals formed depends upon the type of the combining atomic orbitals.
- The filling of MO’s take place according to the same as those of the AO’ i.e., follow Aufbau, Pauli, Hund’s rule.
- Order of energies of various MO’s.
Linear combination of Atomic orbitals (LCAO):
- The formation of molecular orbitals can be explained on the basis of linear combination of Atomic orbitals (LCAO)
- If and are the atomic orbitals of the combining atoms, then the ‘MO’ formed by additive effect of AO’s is called bonding ‘MO’ () and the molecular orbital formed by the subtractive effect of the AO’s is called antiboning molecular orbitals () i.e.,
- A constructive combination of the two atomic orbitals lead to an increased electron density between the two nuclei. This impart stability to the molecule. The electron density for a bonding ‘MO’ is given by i.e.,
- On the other hand destructive (out of phase) combination of the two atomic orbitals leads to a decreased or zero electron density between the two nuclei. This imparts instability to the molecule, the electron density for antibonding ‘MO’ is given by ,
Conditions for the combination of atomic orbitals:
The main conditions for effective combination of atomic orbitals are
- the combining orbitals must have same or nearly the same energies.
- The extent of overlapping between the atomic orbitals of two atoms should be large.
- The combining atomic orbitals must have the same symmetry about the molecular axis.
Formation of Molecular Orbitals:
Molecular orbitals are formed by the combination of suitable atomic orbitals. The addition of the atomic orbitals gives rise to bonding ‘MO’, (), while the subtraction of the ‘AO’s gives rise to the antibonding ‘MO’, (). Formation of molecular orbitals from different atomic orbitals is described below.
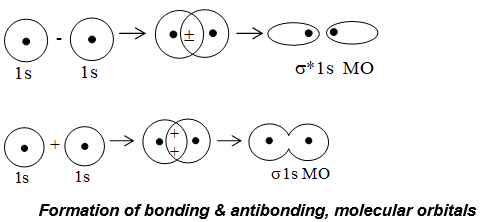
i) Formation of MOs by the combinations of s AO’s:
ii) Formation of MOs by the combination of pz orbitals along internuclear axis:
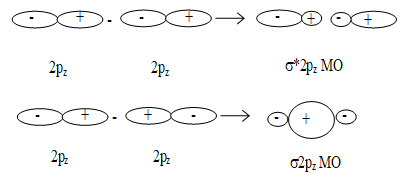
Formation of bonding and antibonding molecular orbitals by the combination of 2pz orbitals.
iii) Formation of MOs by the combination of px or py orbitals due to sideways overlap:
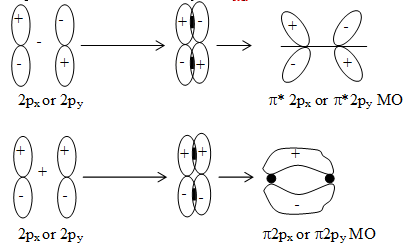
Formation of bonding & antibonding. MO’s from 2px or 2py atomic orbitals.
Differences between bonding and antibonding molecular orbitals
| Bonding MO | Antibonding MO |
| 1) A bonding ‘MO’ is formed by the addition of two atomic orbitals | 1) An antibonding ‘MO’ is formed by subtraction of the atomic orbitals |
| 2) It is formed by the overlap of the atomic orbitals which are in phase. | 2) It is formed by the overlap of atomic orbitals which are out of phase. |
| 3) In bonding ‘MO’, the electron density between the two nuclei is increased. | 3) In antibonding ‘MO’ the electron density between the two nuclei is decreased and might become zero. |
| 4) It is represented by Greek letters or . | 4) It is represented by the greek letters with an asterisk eg. . |
Electronic configuration and molecular behaviour:
a) Bond order:
The relative stability of a molecule can be determined in terms of a parameter called bond order. As per definition bond order is expressed as
Bond order = [no. of electrons in bonding MO’s – no of electrons in anti bonding ‘MO’].
or Bond order =
The bond orders of 1, 2 or 3 correspond to single, double or triple bond. It may be fraction in some cases.
The bond order conveys following important information.
- If Nb > Na; Bond order > 0 ( + ve), then a stable bond is formed. e., molecule is stable.
- If Nb Na; Bond order 0, then the molecule is unstable i.e. In fact such a bond is not formed or not exist.
- The stability of a molecule is measured by its dissociation energy. Dissociation energy of a molecule is proportional to its bond order. Thus, greater the bond order, greater is the bond dissociation energy, greater is the stability.
- Bond length is inversely proportional to the bond order,
e.g. Bond length in nitrogen molecule is shorter than in oxygen molecule, as shown below
| Molecule | Bond order | Bond dissociation energy | Bond length |
| Nitrogen | 3 | 945 kJ mol-1 | 110 pm |
| Oxygen | 2 | 495 kJ mol-1 | 121 pm |
| Fluorine | 1 | 159 kJ mol-1 | 143 pm |
b) Magnetic nature:
The presence of one or more unpaired electrons accounts for the paramagnetic nature of the molecule. The electronic configuration in which all the electrons are paired indicate the diamagnetic nature of the molecule.
Molecular orbital configuration (MOC) of some Homonuclear diatomic molecules & their Nature:
i) Hydrogen molecule (H2): EC of H – 1s1
MOC =
Bond order = 1
Nature = diamagnetic.
ii) Hydrogen molecular cation (H2+) : EC of H = 1s1, H+ = 1s0
MOC =
Bond order = ,
Nature = paramagnetic.
iii) Hydrogen molecular anion: EC of H = 1s1, H– = 1s2
MOC =
Bond order = , Nature – paramagnetic.
Order of stability of H2, H2+, H2– is H2 > H2+ > H2–
iv) Helium molecule : (He2) EC of He = 1s2
MOC =
Bond order = Zero, Nature – diamagnetic (cannot)exist.
v) Helium molecular cation: EC of He = 1s2, He+ = 1s1.
MOC =
Bond order = , Nature – paramagnetic (stable).
vi) Helium molecular anion:
MOC =
Bond order = , Nature – paramagnetic (stable).
vii) Lithium molecule
MOC =
Bond order = 1
Nature = diamagnetic.
Note : The comparison of Li – Li and H – H bonds reveals that the sigma bond in Li2 molecule is weaker and much longer than bond in H2 molecule, because 2s orbital of lithium is bigger in size than the 1s orbital of hydrogen.
viii) Beryllium molecule (Be2) : Be – 1s2, 2s2
Bond order = 0, nature – diamagnetic (does not exist)
ix) Boron molecule (B2) :
BO = 1, Nature = paramagnetic
x) Carbon molecule (C2) :
Bond order = 2, Nature = diamagnetic
xi) Nitrogen molecule (N2):
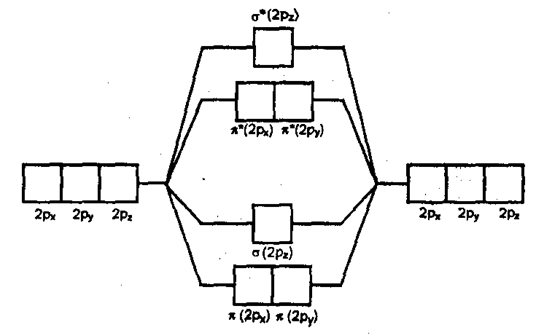
Molecular orbital energy level diagram for N2
BO = 3, Nature – diamagnetic.
Order of bond order of N2, N2+, N2–, N22–

xi) Oxygen molecule (O2) : O – 1s2 2s2 2p4
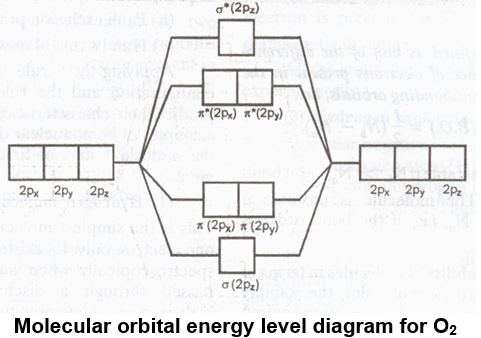
BO = 2, Nature – paramagnetic
Order of bond order of is
xii) Fluorine molecule (F2) : F – 1S2 2S2 2P5
BO = 1, Nature diamagnetic (Stable, BDE = 151 kJ mol-1)
xii) Neon molecular does not exists because Bond order is zero.
Illustration 12 : Which of the two is more stable?
Solution. is more stable because its bond order is +3.
Illustration 13: Arrange the following in the decreasing order of their bond dissociation energies and in the increasing order of bond length O2, O2+, O2–.
Solution. (i) Decreasing order of BDE =
(ii) Increasing order of bond length =
Valence Shell Electron Pair Repulsion Theory (VSEPR theory)
- This theory was given by Sidwick and Powell in 1940, to explain the shapes of molecules and was further improved by Nyholm and Gillespie in 1975.
- According to this theory the shape of the molecule depends upon number of bond pair electrons and no. of lone pair electrons.
Shape of molecule having only bond pairs
| No. of bond pair es– | Shape | Molecule |
| 2 | Linear | BeF2, BeCl2, CO2 etc |
| 3 | Triangular | BF3, BCl3, SO3, CO32- etc |
| 4 | Tetrahedral | CH4, CCl4 etc |
| 5 | Trigonal bipyramidal | PF5, PCl5 etc |
| 6 | Octahedral | SF6, SiF6-2 |
| 7 | Pentagonal bipyramidal | IF7 |
Shape of molecule having bond pairs as well as lone pair electrons.
| No. of bond pairs | No. of lone pairs | Total No. | Shape of Molecule | Example | |
| basic shape | actual shape | ||||
| 2 | 1 | 3 | Triangular | Angular | SnCl2, SO2 |
| 3 | 1 | 4 | Tetrahedral | Pyramidal | NH3, H3O+ |
| 2 | 2 | 4 | Tetrahedral | Angular | H2O |
| 4 | 1 | 5 | Folded square | See Saw | SF4 |
| 3 | 2 | 5 | Trigonal bipyramidal | T-shaped | ClF3 |
| 2 | 3 | 5 | Trigonal bipyramidal | Linear | XeF2 |
| 5 | 1 | 6 | Octahedral | Square pyramidal | IF5, BrF5 |
| 4 | 2 | 6 | Octahedral | Square planar | XeF4 |
| 6 | 1 | 7 | Pentagonal bipyramidal | Distored octahedral | XeF6 |
- A lonepair of electrons occupies more space around the central atom than a bond pair of electrons.
- The bond pairs and lone pairs are oriented (or arranged) in such a way, in the predicted shape, that the repulsion forces among them are minimum. Order of repulsion is LP – LP > LP – BP > BP – BP.
- Double bond causes more repulsion than single bonds, and triple bonds cause more repulsion than double bonds, but multiple bonds between two atoms count the same as single bond because it does not matter how many electrons are present in each direction. Thus, the shape of a molecule can be predicted on the basis of the number of bond pairs and lone pairs on the central atom as follows:
- Total no. of electron pair (ep) =
Illustration 14: Predict the shape of
Solution. (i) NH3
Total no. of electron pairs (ep) =
No. of bond pairs (bp) = 3
No. of electron pair around central atom = 4 – 3 x 0 = 4
lP = Q – bp = 4 – 3 = 1
So, VSEPR notation of NH3 = AX3E, hence it is pyramidal in shape
(ii) H2O :
bp = 2,
Q = 4 – 3 0 = 4
Lp = 4 – 3 = 1
So, VSEPR notation of H2O = AX2E2 & hence it is angular in shape.
(iii) PCl5 :
bp = 6 – 1 = 5
LP = 5 – 5 = 0.
Hence, shape of PCl5 is trigonal bipyramidal.
(iv) ClO3 :
bp = 3
Q = 13 – 3 3 = 4
ep = 4 – 3 = 1
So, VSEPR notation of ClO3– = AX3E, hence it is pyramidal in shape.
(v) ICl4– :
bp = 4
Q = 18 – 3 x 4 = 6
Lp = 6 – 4 = 2.
So, VSEPR notation of ICl4 = AX4E2 and hence square planar in shape.
Hybridization:
The process of mixing of atomic orbitals of same or approx same energy to produce a set of entirely new orbitals of equivalent energy and identical shapes is known as hybridization. The new orbitals are known as hybrid orbitals.
- This is a hypothetical concept and was proposed by pauling and staler.
- The important features of hybridization are :
- Only the orbitals of nearly same energy of an atom or ion can undergo hybridization.
- Number of hybrid orbitals generated is always equal to number of atomic orbitals which are intermixed.
- The hybrid orbitals may differ from one another in orientation in space.
- Like an atomic orbital, hybrid orbital can have two electrons of opposite spins.
- Hybrid orbitals form bonds and unhybridized orbitals form bonds, molecule has a regular geometry, if all the hybrid orbitals after overlapping contain shared pair of electrons.
- If, however, the central atom is surrounded by one or more orbitals containing lone pairs of electrons in the valence shell, the geometry of the molecule would be distored to some extent.
- The distribution of electrons into hybrid orbitals is also as per the pauling exclusion principle and Hund’s rule of maximum multiplicity.
Types of hybridization and shapes of molecules:
| Hybridization | sp | sp2 | sp3 | sp2d or dsp2 | dsp3 or sp3d | d2sp3 or sp3d2 | d3sp3 or sp3d3 |
| No. of bonded atoms | 2 | 3 | 4 | 4 | 5 | 6 | 7 |
i. sp – hybridization :
One s + one p → 2 sp hybrid orbitals.
e.g.. BeCl2 (No. of bonded atoms 2) ‘Be’ is a central atom which undergoes sp – hybridization. Shape of BeCl2 Linear
sp2 – hybridization :
One s + two p → three hybrid orbitals.
e.g. BCl3. (no. of bonded atom = 3) In BCl3, B’ undergoes sp2 hybridization. Shape of the BCl3 is a triangular planar.
iii. sp3 – hybridation :
One s + three p → 4 hybrid orbitals
e.g.1. CH4 (bonded atoms = 4): In CH4 ‘C’ is central atom which under goes sp3 hybridization. Shape of CH4 is tetrahedron.
e.g. 2. NH3 (bonded atoms = 3, LPE = 1) : N undergoes sp3 hybridization, it has pyramidal shape. The expected bond angle would be 109028’ but the actual bond angle is 106045’ due to repulsion between lone pair & bond pairs.
iv. sp3d hybridization: One s + three p + one dZ → 5 hybrid orbitals.
e.g. PCl5 (bonded atoms = 5) : P undergoes sp3d hybridization. It has Trigonal bipyramidal shape.
v. sp3d2 hybridization:
one s + three p + two d → 6 hybrid orbitals
e.g. SF6 (bonded atom = 6): S undergoes sp3d2 hybridization. Hence, it has octa hedral structure.
vii. Sp3d3 hybridization:
one s + three p + three d → 7 hybrid orbitals
e.g., IF7 (bonded atom = 7) : Hybridization of I = sp3d3. Therefore shape of IF7 is pentagonal bipyramidal.
Common hybridization with shapes & examples.
| Types of hybridization | Atomic orbitals involved | Shape of the molecule | Bond angles | Examples |
| 1) sp | One s + one p | Linear | 1800 | BeCl2, BeH2, C2H2, HgCl2 etc. |
| 2) sp2 | One s + two p | Triangular planar | 1200 | BF3, BCl3, SO2,C2H4, NO–3, CO32- etc |
| 3) sp3 | One s + three p | Tetrahedral | 1090, 28/ | CH4, CCl4, SnCl4, NH4+, C2H6, (NH3 & H2O bond angle decreases) |
| 4) dsp2 | One d (dx2 – y2) + one s + two p | Square planar | 900 | [Pd Br42-] [Ni (W)42-, [Pt U4] |
| 5) sp3d or dsp3 | One s + three p + one d (dz2) | Trigonal bipyramidal | 900, 1200, 1800 | Example. PF5, PCl5 |
| 6) sp3d2 or d2sp3 | One s + three p + two d (dx2y2, dz2) | Octahedral (Square pyramidal) | 900, 1800 | SF6 |
| 7) sp3d3 or d3sp3 | One s + three p + three d (dxy, dyz, dzx) | Pentagonal bipyramidal | 900, 720 | IF7 |
Method for finding the hybridization:
The type of hybridization of the central atom can be predicted by using the following generalized formula
- , where V = No. of monovalent atoms are groups attached to the central atom. G = No. of valence electrons of the central atom. a = charge on anion, c = charge on cation, z = no. of electron pairs.
| Value of Z | 2 | 3 | 4 | 5 | 6 | 7 |
| Hybridization | Sp | sp2 | sp3 | sp3d | sp3d2 | sp3d3 |
- Hybridization of ‘O’ in
- Hybridization of N in
- Hybridization of C in
- Hybridization of S in
- Hybridization of S in
- Hybridization of O in
- Hybridization of N in
- In complex compounds, the type of hybridization of central metal ion is predicted as:
| No. of legends | 2 | 3 | 4 | 5 | 6 |
| Hybridization | Sp | sp2 | sp3 & dsp2 | sp3d or dsp3 | sp3d2 or dsp3 |
Illustration 15: Predict the type of hybridization of underline atoms in the following species. (i) SO2 (ii)
Solution.
Illustration 16: In which of the following molecules/ions , the central atom is sp2 hybridized?
(A) (B)
(C) (D)
Solution: (D) are sp2 hybridized molecule/ion.
Some important bond characteristic (Bond parameters) :
i. Bond energy:
- The amount of energy required to break one mole of bonds of a diatomic molecular substance, in the gaseous state into its free constituent atoms, is called bond energy or simply bond dissociation energy.
- It is usually expressed in kJ mol-1.
- Bond dissociation energy and bond energy are same in diatomic molecule, as they have only one bond.
- But in molecules having many bonds, bond dissociation energy is the average value of bond dissociation energy. e., BE = Av BDE.
- The strength of bond is measured on the basis of bond energy.
- It increases as the number of bonds between two atoms increases.
- It increases as the EN between two atoms increases as
- Shorter the bond length, higher is the bond energy.
- Resonance in the molecule affects increases the bond energy i.e. If resonating structures are present in the molecule, its bond energy increases, stability increases and bond length decreases. e., reactivity of the substance decreases.
- e.g., CO2, N2 etc are stable gases in the atmosphere because their bond energies are high.
- It increases in the following order. s < p < sp < sp2 < sp3
Bond length:
- The distance between the nuclei of two atoms bonded together is called bond length.
- It is expressed in angstrom (Å) or picometer (pm) units. [Å = 10-8cm, 1pm = 10-10cm ].
- It is measured by X-ray diffraction of solids and electron diffraction of gases.
- It can be obtained by adding the covalent radii of the bonded atoms.
- When the number of bonds between two atoms increases, the bond length decreases i.e., multiplicity increases.
- Bond length decreases with increase in S-character as
- When the EN between two atoms increases, the bond length decreases as
iii. Bond Angle:
- The angle between two adjacent bonds in a molecule is called bond angle.
- It is expressed in degrees, minutes and seconds.
- The value of bond angle largely depends on the nature of the bonds concerned.
- It increases, as the s- character increases in hybrid bonds or orbitals i.e., depends on the state of hybridization of the central atom. e.g.,
| CH4 | BCl3 | BeCl2 |
| sp3 | sp2 | Sp |
| 25% s – character | 33.3% s. character | 50 % s – character |
| 1090.28 | 1200 | 1800 |
- It decreases, as number of lone pair electrons increases on central atom, due to increase in repulsion between lone pairs.
- It decreases, as the electro negativity of the central atom decreases.
- It increases with the decrease in electro negativity of the surrounding atom, in case of central atom remains the same.
- The bond angle gives an idea of the shape of the molecule.
Bond Angles of some molecules
| Molecule | Bond angle | Molecule | Bond angle |
| CH4 | 1090.28′ | H2O | 1040, 31’ |
| C2H4 | 1200 | NH3 | 1070,8’ |
| C2H2 | 1800 | BeCl2 | 1800 |
| C2H6 | 109028′ | BCl3 | 1200 |
| PCl5 | 120 & 900 | BF3 | 1200 |
| XeF4 | 900 | SF6 | 900 |
Polarity of covalent bond : (Ionic character of covalent bond) :
- A covalent bond, in which electrons are shared unequally and the bonded atoms acquire a partial positive and negative charge is called a polar covalent bond or a covalent bond between two dissimilar atoms is a polar bond and such molecules are called polar molecules.
- A polar bond is formed between two different electronegative atoms.
- Polar covalent bonds may be thought of as being intermediate between the non-polar bonds and pure ionic bonds.
- Two kinds of notation are used to indicate a polar covalent bond i.e.,
- Polarity of a covalent bond is described in terms of ionic character which usually increases with increasing difference in the electro negativity between bonded atoms e.g.,
| H – F | H – Cl | H – Br | H – I |
| EN ⇒ 1.9 | 0.9 | 0.7 | 0.4 |
Mathematical equations for calculating the % ionic character
a. Pauling equation :
% ionic character of covalent bond where XA & XB are the electronegativities of atoms.
b. Hannary and smith equation:
% of ionic character covalent bond where XA & XB are the electronegativities of atoms.
This equation gives approximate calculation of percentage of ionic character.
Table : Percentage ionic character and EN.
| XA – XB | % of ionic character | Nature of A – B bond |
| 0 | O | Purely covalent |
| 0.1 to 0.8 | 0.5 – 15 | Covalent |
| 0.9 to 1.6 | 19 – 47 | Polar covalent |
| 1.7 | 50 – 54 | 50% ionic & 50% covalent |
| 1.8 to 3.2 | 55-93 | Ionic |
Illustration 17: Calculate the percentage of ionic character in H – F bond in HF molecule. The electronegativity values of H and F are 2.1 and 4.0 respectively.
Solution : From Hannary and Smith equation.
% of ionic character in H – F bond = 16 (XA – XB) + 3.5 (XA – XB)2
Illustration 18: Arrange the molecules HF, HCl, HBr & HI in the increasing order of the percentage of ionic character. (Given EN : H = 2.1, F = 4.0, Cl = 3.0, Br = 2.8 I = 2.5)
Solution: % of ionic character .
Dipole moment:
- The product of magnitude of negative or positive electric charge ‘q’ and the distance ‘d’ between the two poles of a polar bond is called dipole moment.
- It is expressed by .
- Mathematically,
= electric charge X bond length
.
where, order of q = 10-10 esu
order of d = 10-8 em
order of = 10-18 e.s.u. cm
Unit of dipole moment = Debye (D)
1D = 1 x 10-18 e.s.u cm or 1D = 3.335 10-30 coulimb metere (SI unit)
In SI unit 1e.s.u = 1.602 10–19C/4.803 10–10 = 3.335 10–10C
Dipole moment of an electron separated from unit positive charge by a distance.
1A0 is 4.80 10-10 esu 10-8 cm = 4.8 10-18 seu cm = 4.8 debyes.
- It a vector quantity i.e., it has magnitude as well as direction.
- It is represented by an arrow with its tail at the positive centre (pole) and head pointing towards the negative centre (pole) and i.e., (+ → –).
- In case of polar diatomic molecules there is one polar bond so, dipole moment of molecule = dipole moment of the polar bond.
- In case of polyatomic molecules, there are more than one polar bonds, so,
- Dipole moment = Resultant dipole moment of all individual polar bonds.
- Resultant dipole moment may be calculated by the vectorial addition of the bond moments, as
Case . i. If, = 0, the resultant is maximum
Case . ii. If, = 1800, the resultant is minimum.
e.g. (i) Dipole moment of water is 1.84D, which is equal to the resultant dipolemoment of two O – H bonds.
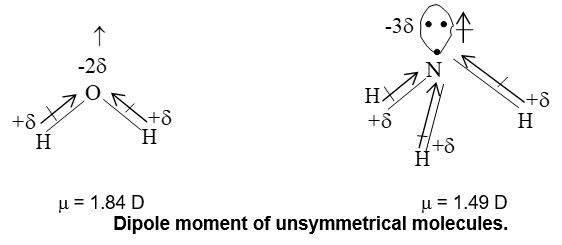
e.g. (ii) Similarly dipole moment of Ammonia is 1.49D, which is equal to the resultant of dipole moments of there N – H bonds.
- The molecule having zero resultant dipole moment are said to be non polar molecules.
e.g. i. CO2, BeCl2, BeF2, CS2, BF3, BCl3, CH4, CCl4 etc having zero dipole moment however the contain to polar bond.
e.g. ii. BF3, CH4, CCl4 etc., are also having zero dipole moment.
- The molecules having certain dipoloments are said to be polar molecules.
e.g.
Fig. unsymmetrical molecules with certain dipolments.
Dipolement of some common molecules:
| Molecule | (D) | Molecule | (D) |
| HF | 1.92 | SO2 | 1.60 |
| HCl | 1.03 | H2S | 0.92 |
| HBr | 0.79 | CH3Cl | 1.86 |
| HI | 0.38 | C2H5OH | 1.68 |
| H2O | 1.84 | C6H5Cl | 1.57 |
| NH3 | 1.49 | CH3COCH3 | 1.30 |
| CO2 | 0.00 | CH3I | 1.35 |
| CH4 | 0.00 | C6H5NO2 | 4.08 |
| NF3 | 0.24 | C6H5OH | 1.70 |
| H2 | 0.95 | PCl5 | 0.0 |
| CS2 | 0.0 | CCl4 | 0.0 |
| HCN | 2.93 | PH3 | 0.59 |
| H2S | 0.92 | H2O2 | 1.84 |
| N2O | 0.17 | CH3OH | 1.65 |
| CO | 1.11 | O3 | 0.52 |
Molecular geometry and Dipole moment
| General formula | Geometry | Dipolemoment | Examples |
| AX | Linear | May be non zero (> 0) | HF, HCl |
| AX2 | Linear | Zero | CO2, CS2, BeF2 |
| AX3 | Triangular planar | Zero | BF3 |
| Pyramidal | > 0 | NH3, PCl3 NF3 | |
| T – shaped | > 0 | ClF3 | |
| AX4 | Tetrahedral | Zero | CH4CCl4 |
| Square planar | Zero | XeF4 | |
| See saw | non – zero | SF4 | |
| AX5 | Trigonal bipyramidal | Zero | PCl5 |
| Squarpyramidal | non zero (0) | BrCl5 | |
| AX6 | Octahedral: | non zero | XeF6 |
| Distorted octahedral | Zero | SF6 | |
| AX7 | Pentagonal bipyramidal | Zero | IF7 |
Applications of Dipole moment:
i. In determination of polarity of the bond:
Molecules having zero dipole moment () are said to be nonpolar molecules and those having are polar in nature.
e.g., H2, N2, O2 etc are nonpolar () and HF, HCl, HBr, HI, etc are polar molecules ().
Thus, dipolemoment can also be used to distinguish between polar and nonpolar molecules.
ii. In calculation of percentage of ionic character:
Ionic character can be determined using dipolemoment.
e.g. Experimental dipole moment for HCl = 1.03
Suppose charge on H+ or Cl–, q = 4.8 x 10-10 esu, d = 1.27A0
theoretical value of
Thus, percentage of ionic character in H – Cl bond in HCl molecule
In general, % ionic character
iii. In determining the geometry of molecules:
- CO2, CS2 molecules are linear
- H2O is not a linear molecule . Actually, it has V shaped structure
- NH3 has pyramidal structure .
iv. To distinguish between cis and trans isomers:
In general, cis isomer has certain dipolemomnet but trans isomer has zero or very low dipolemoment. e.g. dipole moment of cis -1, 2 – dichloroethyne ( = 1.9D) is greater than trans – 1, 2 dichloroethyne ( = 0).
It should be noted that, if two group have opposite inductive effect then trans isomer will have greater dipolement e.g. dipolemoment of trans 1 chloropropene is greater than cis 1 chloropropene i.e.,
v. In determining orientation in benzene ring (o > m > p):
In ortho isomers, experiment value of dipolemoment is found different from theoretical value of dipolemoment, due to dipole – dipole interaction of two groups. e.g., dipolemoment of o- dichlorobenzene is greater than m-dichlorobenzene and p – dichlorobenzene.
observed = 2 (bond moment) .
Where = bond angle.
vi. Indetermining Bond moment:
Suppose, observed dipolemoment of water is 1.85D and is 104.50 then bond moment may be calculated as
vii. Dipolemoment can be used in predicting hybridization:
eg(i) If a molecule AB2 has and orbitals are used by A (z < 21), must be Sp-hybridised. Example. BeF2.
(ii) If a molecule AB3 has , the orbitals are used by A (z < 21) must be sp2 hybridized eg. BF3.
iii) If a molecule AB4 has , the orbitals also used by A (Z < 21) must be sp3 hybridized are used by A (z < 21) must be sp3 eg. CCl4.
Illustration 19: The dipolemoment of H2S is 0.95 D. Find the moment if the bond angle is 970 (given cos 48.50 = 0.662) (Hint = 2 (bond moment ))
Solution. H2S has bent structure with
= 2 (bond moment) cos 48.50
0.95 = 2 (bond moment) 0.662
0.95 = 2 ( – H) x 0.662
bond moment = 0.72 D
Hydrogen bond:
A bond between hydrogen atom of a molecule and a highly electronegative atom (i.e., F, O, N) of the same molecule or different molecule is called hydrogen bond.
- The hydrogen bond was proposed by Moore and Winmill and it was introduced by Latimer and Rodebus, in 1920.
- A hydrogen bond is represented by a dotted line.
e.g. i) Hydrogen bonding in H – F molecules.
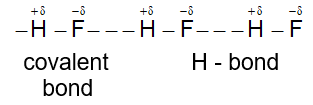
ii) Hydrogen bonding in water
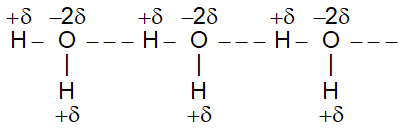
Nature of H – Bond:
Hydrogen bond is a slight electrostatic attraction between hydrogen of one molecule and a more electgronegative atom of same or different molecules.
Strength of H – bond:
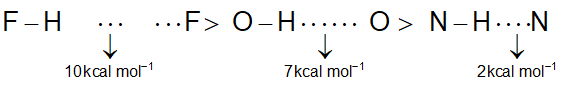
Conditions for H – bonding:
i) Hydrogen atom should be bonded to a highly electronegative atom (i.e., F, O, N).
ii) For strong bonding, the size of the more electronegative atom should be small.
Types of H – Bond: H – bonds are of two types.
i) Inter molecular H – bond
ii) Intra molecular H – bond
i) Intermolecular H – bond : The H – bond formed between two or more molecules of the same or different substances is called intermolecular hydrogen bond.
Examples of intermolecular H – bonding.
i) Intermolecular H – bonding in Hydrofluoric acid (HF) : In solid state – zig – zag chain structure.
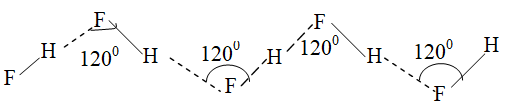
e.g. ii. Intermolecular H – bonding in water:
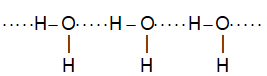
e.g. iii . Intermolecular H – bonding in NH3
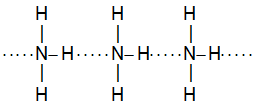
e.g. iv. Intermolecular H – bonding also present in formic acid (HCOOH), alcohols (R – OH), carboxylic acids (R – COOH) and p – hydroxyl benzaldehyde (etc)., Carboxylic acids form dimer.
II) Intramolecular H – bond: Hydrogen bond formed between the atoms of the same molecule is known as intra molecular H – bond and it is also knows as chelation.
Examples of intramolecular H – bonding.
i) Intramolecular H – bonding in o – Chlorophenol (a) and o – Nitrophenol (b).
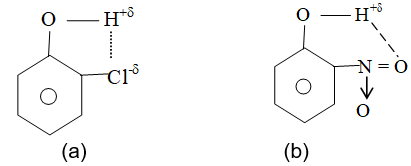
ii) Intramolecular H – bonding is also present in salicylaldehyde, o- Nitro aniline, etc.,
Effect of H – bonding on properties:
Following properties increases on increasing the no.of H – bonds:
- Melting points and boiling points ⇒ increses
- Solubility of substances – increases.
- Solvation power of solvent ⇒ increases
- Viscosity of substances = increase
Comparison of boiling points of NH3 and HCl:
NH3 has a higher boiling point than HCl even though nitrogen and chlorine have nearly the same electronegativity values. NH3 forms hydrogen bonds while HCl does not. This is because of the smaller size of the nitrogen atom compared to the chlorine atom.
The abnormal melting and boiling points of H2O or HF or NH3 are due to hydrogen bonding between molecules.
Comparison of boiling points of water and hydrogen fluoride:
Practically water has higher boiling point than HF. To explain this fact it is suggested that because of two O – H bond in H2O molecule the number of hydrogen bonds per water molecule are almost twice than those per HF molecule. Therefore more energy is required to break greater number of H-bonds in water. Secondly HF exists as (H – F)6 even in vapour state i.e., all hydrogen fluoride before it is converted into vapour, but by the time vapour is formed water molecules become independent with all hydrogen bonds broken.
Hydrogen bonding is responsible for the association of molecules in water. For this reason only water is a liquid. Hydrogen bonding occurs in both liquid and solid states of water.
Illustration 20: Water is liquid while H2S is gas, why?
Solution. Water is liquid due to presence of intermolecular hydrogen bonding while hydrogen sulphide (H2S) is a gas due to absence of H – bonding.
Illustration 21: NH3 has a higher boiling point than HCl why?
Solution. NH3 form hydrogen bonds while HCl does not, form because of its smaller size than clorine atom hence due to formation of H – bonds in ammonia its boiling point is higher than HCl.
FORMULAE AND CONCEPTS AT A GLANCE
1. Chemical bond is a force that acts between two or more atoms to hold them together as a stable molecule.
2. Valency of an element depends mainly on the number of electron present in the outermost shell
3. Favourable conditions for forming ionic bond are
(i) low ionization potential
(ii) high electron affinity
(iii) high lattice energy of the compound
4. Crystals of different ionic compounds having same geometry are known as isomorphs.
5. Covalent bond is formed by the mutual sharing of the electron by different or same type of atoms.
6. Covalent compounds show structural and space isomerism.
7. Dative bond is a special type of covalent bond where both the electrons forming the bond are contributed by one atom but are shared by both the atoms.
8. For a diatomic polar molecule, the product of the magnitude of the positive or negative charge on each atom of the molecule and the distance between the centres of the two atom is called dipole moment.
9. The ability of a cation to polarize the nearby anion is called its polarizing power and the tendency of an anion to get distorted or polarized by the cation is called its polarizability.
10. According to valence bond theory, there is a condition of maximum atomic orbital overlap leading to maximum bond strength at a particular internuclear distance (bond length).
11. Hybridization of atomic orbitals is the concept of intermixing of orbitals of same energy or of slightly different energy to produce entirely new orbitals of equivalent energy and symmetrically disposed in space. New orbitals thus formed are called hybrid orbitals.
12. VSEPR theory helps us in explaining the shapes of molecules having lone pair of electrons.
13. Molecular orbital theory is based on the concept of wave mechanics.
SOLVED PROBLEMS
Prob 1. What is true about N2 and CN –?
(A) Both are isoelectronic (B) Both chemically inert
(C) Both are highly reactive (D) Both have same polarity
Sol: (A). Number of electrons in N2 = 7 + 7 = 14 and number of electrons in CN – = 6 + 7 + 1 = 14
So both are isoelectronic.
Prob 2. The bonds are N2O5 are
(A) Only ionic (B) Only covalent
(C) Covalent and ionic (D) Covalent & Coordinates
Sol: (D). The structure of N2O5 is
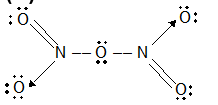
If has covalent and coordinate bond
Prob 3. Bond angle is minimum is
(A) H2O (B) CO2
(C) NH3 (D) CH4
Sol: (A). In CO2 molecule, structure is linear O = C = O. So bond angle is 180°
In H2O, NH3 & CH4 the central atom is sp3 hybridised
In CH4 4 bond pair of electrons and no lone pair of electron is present.
In NH3 3 bond pair of electron and 1 lone pair of electron is present.
In H2O 2 bond pair of electron and 2 lone pair of electron is present.
Greater is no. of lone pair greater is the lone pair bond pair repulsion smaller is the long angle.
So bond angle is least in case of H2O
Prob 4. According to M.O.T. bond order of N2 is
(A) 1 (B) 2
(C) 3 (D) 4
Sol: (C). Molecular orbital configuration of N2 is
Bond order
Prob 5. The number of p-orbital not involved in the hybridisation of middle carbon atom in CH2 = C = CH2 is
(A) 1 (B) 3
(C) 2 (D) None of the above
Sol: (C). The middle carbon atom is sp hybridised therefore the number of p-orbitals not involved in hybridisation is 2
Prob 6. Which of the following structure is most expected for the olecules XeOF4?
(A) Tetrahedral (B) Square pyramidal
(C) Square planar (D) Octahedral
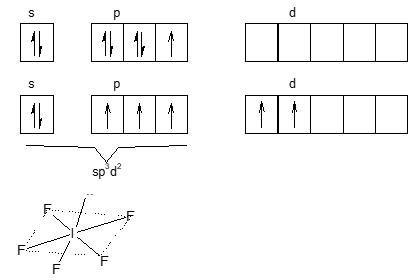
sol: (B). Electronic conf. of I
O.S. of I = 5
Electronic conf. after excitation
shape is square pyramidal
Prob 7. In OF2, oxygen has hybridization of
(A) sp (B) sp2
(C) sp3 (D) none of these
Sol: (C). OF2 has sp3 hybridization
Prob 8. The boiling points of methanol, water and dimethyl ether are respectively 65°C, 100°C and 34.5°C. Which of the following best explains these wide variations in b.p.
(A) the molecular mass increases from water (18) to methanol (32) to diethyl ether (74)
(B) the extent of H-bonding decreases from water to methanol while if is absent in ether
(C) the extent of intermolecular H-bonding decreases form ether to methanol to water
(D) the number of H atoms per molecule in creases form water to methanol to ether.
Sol: (B). Statement (B) is correct.
Prob 9. Which of the following is an example of super octet molecule?
(A) CF3 (B) PCl5
(C) IF7 (D) All the three
Sol: (D). All the molecules are supper octet molecule since in all these molecules; the central atom has more than 8 electrons
Prob 10: According to Fajan’s rule formation of a covalent bond is favoured by
a) large cation and large anion b) small cation and small anion
c) small cation and large anion d) large cation and small anion
Sol: (C) According to Fajan’s rule formation of covalent bond is favoured by small cation and large anion.








