Introduction:
Polymers are the chief products of modern chemical industry which form the back bone of present society. i.e., life began with and is being mainted by polymers.
But the systematic study of polymer science and technology started as late as the middle of the 20th century with the pioneering work of Herman staudinger.
The materials made of polymers find multifarious uses and applications in all walks of our life.
They have influenced our day to day life to such an extent that it is impossible to get through the day without using a material based on polymers.
Common examples of polymers are rubber, plastics, foams, fibers, proteins, nucleic acids, cellulose i.e., plastic dishes, cups, non-stick pans; TV and computer cabinates; with range of synthetic fibres for clothing, synthetic glues, flooring materials and materials for biomedical and surgical operations.
Polymers and Monomers:
Polymers are complex and giant molecules of high mm.
A polymers may be defined as a large molecule built up by repeating structural units joined by the covalent bonds.
The word polymer has been derived from the Greek word (poly = many; mer = parts).
The smallest unit or molecule that repeatedly combines to form the polymer is known as monomer or monomer unit.
The process by which the monomer units are converted into polymers called polymerization.
For example: Polyethylene is a polymer which is obtained by the polymerisation of ethene monomer.
Depending upon the nature of the repeating structural units polymers may be homo polymers and copolymers.
Classification of polymers:
I. Classification of polymers on the basis of source of availability:
On the basis of source or origin, the polymers are classified into three types.
1. Natural polymers 2. Synthetics 3. Semisynthetic
II. Classification of polymers on the basis of structure:
On the basis of structure of polymers, these can be classified as:
(1) Linear polymers (2) Branched chain polymer (3) Cross linked polymers.
1. Linear polymers:
These are polymers in which monomeric units are linked together to form linear chains.
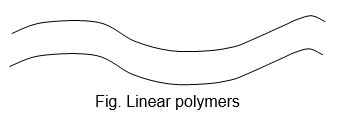
As they are well packed and therefore have high densities, high tensile (pulling) strength and high melting points.
Some common examples of linear polymers are: Polyethylene, nylons, polyesters etc.
2. Branched chain polymers:
These are polymers in which monomeric units are linked to form long chain with side chains (branches) of different length.
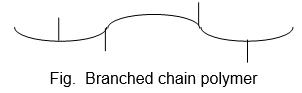
As they are irregularly packed and therefore have low tensile strengths and melting points than linear polymers.
Some common example are: low density polythene, glycogen, starch etc.
3. Cross-linked polymers:
These polymers are hard, rigid and brittle because of network structure. Some common example are – Bakelite, melamine formaldehyde resin, etc.
III. Classification of polymers on the Basis of molecular forces:
A large number of applications of polymers depend upon their mechanical properties such as tensile strength, elasticity, toughness etc.
These mechanical properties depend upon intermolecular forces like van der Waal’s forces, hydrogen bonds and dipole – dipole intractions, existing in the molecules.
Although these intermolecular forces are also present in simple molecules.
This is because in the polymers, these forces extend all along the chain, resulting significant combined effect.
Thus, longer the length of polymer chain, stronger is the effect of intermolecular forces.
Depending upon the intermolecular forces, the polymers have been classified into four types.
1. Elastomers:
• The polymers that have elastic character like rubber are called elastomers. The must important example of elastomer is natural rubber.
2. Fibres:
The polymers which are used for making fibres, possess high tensile strength and high modulus. This can be attributed to the strong inter molecular forces like hydrogen bonding which, for example operate in polyamides (i.e., nylon 66).
3. Thermoplastic:
• The intermolecular forces of attraction in thermoplastic polymers are intermediate between elastomers and fibres.
• As a result, these can be easily moulded by heating.
• sealing wax etc.
Note: Plasticizers: Certain plastics do not soften very much on heating. These can be easily soften by the addition of some organic compounds which are called plasticizers. For example: Polyvinyl chloride (PVC) is a very stiff and hard but it is made soft and workable by adding di-nbutyl phthalate a plasticizer. The plasticizing effect is due to solubilization action and an accompanying reduction in intermolecular forces which permits free movement of molecules relative to each other.
Some other common plasticizer are dialkyl phthalates cresyl phthalates, triceryl phosphate, triphenyl phosphate, camphor etc.
4. Thermosetting polymers:
These polymers are normally made from relatively low molecular mass semi-fluid substances which on heating in a mould become infusible and form an insoluble hardmass. This happens due to extensive cross linking between different polymer chains forming three dimensional network of bonds.
Some common thermosetting polymers are: Bakelite, Polysiloxane, Malamine formaldehyde, etc.
IV) Classification of Polymers on the Basis of heat treatment: Depending on heat treatment the polymers have been classified into two types.
|
a) Thermoplastic polymers Difference |
b) Thermo setting polymers |
|
(i) Thermoplastic polymers can be softened repeatedly by heating and hardened on cooling without change in properties. |
(i) Thermosetting polymers can be heated only once because it undergoes permanent change on melting and sets into a new solid which can not be remitted. |
|
(ii) Thermoplastic polymers can be moulded to any desired shape and can be processed again and again. |
(ii) Thermosetting polymers can not be moulded easily into desired shape and can not be reprocessed. To form articles with the desired shapes from thermosetting materials, the cross linking must be allowed to occur during the fabrication of the articles. |
V. Classification of polymers based on mode of polymerization or mechanisms of polymerization:
1. Homopolymers and copolymers:
2. Addition and condensation polymers: Synthetic polymers are also classified according to their mode of formation and are distinguished as addition polymers and condensation polymers.
(i) Addition polymers: Addition polymers are formed by reactions between monomer units possessing multiple bonds with out the elimination of any by-product. For example: Polythene, polypropylene, polyvinyl chloride polyacryolonitrle (orlon) Teflon, SBR etc.
2. Condensation polymers:
The condensation polymers are formed by condensation between monomeric units with the elimination of small molecules such as water, ammonia, alcohol etc.
For example:
(i) Nylon: Nylon 66 is formed by condensation between hexamethylene diamine and adipic acid.
(ii) Dacron (Polyester): It is a condensation polymer of ethylene glycol and terephthalic acid, monomers.
(iii) Bakelite: It is a condensation polymer of phenol and formaldehyde monomers.
VI. Classification of polymers based on polarity:
1. Cationic polymerization polymers: e.g., Polystyrene, polyvinyl ethers, polyisobutene, polypropylene, polyvinyl acetate etc.
2. Anionic polymerization polymers: e.g., Bunatype synthetic rubbers poly isoprene, polyacryonitrile, polyvinyl chloride PMMA etc.
Polymerization process: The process by which the monomer units are converted into polymers is called polymerization
The smaller species is formed by the process of polymerization of monomer units are referred to dimer, trimer, tetramer, pentomers ….. polymer, the depending on the nature of monomer units.
Generally polymerization process involves addition and condensation polymerization.
According to carothers (1929), Mark (1940) and Flory (1950) all polymers could be divided into two categories depending upon the mechanism of polymerization i.e., Addition polymers & condensation polymers.
1. Addition polymerization: When the molecules of same monomer or different monomers simply add to gather to form a polymer, this process s called addition polymerization.
In this process a polymer so formed with out elimination of some by-product molecules is called addition polymer.
The monomers used here are unsaturated compounds such as alkenes, alkadienes and their derivatives.
This mode of polymerization can take place through formation of either radicals or ionic species such as carbanions and carbocations.
This process is also called chain polymerization or chain growth polymerization because it takes place through stages leading to increase of chain lengths and each stage produces reactive intermediate for use in next stage of the growth of the chain.
The addition polymerization reaction very rapid and also characterized by three steps.
(i) Initiation (ii) Chain propagation (iii) Chain termination
On the basis of initiator used to produced reactive species like ore radical, cation and anion addition polymerization can be broad by subdivided into two type.
1) Free radical addition polymerization
2) Ionic polymerization i.e., cationic and anionic polymerization
Free radical addition polymerization:
A variety of unsaturated compounds, alkenes or deines or their derivatives are polymerised by free radical addition polymerization. It is catalysed by organic peroxides or other reagents which decomposed to give free radicals.
Some examples of free radicals polymerization are discussed below.
1. Alkene polymerization: This type of polymerization is performed by heating the monomer with a small amount of initiator, commonly peroxides or by exposing the monomer to light.
Mechanism: The free red polymerization reaction involves the following three steps.
(i) Chain initiation step: Organic peroxides undergo homolytic fission to form free radicals which act as initiator (In).
The initiator adds to the carbon – carbon double bond of an alkene molecule to form a new free radical.
(ii) Chain propagation step:
The new free radical adds to a double bond of monomer to form a larger free radical. The radical formed adds to another alkene molecule to form an even large free radical. This process continuous until the radical is destroyed. These steps are called propagation steps.
(iii) Termination step: The above chain reaction terminates when free radical combines with another free radical.
2. Vinyl polymerization: Most of the commercial addition polymers are vinyl polymers obtained from alkenes and their derivatives
This type of polymerization is performed by heating the monomer with only a very small amount of the initiator or by exposing the monomer to light free radical.
3. Conjugate Diene polymerisation:
(i) 1, 4 – polymerization:
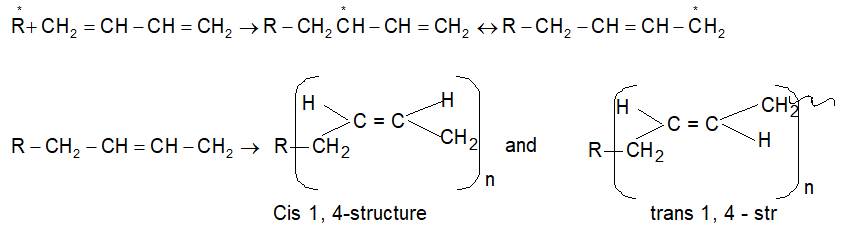
(ii) 1, 2 – Polymerization:
Some commonly used radical initiators:
|
|
Name of Initiator |
Structure |
|
(i) |
Hydrogen peroxide |
|
|
(ii) |
Cumyl hydroperoxide |
|
|
(iii) |
Rotassium persulphate |
|
|
(iv) |
Dibenzoyl peroxide |
Illustration 1: How does the presence of benzoquinone inhibit the free radical polymerisation of a vinyl derivative?
Solution: Benzoquinone traps the radical intermediate to form a non-reactive radical, which is highly stabilized by resonance. Because of the lack of reactivity of this intermediate, further progress of the chain reaction is interrupted and the reaction stops.
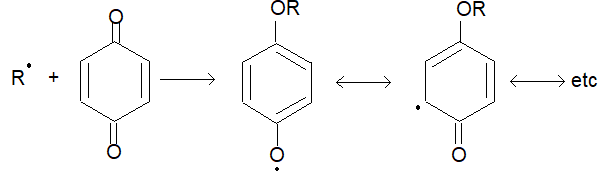
Illustration 2: Why should one always use purest monomer in free radical polymerisation reactions?
Solution: In free radical polymerization reaction, the impurities can acts as chain transfer agent and may combine with the free radical to slow down the reaction or even stop the reaction.
Illustration 3: Write the structure of reagent used for initiating a free radical chain reactions. How does it act?
Solution: Tertiary butyl peroxide is used for initiating a freeradical chain reaction. It decomposes under mild conditions to firm tert-butoxide free radical which initiates the polymerization reaction.
Ionic polymerization:
(i) Cationic polymerization:
When the initiator is cationic in nature, it adds to the double bond, to generate a cationic intermediate for propagating the addition chain process and is termed as cationic addition polymerisation.
• The process is initiated by an acid the important chain initiator used for the cationic polymerisation are acids like H2SO4, HF and lewis acids such as BF3, AlCl3 or SnCl4 in presence of small amount of water. The acids generate protons as
• The simple example for this kind of polymerization is the formation of a vinyl compound from its monomer.
Mechanism: The chain reaction involves the following steps.
(i) Chain initiation step:
The proton (cation) adds to the carbon-carbon double bond of alkenes to form a stable carbo cation.
(Where R = G = electron donating group)
(ii) Chain propagation step:
Carbocation on further addition tot eh double bond, a bigger carbocation is formed and sequence of steps propagates the chain to the polymeric cation.
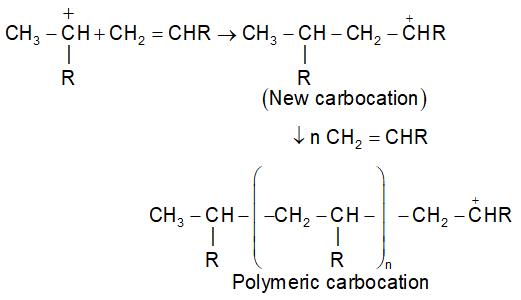
(iii) Chain termination step:
The chain reaction may be terminated by combination of carbocations with negative ion (A–) or by loss of a proton.
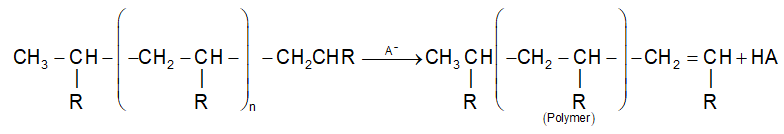
the complete reaction may be written as
It may be noted that cationic polymerisation is effective only with vinyl monomers which contain an electron releasing group (G). The electron donating groups will be able to stabilise the chain carrying carbocation intermediate.
(2) Anionic Polymerization:
This type of polymerization is initiated by anion, which may be bases or other nucleophile. Here the active centre of the propagating species is negatively charged. Hence, it occurs easily in case of vinyl monomers containing electron with drawing groups such as phenyl, nitrile, etc., which are able to stabilize the propagating species. Greater is the stability of the carbanion intermediate, more facile is the anionic polymerization. The initiation can be brought about by reagents such as n-butyl lithium or potassium amide, KNH2.
Mechanism: The anionic polymerization involves the following steps.
(i) Chain initiation step: The base adds to the double bond to form a carbanion.
(Where, X = R = Electron with drawing group)
(ii) Chain propagation step: The carbanion adds to the double bond and the process goes on getting repeated to form a polymeric carbanion.
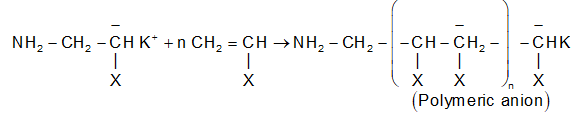
The formation of polystyrene from styrene in the presence of KNH2 is an important example of this category of polymerization.
Polymerization of acrylonitrile: (Anionic addition polymerization) is
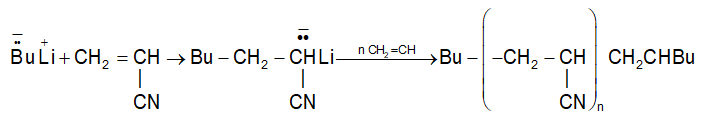
Some common examples of alkenes that undergo anionic polymerization are: vinyl chloride, acrylonitrile, methylmethacrylate, and styrene.
Note: The anionic polymerization is not limited to only vinyl type involving addition to carbon – carbon double bonds. It can be carriedout even with ethylene oxide in the presence of small amount of base forming polymer as
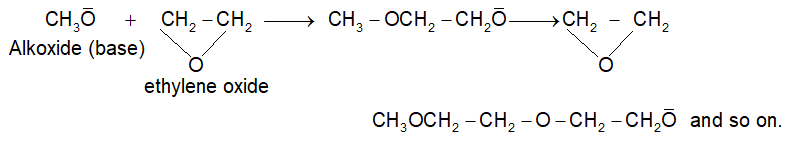
Illustration 4: Will you prefer to polymerise acrylonitrile under anionic or cationic conditions. Examples.
Solution: Acrylonitrile (CH2 = CH-CN) contains an electron with drawing (-CN) group which can stabilize the carbanion intermediate. Since in anionic polymerization, carbanion inter mediates are produced, therefore polymerisation of acrylonitryl must be carried out under anionic conditions.
Condensation polymerisation: The condensation polymerization, in general can be represented as follows:
For example:
(i) Proteins, starch, cellulose, etc are the examples of natural condensation polymers.
(ii) Polyester: It is along chain condensation polymer of ethylene glycol and terephthalic acid.
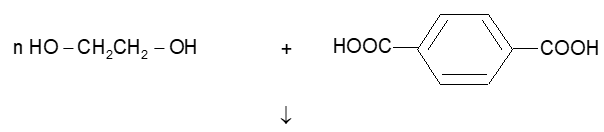
Polyamides: Some examples of polyamides are:
(i) Nylon 66: It is long chain condensation polymer of hexamethylenediamine and adipic acid each of which possess is six carbon atoms and these product as 66.
Molecular mass of the polymer is controlled in the range 12000 to 20000.
(ii) Nylon 6, 10:
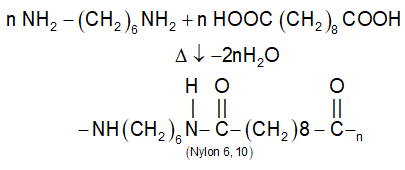
Nylons are insoluble in common solvents, have good strength & absorb little moisture.
Nylon fibres are stronger than natural fibres. So these are used in making garments, carpets cords and ropes & tyres etc.
Polyesters:
(i) Terylene or Dacron:
Terylene is best known polyester, which is prepared by condensing ethylene glycol and terephthalic acid monomers.
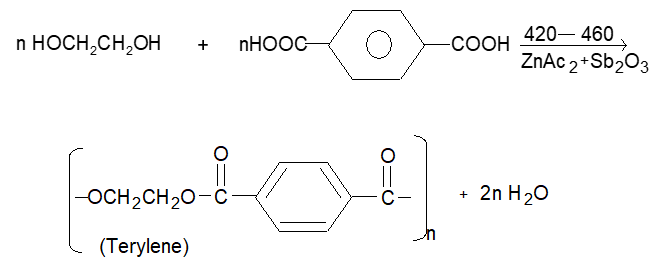
The terylene fibre is crease resistant and has low moisture absorption. It has high tensile strength. It is mainly used is making wash & wear garments, in blending with wool to provide better crease and wrinkle resistance.
(2) Glyptal or Alkyds: It is a cross linked and thermosetting plastic polymer.
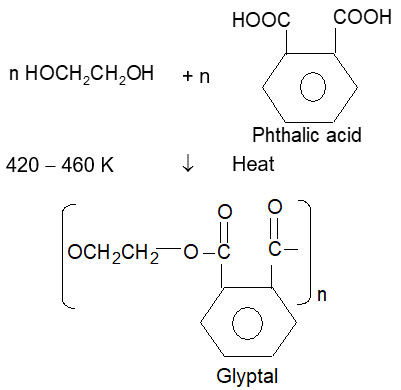
When its solution in a suitable solvent is evaporated it leaves a tough but nonflexible film. It is therefore used in the manufacture of paints & lacquers.
Formaldehyde resins:
(i) Phenol formaldehyde resins (Bakelite & related polymers): These are oldest synthetic polymers and are still extensively used. Phenol formal dehyde resins are obtained by the reaction of phenol and formaldehyde in presence of either an acid or a base.
(a)
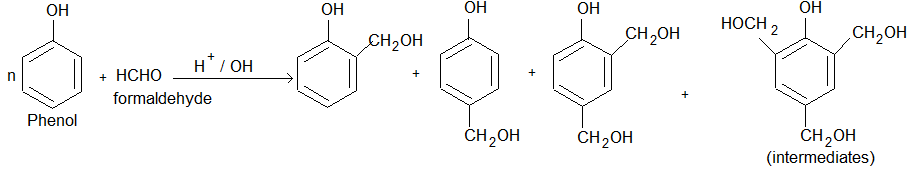
(ii) The condensation of O-hydroxybenzyl alcohol or p-hydroxy benzyl alcohol gives a linear polymer.
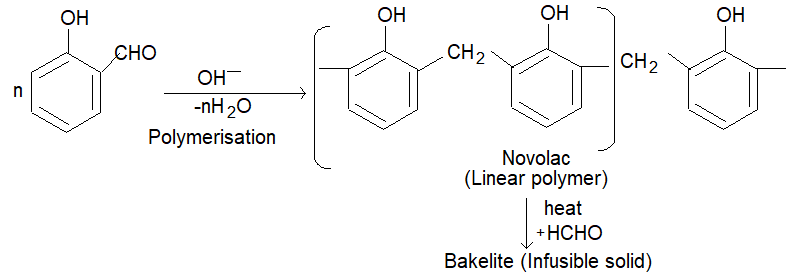
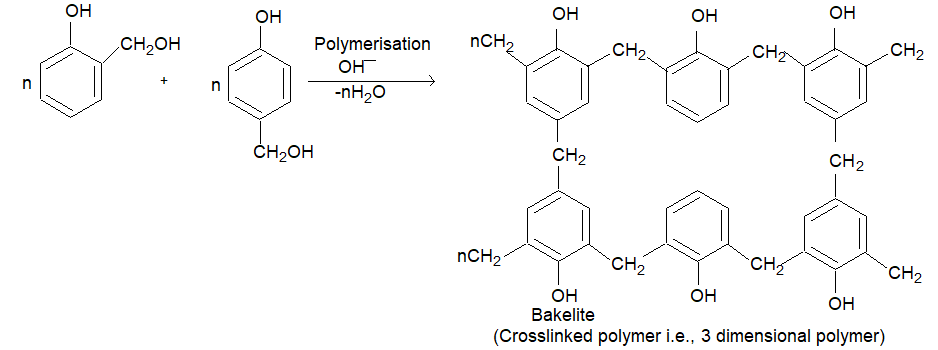
Bakelite is a cross linked thermosetting polymer. Soft bakelites with low degree of polymerization are used as bonding glue for laminated wooden planks, in the preparation of varnishes and lacquers. High degree polymerisation gives hard backelite which is used for making combs, fountain pens, barrels, electrical good, gramophones, formica table tops and many other products. Sulphonated backelites are used as ion-exchange resins for softening of hard water.
(2) Urea formaldehyde resin:
It is a polymer formed by the condensation of urea and formaldehyde.
It is used in making unbreakable caps and plates.
(3) Melamine formaldehyde resin:
Melamine and formaldehyde copolymerise to give a metamine polymer.
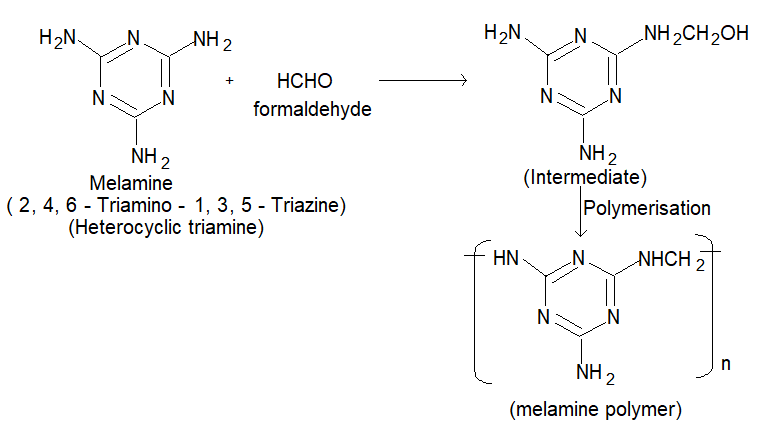
Uses: It is used in making plastic crockery, cups & plates are hard and do not break on dropping.
Rubber: It is a common example of elastomer. The rubber obtained from natural sources is called natural rubber and the polymers prepared in laboratory which are similar to natural rubber are known as synthetic rubbers.
(i) Natural rubber:
It is a natural polymer and possesses remarkable elasticity.
The elasticity makes it valuable for a variety of uses i.e., multifarious uses.
Natural rubber is obtained from rubber trees in the form of a milky sap known as latex. Latex is a suspension of rubber in water, which is obtained by making incisions (i.e., cuts) in the bark of rubber trees found in tropical and subtropical countries such as India (Southern Part), Indonesia, Malaysia, Sri Lanka, South America etc. The latex is coagulated with acetic acid or formic acid. The coagulated mass is then squeezed.
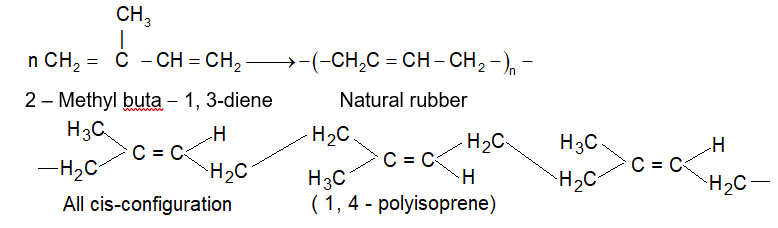
Vulcanization of rubber:
Natural rubber is a soft, sticky thermoplastic having no cross links between the polymer chain.
It has large water absorption capacity and is non resistant to non-polar solvents and is attracted by oxidising agents.
Accidently, charless Goodyear (1893) found that the physical properties of natural rubber can be modified and improved by the addition of sulphur or compound of sulphur like SF6 to hot rubber. This process of heating natural rubber with sulphur or SF6 to modify and improve its properties is called valcanization.
Originally it was performed by heating a mixture of raw rubber and sulphur in the temperature range 373 to 415 K. This was a slow process.
Now a days some additives such as zinc oxide, etc are used to accelerate the rate of vulcanisation.
During vulcanization, sulphur cross links the reactive sites of rubber i.e., double bonds, in the rubber molecule act as reactive sites. The allylic -CH2 (alpha C to double bond) is also very reactive.
As a result, rubber gets stiffened and intermolecular movement of rubber spring is prevented resulting in change of physical character of rubber. i.e., The formation of cross links makes rubber hard, tough with greater tensile strength.
The extent of stiffness of vulcanized material depends upon the amount of sulphur added. E.g., about 5% sulphur is used for making tyre rubber while 30% of sulphur is used for making battery case rubber.
Characterics of vulcanized rubber: The vulcanized rubber has
(i) excellent elasticity
(ii) low water absorption tendency
(iii) resistance to oxidation and organic solvents.
Uses:
(i) Natural rubber is used for making shoes, water proof coats and golf balls.
(ii) Vulcanised rubber is used for manufacturing rubber bonds gloves, tubing conveyor belt and car tyres.
Comparison of main properties of natural rubber and vulcanized rubber.
|
Natural rubber |
Vulcanized rubber |
|
(i) Natural rubber is soft and sticky. |
(i) Vulcanized rubber is hard and non-sticky. |
|
(ii) It has low tensile strength. |
(ii) It has high tensile strength. |
|
(iii) It has low elasticity. |
(iii) It has high elasticity. |
|
(iv) It can be used over a narrow range of temperature (from 100C to 600C). It becomes soft at higher temperatures and brittle at low temperatures. |
(iv) It can be used over a wide range of temperature (-400 C to 1000C) |
|
(v) It has low wear & tear resistance. |
(v) It has wear and fear resistance. |
|
(vi) It is soluble in solvents like ether, carbon tetra chloride, petrol etc. |
(vi) It is insoluble in all the common solvents. |
Synthetic Rubber:
1) Neoprene Rubber:
It is a first synthetic rubber manufactured on a large scale. It is prepared by polymerization of chloroprene monomer.
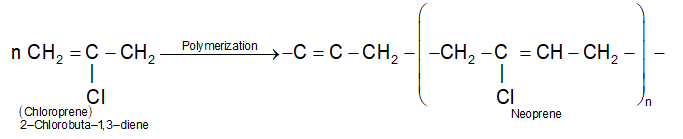
It polymerize very rapidly (700 times faster than isoprene) and the reaction occurs by 1, 4-addition of one chloroprene molecule to the other. The starting material, chloroprene is obtained by dimerisation of acetylene by passing it through aqueous solution of NH4Cl and cuprous chloride at 343K followed by treatment with HCl.
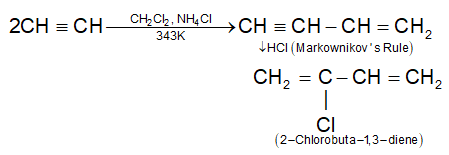
2) Nitrile rubber (Buna-N):
It is obtained by polymerisation of 1, 3-butadiene and acrylonitrile.
It is used for making oil seals, manufacture of hoses and tank linings.
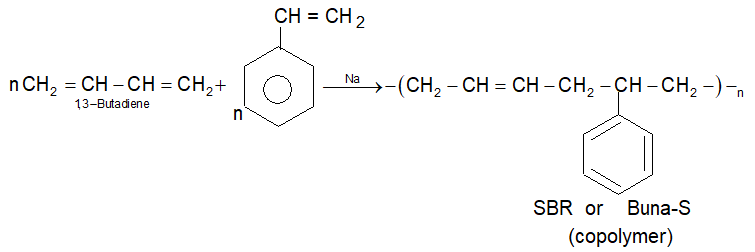
3) Styrene Butadiene Rubber (SBR):
• It is prepared by the polymerisation of buta-1-3-diene and styrene in the ratio of 3 : 1 in the presence of sodium.
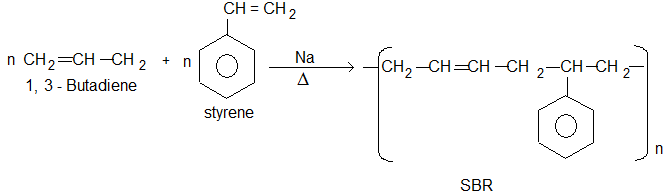
• It is also called as Buna-S, in which Bu stands for butadiene, Na for sodium and S stands for styrene. It has slightly less tensile strengths than natural rubber.
• It is used for making automobile tyres and foot near.
• Bubble gum contains synthetic styrene butadiene rubber.
Difference between natural and synthetic polymers:
|
|
Property |
Natural polymer |
Syntheticpolymer |
|
1. |
Preparation source |
Prepared in nature. E.g., wool, Silk, Jute etc. |
Syntheticpolymer prepared in the labs. |
|
2. |
Length of polymer chain |
Non uniform length |
Can be prepared of uniform length |
|
3. |
Affinity for sulphur & Vat dyes |
Possess very high affinity |
Possess low affinity. |
|
4. |
Fixing quality |
Low |
High |
Some commonly used polymers:
(i) Kelvar: It is a polyamide. It is obtained by condensation of terphthalic acid
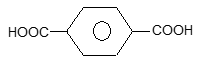
and p – phenylenediamine. The fibres of this polymer are very strong and are used in making light weight bullet proof vests.
(ii) Nomax: It is also a polyamide made from m-phthalio acid and m-phenylenediamine (1, 3-benznediamine). This polymer has fire resistance properties and is used in preparing fireproof coats for fire fighters. It is also used in clothing for astronaughts and race-car drivers.
(iii) Lexan: It is a polyester and is obtained by condensation of diethyl carbonate and bi-phenol -A. It is used for making of crash helmets, bullet proof windows etc, because of its unusually high impact strength.
(iv) Super Glue: It is polymer of a-cyanoacrylate and possess high tensile strength. One drop of super glue can support a mass of 2,000 1bs. (1bs = pound, 1 pound = 0.454 kg).
(v) Ebonite : It is highly vulcanised rubber having about 20-30% of sulphur.
(vi) Polyurethanes: These are condensation polymers of toluene – 2, 4-diisocyanate and ethylene glycol. During polymerisation a low boiling liquids such as Freon-11 are added to reaction mixture. The heat liberated during polymerisation causes vaporisation of low, boiling liquid producing bubbles which converts viscous polymer to form polyurethane foams.
Resins and Plastics:
(i) Resins: Resins are amorphous organic solids or semisolids which usually have a typical metallic lustre and are often transparent or translucent. The common examples are shellac secreted by lac insect.
(ii) Plastic: A plastic is a substance which is capable of being moulded whereas a resin lacks this property. Typical plastic on reacting give mobile melts. Plastics usually have much higher molecular mass than resin. Never the less, this differentiation is not very clear and the two terms are obten used inter changeably.
Artificial silk or rayon:
The synthetic fibres which are manufactured from cellulose are known as rayon. It is manufactured by a number of different process such as
(i) Cellulose nitrate process
(ii) Cellulose acetate process
(iii) Cuprammonium process
(iv) Viscose process
Among these processes the most commonly used method for preparation is the viscose process and the rayon obtained by this method is called viscose yarn or viscose rayon.
Molecular Mass/Weight of Polymers:
Different polymers/macromolecules have different degree of polymerization i.e., they have different chain length viz the number of monomer units per molecule varies from sample to sample.
Thus, the molecular masses of the individual macromolecules in a particular sample of polymer are different.
Hence, an average value of molecular mass is taken.
In contrast, natural polymers such as proteins contain chains of identical length and hence have definite molecular mass.
In polymer chemistry the molecular mass/weight of a polymer is merely a statistical average.
Several methods are used to determine the molecular mass/weight of polymers.
(i) Number average molecular mass/weight
(ii) Weight average molecular mass/weight
(iii) Z – average molecular mass/weight
(iv) Viscosity average molecular mass/weight
Out of these average molecular masses only number – average molecular masses and weight average molecular masses/weights are commonly used.
(i) Number average molecular mass/weight :
When the total mass of all the molecules of a sample is divided by the total number of molecules, the result obtained is called the number average molecular mass/weight.
e.g., Let us consider a polymer sample and thought of it consist of monomers, dimers or polymers.
If there be N1 monomers each of mass M1, N2 dimers of mass M2 each; …. Ni polymers of mass Mi each.
Then, the mass of the sample is equal to =
=
and total number of particles in the system =
Hence, the number average molecular mass/weight of polymer is given as
, depends on number of molecules/particles present in sample polymer and is generally determined by osmotic pressure measurement.
Note: can be determined chemically by end group analysis method or physical methods based on the number particles present i.e., by use of any colliquative property.
Experimental determination:
By osmotic pressure method:
A solution of polymer (1g/1 lr) is prepared. A series of solutions of known concentrations (C) are prepared from this solution by diluting with a suitable solvent.
• The osmotic pressure (π) of each of the solutions is measured.
• The value of π/C (where ‘π’ is in cm and c is in g/litre) is plotted against C. A straight line is obtained, which is extrapolated to zero concentration.
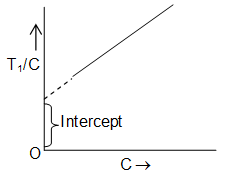
The value of π/C at C = 0 is noted as (π/C)O
(ii) Weight average molecular mass/weight:
When the total mass of groups of molecules having different molecular masses are multiplied with their respective molecular masses, the products are added and the sum is divided by the total mass of all the molecules, the result obtained is called the mass average molecular mass .
Let, there be N, monomers of mass M1 each, N2 dimers of mass M2 each; ….. Ni polymers of mass Mi each, are present in sample polymer.
Then, the total mass of the sample
=
Sum of the products of each molecular unit =
=
Hence, weight average molecular mass/weight of polymer is given as
⇒ of polymer depends on the weight of the polymer in a given volume of the polymer solution.
⇒ is generally determined by techniques like ultra centrifugation or sedimentation, viscosity measurements and scattering of light.
Illustration 5: Calculate of a polymer sample in which 80% molecules have a molecular mass of 20,000; 40% have 30,000; and the rest have 60,000.
Solution:
Illustration 6: In a particular sample of polymer, 100 molecules have molecular mass 103 each, 200 molecules have molecular mass 104 each and 200 molecules have molecular mass 105 each. Calculate the number – average and mass average molecular mass.
Solution:
Poly dispersity Index (PDI):
The ratio of weight and number average molecular masses is called Poly Dispersity Index (PDI). i.e.,
It gives the idea about homogeneity of a polymer.
Polymers whose molecules have narrow range of molecular masses are said to be monodispersed and polymers which have wide range of molecular masses are called polydisperse.
For some natural polymers, the PDI is unity
It means that such natural polymers are generally monodispersed i.e., usually consisting of monomers only and are more homogeneous.
In the case of synthetic polymers, polymeric species is always dominant over monomeric species and so that PΔi for the such polymers is greater than unityi.e., is always higher than
It means that such polymers (synthetic) are less homogeneous.
Polymers and Environmental Pollution:
In recent times, one of the common use of synthetic polymers is in the form of plastic has assumed alarmingly high levels.
Plastic is frequently used in abundance in the form of packing material and throw away bags and glasses etc (i.e., use and throw bags or glasses).
Now the plastic materials are thrown away and they are not recycled in atmosphere due to inert nature of the polymer towards biodegradation even after a long time i.e., they do not disintegrate automatically in the atmosphere. Over the period of time 1/12 they are non-biodegradable polymers.
This inertness, stability, durability of plastics mainly polythene, poses a serious waste disposal problems and create acute environmental problems. i.e., some whee responsible for global warming.
With ever increasing use of plastics and its non biodegradable nature, the entire mankind may get buried under the pile of plastic debris.
Scientist these days are carrying on researches to develop such a plastic which is capable of biodegradability over the period of time by using innovative cleaner technology and concept of green chemistry. (Based on recycling of starting materials).
Many of the country have put a ban on the use of polythene bags in daily routine.
Biopolymers:
Nature provides many polymeric species, which are essential for life and are called biopolymers, Polysacharides, proteins and nucleic acids constitute important biopolymers.
Biopolymers degrades quicklyin living systems by enzymatic chemical reaction s like oxidation or hydrolysis.
Biodegradable polymers:
Generally, synthetic plastic polymers are non-bio degradable or inert towards strong acids bases etc, in atmosphere & create environmental problems.
On the other hand biopolymer degrades quickly in living system by enzymatic chemical reactions like oxidation or hydrolysis.
At this point of time to solve the disposal problem of the polymer waste and make them eco friendly for other safe uses in human system, biodegradable synthetic polymers have been developed.
Thus, biodegradable polymers are those synthetic polymers which degrade in atmosphere by enzymatic chemical reactions (i.e., enzyme – catalysed reaction) over the period of time.
These biodegradable polymers mostly have functional groups prevalent in biopolymers and lipids.
It is noted that, to make a polymer, biodegradable we have to insert certain hydrolysable we have to insert certain hydrolysable bonds like ester linkages in the polymer chain so that these can be easily broken by the enzyme present in atmosphere.
Aliphatic polyesters are one important class of biodegradable polymers as several of them are commercially potential biomaterials.
The condensation reaction between carboxylic acid (-COOH) and alcohols (-OH) form esters. This principle was first utilized by carothers to prepare biodegradable polyester and polyamides.
Some other examples of biodegradable polymers are described below.
(i) PHBV (Polyhydroxy butyrate – CO-β-hydroxy valerate:
It is a copolymer of 3-hydroxy butyric acid and 3-hydroxy pentanoic acid in which the monomer units are connected by ester linkages.
The physical properties of PHBV vary (change) according to the ratio of both the acids (i.e., relative amounts).
3-hydroxybutanoic acid provides stiffness/toughness and 3-hydroxy pentanoic acid imparts flexibility the copolymer.
It is used in specialty packaging, orthopaedic devices and controlled drug release i.e., immensely used in the field of medicine in making capsule.
When a drug is put in the capsule of PHBV, it is released only when the polymer is degraded. This polymer has an additional advantage that it also undergoes bacterial degradation in the environment.
(ii) Poly (Glycolic Acid) and Poly (Lactic Acid):
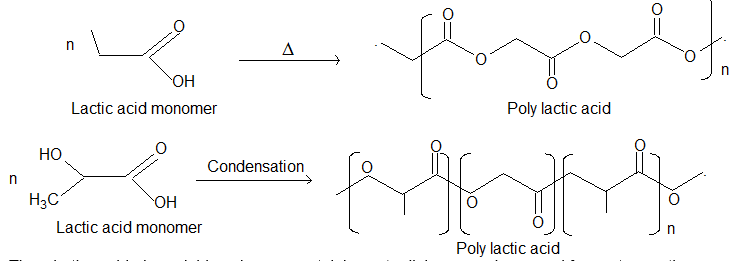
Thus, both are biodegradable polymers containing ester linkages and are used for post operative stiches.
They are commercially successful biodegradable polymers used as sutures.
Dextron was first polymer used as biodegradable materia i.e., biodegradable sutures, made from biodegradable polyesters for post operative stiches. Now, several of the synthetic biodegradable sutures have come into the market.
(iii) Nylon-2-Nylon–6: It is an alternating biodegradable polyamide copolymer of glycine and amino caproic acid.
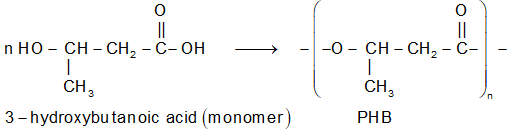
(iv) Polyhydroxybutyrate (PHB): It is obtained from 3-hydroxy butanoic acid monomer.
(v) Poly (e – caprolactone) (PCL): Poly ( – caprolactone) is obtained by chain polymerisation of the lactone of 6-hydroxy hexanoic acid.
All these 1-5 polymers are polyether and are therefore susceptible towards hydrolysis of their ester links.
Note: Copolymers of PGA and PLA (90 : 10) is used to make absorbable sutures to close an internal or external wound and has replaced catgut (thread used in stitching): These are completely degraded and absorbed by the body within 15 days to one month of the surgery.
These are also finding uses in agriculture material (such as film, seed containing, fast food wrappers, personal hygience products, etc.)
FORMULAE AND CONCEPTS AT A GLANCE
Polymer: A giant molecular with high molecular mass.
Polymerization: It is a process of forming high molecular mass substances called polymers by combining monomers.
Homopolymer: A polymer formed by the union of same type of monomers.
Co-polymer: A polymer produced by polymerizing two or more different polymers.
Addition Polymer: Polymer formed by the addition polymerization of monomers containing unsaturated bonds.
Condensation Polymer: Polymer formed by condensation of monomers containing at least two functional groups.
Elastomer: A polymer in which there are weak intermolecular forces between long polymer chains.
Plastic: A polymer in a readily deformation state which can be moulded into any shape.
Thermosetting: A polymer in powder form is fused irreversibly by heat and pressure and cannot be softened by reheating. E.g., Bakelite.
Thermoplastic: A plastic that softens or melts on heating, e.g., polyester.
Plasticizer: A substance that improves the properties of finished plastic products.
Rubber: Natural rubber is a polymer of isoprene.
Latex: A milky juice occurring in plants. It is a colloidal solution of rubber in water.
Resilience Property: The property of returning to the original shape after distortion within elastic limits.
Vulcanization: Heating of natural rubber with sulphur to make it strong.
Neoprene: Monomer of synthetic rubber, 2-chlorobutadiene.
Thiokol: Synthetic rubber containing chloroprene units.
Fibres: It is a fine thread like piece of cotton jute or asbestos.
Cotton: A natural polymer obtained as hair of the seeds of cotton.
Rayon: A regenerated textile fibre.
Silk: A natural fibre obtained from cocoons of silk works.
Wool: A condensation polymer of adipic acid and hexamethylene diamine.
Nylon: A condensation polymer of adipic acid and hexamethylene diamine.
Terylene: A condensation polymer of terephthalic acid and ethylene glycol.
Poly Dispersity Index: It is equal to the ratio Mw/Mn. For monodispersed polymers, PDI – 1 and for polydispersed polymers, PDI > 1.
Number average molecular mass is Mn and weight average molecular mass is Mw.
SOLVED PROBLEMS-1
Prob 1: What is a co-polymer? Give one example.
Sol: Copolymers are polymers formed by the union of two different monomers, e.g., Nylon – 66.
Prob 2: What are natural polymers? Give two examples.
Sol: Polymers formed in nature are called natural polymers. E.g., (i) Starch (ii) Cellulose
Prob 3: What are inorganic polymers? Give two examples.
Sol: Polymers made from inorganic molecules are called inorganic polymers. Examples are silicates and silicones.
Prob 4: What do you understand by macromolecules?
Sol: Macromolecules are long-chain organic polymers consisting of thousands of monomer units.
Prob 5: What is vulcanization?
Sol: Vulcanization is a process in which rubber is heated with sulphur. The polymer chains are cross-linked through sulphur.
Prob 6: Write the full name and formula of PVC.
Sol: PVC stands for Polyvinyl chloride. It is an addition polymer with the formula:
Prob 7: Name the monomer of synthetic rubber.
Sol: Chloroprene,
Prob 8: What is the full name of PMMA? Write its uses.
Sol: Full name of PMMA is poly-methylmethacrylate. It is used for making lenses, aircraft windows, etc.
Prob 9: What is a polyester? Give two examples of polyester polymers.
Sol: Polymers having ester linkages are known as polyesters. Examples are glyptal and dacron.
Prob 10: What do the abbreviations stand for PVC, PTFE, PMMA?
Sol: PVC – poly-vinyl-chloride
PTFE – Poly-tetra-fluoroethylene
PMMA – Poly-methyl methacrylate
SOLVED PROBLEMS-2
Prob 1: Synthetic polymer prepared from caprolactum is known as
(A) Nylon 610 (B) Teflon (C) Terylene (D) Nylon – 6
Sol: (A)
Prob 2: Which one of the following pairs is not correctly matched?
(A) Terylene – condensation polymer of terephthalic acid and ethylene glycol
(B) Teflon – thermally stable cross linked polymer of phenol and formaldehyde
(C) Perspex – A homopolymer of methyl methacrylate
(D) Synthetic rubber – A copolymer of butadiene and styrene
Sol: (B)
Prob 3: Ebonite is
(A) Natural rubber
(B) Synthetic rubber
(C) Highly vulcanized rubber
(D) Polypropene
Sol: (C)
Prob 4: The catalyst used in the manufacture of polyethene by Zeigler method is
(A) lithium tetrachloride and triphenyl aluminium
(B) titanium tetrachloride and trimethyl aluminium
(C) titanium oxide
(D) titanium isoperoxide
Sol: (B)
Prob 5: Which of the following is not an example of addition polymer?
(A) Polyethene (B) Polystyrene (C) Neoprene (D) Terylene
Sol: (D)
Prob 6: What is not true about polymers?
(A) Polymers do not carry any charge
(B) Polymers have high viscosity
(C) Polymers scatter light
(D) Polymers have low molecular weights
Sol: (D)
Prob 7: Teflon, styron and neoprene are all
(A) Copolymers
(B) Condensation polymers
(C) Homopolymers
(D) Monomers
Sol: (C)
Prob 8: Natural rubber is which type of polymer?
(A) condensation of polymer
(B) addition polymer
(C) co-ordination polymer
(D) None of these
Sol: (B)
Prob 9: The number average molecular mass and mass average molecular mass of a polymer are respectively 30,000 and 40,000. The poly dispersity index of the polymer is
(A) < 1 (B) > 1 (C) – 1 (D) 0
Sol: (B)
Prob 10: Which of the following is fully flourinted polymer?
(A) Neoprene (B) Teflon (C) Thiokol (D) PVC
Sol: (B)








