HALOGENS
Introduction:
The elements of group 17 are collectively known as halogens, Greek : halo – sea salt , gene – producer i.e., sea salt producer because the (first) three members occur as salts (chlorides, bromides, and Iodides) in sea water(F → super halogen). The group 17 consist of F,Cl,Br,I and as tatine. These are p-block elements as the last differentiating electron enter into p orbital of n shell. These elements have seven electrons in their valency shell and thus placed in group 17 or VIIA of periodic table.
These are most reactive non – metallic elements.
The last member of family, Astatine is radioactive (very short life) nature (artificially prepared)
Occurrence: The halogens are very reactive and therefore do not occur in the free state.
In combined state they occur as a halide ions ()
Chlorine occurs as : Sodium chloride – NaCl. (rock salt) or in sea water (NaCl mixed with etc)
Bromine occurs as : Sea water, (NaBr, KBr, MgBr2), bromine is relatively less abundant in crystal rocks than either fluorine and chlorine.
Iodine occurs as : Iodides of alkali metals in sea water. Sodium iodate in chile salt peter.
General characteristics of halogens:
1. Electronic configuration:
Element Atomic number Electronic configuration
F 9 [He]
Cl 17 [Ne]
Br 35 [Ar]
I 53 [Kr]
At 85 [Xe]
2. Physical state:
are gases, volatile or possessive liquid and iodine is volatile solid. This is due to weak inter molecular forces in halogen.
3. Atomic and Ionic radii:
The halogens have the smallest atomic and ionic radii in their respective periods due to maximum effective nuclear charge and increase regularly down the group from fluorine to iodine because new electronic shell are added step wise step.
4. Ionization energies:
The ionization energies of halogens are very high. This indicates that they have very little tendency to lose electrons. On going down the group from fluorine to astatine, the ionization enthalpy decreases. This is due to gradual increase in atomic size which is maximum for iodine.
5. Melting and boiling points:
The melting and boiling points of halogens increase with increase in atomic member as we go down the group.
Reason: The heat of fusion as well as heat of vapourisation also increase with the increase in atomic number. This indicates that the strength of van der Waal’s forces of attraction between the halogen molecules increases down the group, hence their mp & bp increase from F to At.
6. Electron affinities:
These elements have high value of electron affinities because they have strong tendency to accept the electrons. On moving down the group electron affinity values decrease. This is due to the fact that the effect of increase in atomic size is much more than the effect of increase in nuclear charge and thus, the additional electron feels less attraction by the large atom. Consequently, electron gain enthalpy decrease. Thus order of electron affinity is F < Cl > Br > I.
Chlorine has the highest electron affinity in the periodic table.
7. Electro negativity:
Halogens have large electronegativity values. On moving down the group electronegativity values decrease from fluorine to iodine because the atomic size increases and the effective nuclear charge decreases. It is to be noted that fluorine is the most electronegative element in the periodic table.
8. Colour:
All the halogens are coloured. The colours of different halogens are given below :
|
Halogen |
Fluorine |
Chlorine |
Bromine |
Iodine |
|
Colour |
Pale yellow |
Greenish Yellow |
Reddish brown |
Dark violet |
Reason: The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy level.
9. Oxidation states:
General electronic configuration of halogens is , hence, they have tendency to acquire noble gas electronic configuration either by gaining one electron to form uninegative ion or by sharing one electron with other atoms.
Thus, halogens show an oxidation state of -1 or +1, depending on whether the element combining with halogens is less electro negative or more electro negative than halogens.
Since fluorine is most electronegative element, is always shows an oxidation state of -1 and never shows any positive oxidation state.
The other elements also show positive oxidation states of +1, +3, +5, and +7, the higher oxidation states of chlorine, bromine and iodine are due to the presence of vacant d-orbitals in their valency shells.
As a result the outer s or p electrons can easily be promoted to the vacant d-orbitals.
Oxidations states of halogens:
|
Halogens |
F |
Cl |
Br |
I |
Chemical properties: All the halogens are very reaction but reactivity due to their low dissociation energies and high electron enthalpies but amongst them fluorine is the most reactive. On going down the group reactivity decreases. This is due to the decrease in electronegativity and increase in bond dissociation energy. F2 >> Cl2 > Br2 > F2
Bond dissociation enthalpy
|
Molecule |
F2 |
Cl2 |
Br2 |
I2 |
H2 |
O2 |
N2 |
|
BDE |
158.8 |
242.6 |
132.8 |
151.1 |
458 |
495 |
541 |
Reduction potentials of halogens
|
X2 |
F2 |
Cl2 |
Br2 |
I2 |
|
2.87 |
1.40 |
1.09 |
-0.3 |
Order of oxidizing power is F > Cl > Br > I.
Some important chemical reaction of halogens are discussed below
1. Formation of hydrides (Covalent):
a) By the action of hydrogen:
Halogen combine with hydrogen to form volatile halides
i)
This reaction takes place even in dark & highly energetic.
ii)
This reaction is slow is dark but fast in sun light
iii)
This reaction does not take place at room temperature but takes place at in sunlight.
iv)
This reaction takes place in the presence of Pt catalyst at and is reversible change.
b) By the action of water:
The solubility in water decreases from fluorine to Iodine.
* Fluorine decomposes water very vigorously and form and (ozonized oxygen)
* Chlorine dissolves in water giving the solution known as chlorine water. A fresh solution of chlorine water contains HCl and HOCl.
HOCl being unstabl, dissociates to give nascent oxygen
HOCl → HCl + [O]
* Bromine is not freely soluble in water but when allowed to stand it the gives bromine water.
* does not react with water at ordinary temperature or dissolve in water.
This is attributed to positive free energy change (+ΔG)
* On the other hard HF, HCl & HI can be manufactured as follows.
HBr & HI cannot be prepared satisfactorily by the treatment of metal bromides or iodides with conc. because HBr & HI formed are oxidized by and the product is contaminated with the respective halogen. The difficulty can be solved by using a non-oxidizing acid like .
* HI can also manufactured by the reaction of HI with hydrazine.
Some characteristics of hydrides:
i) Physical nature: Except HF, all other hydrides Viz HCl, HBr, HI are gases. HF is a liquid. (BP =19C) because of intermolecular hydrogen bonding i.e. it exist as a associate molecule (HF)n.
-H-F……………..H-F……………….H-F………….H-F………………
ii) Acidic strength:
In gaseous state hydrides of halogens are covalent in nature but in aqueous solutions they ionize and act as acids the acidic strength of these acids increase from f to I. Thus order of acidic strength is.
HF < HCl<HBr < HI
Therefore, HF is the weakest acid and HI is the strongest acid among these halogen hydrides.
Reason:
* On the basis of electronegativity, the above order of basic strength should be reversed.
* Fluorine is the most electronegative halogen; therefore, the electronegativity difference will be maximum in HF and should decrease gradually down the group. Thus, HF should be more ionic in nature and consequently it should be strongest acid. But it does’t happen.
* Although many factors contribute towards the relative acidic strengths, the major factor is the bond dissociation enrgy, which decreases from HF to HI
|
Halide |
HF |
HCl |
HBr |
HI |
|
Bond dissociation energy (kJ mol-1) |
574 |
432 |
363 |
295 |
Hence, strength of H-X, bond decreases as HF > HCl > HBr > HI
* Since H – I bond is weakest, it can be easily dissociated into H+ and I– ions, therefore, HI is the strongest acid.
* On the other hand H-F bond is strongest, hence it is the weakest acid among all the halogen acids.
III. Reducing character:
* The reducing character of these hydrides decreases down the group. It is because of the strength of H-F and decreases from HF to HI
* Lower is the strength of bond greater is the ease of cleave of H-F bond and hence greatehr is the reducing character.
* Thus, the order of reducing character is
HF < HCl < HBr < HI (strongest reducing agent)
1. Thermal stability:
* Thermal stability of these hydrides decrease from HF to HI.
* HF is most stable where as HI is least stable eg. HF & HCl are stable upto 1500K while HBr dissociates to the extent of 10% and HI is dissociated to the extent of 20% at 700 K
* The decrease in stability of the hydrides is due to decrease in bond strength which decrease when we go down the group.
2. Formation of Halides:
* The halogens, in general, react with almost all the metals and non metals except He, Ne and Ar to form a wide variety of binary halides.
* These may either be simple or complex molecules which differ from each other in their stoichiometrics and hence in structures as well.
i) Halogens on reaction with metals having low I.E such as Na, K, Mg etc form ionic halides of high mp & bp. As the EN of the halogens decreases down the group the ionic charcter decreases as (more ionic) MF > MCl > MBr > MI. (less ionic)
ii) Halogens on reaction with metals having high IE. Such as Sb, Sn, Pb etc form covalent halides.

iii) Halogen on reaction with non metals such P, As and S readily form covalent halides such as, etc.
iv) On reaction with NaOH they form different compounds.
form either hypohalites or halites in addition to the halide.
Eg.
In this reaction oxidation state of halogen changes from `0’ to -1 and +1 states.
In this reaction oxidation state of chlorine changes from `0’ to -1 and +5 states.
3. Formation of Oxides:
* Halogens forms many compounds with oxygen (indirectly) but most of these are unstable.
* Fluorine forms two oxides which are called oxygen fluorides.
* The compound of oxygen with fluorine are called as fluorides because fluorine is more electronegative then oxygen.
e.g. Oxygen difluoride is prepared by the action of 2% NaOH solution.
* On the other hand oxides of Cl, Br and I are called oxides. They form oxides from -1 to +7 oxidation states.
* Chlorine form five oxides .
|
Oxidation state |
Fluorene |
Chlorine |
Bromine |
Iodine |
|
-1 |
OF2, O2F2 |
– |
– |
– |
|
+1 |
– |
Cl2O |
Br2O |
|
|
+2 |
– |
– |
– |
– |
|
+3 |
– |
Cl2O3 |
– |
– |
|
+4 |
– |
ClO2 |
BrO2 |
I2O4 |
|
+5 |
– |
– |
– |
I2O5 |
|
+6 |
– |
Cl2O6 |
– |
– |
|
+7 |
– |
Cl2O7 |
– |
– |
* All the oxides are powerful oxidizing agents (unstable) and decompose explosively when subjected to mechanical shock or heat.
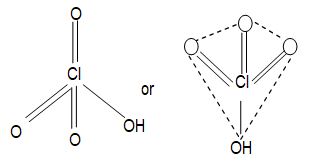
Structures of oxides of halogens: The structures of are all related to a tetrahedron with two positions occupied by lone pairs of electrons.
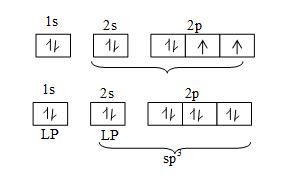
Repulsion between the lone pairs reduces the bond angle in from the tetrahedral angle of to
In ( and presumably ) the bond angle is increased because of steric crowding of the larger halogen atoms.
4. Formation of Oxoacid: Four series of oxiacid are known. Fluorine forms one oxyacid, hypofluorous acid (HOF). Chlorine bromine and Iodine form four, series of acids with the formula HOX, HXO2, HXO3 and HXO4.
Oxoacids of halogens:
|
Oxoacids |
Oxidation state Fluorine |
Chlorine |
Bromine |
Iodine |
|
|
Hypohalous acid |
+1 |
HOF |
HOCl |
HOBr |
HOI |
|
Halous acid |
+3 |
– |
HClO2 |
– |
– |
|
Halic acid |
+5 |
– |
HClO3 |
HBrO3 |
HIO3 |
|
Perhalic acid |
+7 |
– |
HClO4 |
HBrO4 |
HIO4 |
i) Hypo halous acid (HoX):
a) HOF is a colourless unstable gas.
Prep :
Prop : i) It is unstable gas and decomposes on its own to HF &
ii) It is a strong oxidizing agent and oxidize . (Quite readily).
iii) The compounds containing – of group are also act as oxydising agent i.e.
etc.,
b) HOCl, HOBr and HOI are weak acids (not very stable) and are known in aqueous solutions.
Prep : (i) They can be formed in aqueous solutions by disproportionation of the halogen water. ( X = Cl, Br, I). Order of reactivity of halogen towards is Cl > Br > I
(ii) They can be prepared by shaking the halogens with freshly precipitated HgO in water
e.g..
Hypochlorous acid is the most stable.
⇒The salts of these acids are known as hypohalites
e.g. bleaching power (CaO Cl2) is a common example of this category.
The structure of HOCl and HOF along with structural parameters are shown below
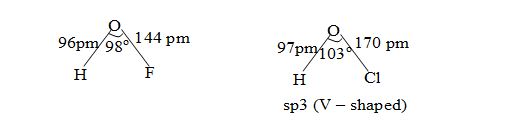
ii) Halous acids (HXO2):
• Only chlorous acid (HClO2) is known and only exist in solution.
• It is a weak acid but is stronger than HOCl
Prep :
Prop : Salt of are called chlorites and are made either from .
Chlorites are used as bleaches and are stable in alkaline solution even when boiled.
In acidic solution they disproportionate particularly when they heated
iii) Halic acids:
• are known in solution but iodic acid (HIO3) exist as a white solid.
• The stability of these acids increases with increase in atomic number of the halogen.
Prep: can be prepare by oxidizing with concentrates are made by treating the barium helates with .
Prop : These acids act as strong oxidizing agent eg these oxygen halides, oxidise to give halogens. in acid medium
• The salts of these acids are called as halates.
• Amongst the halates, sodium chlorate and potassium chlorate are prepared on industrial scale
e.g.
• Chlorates and bromates decompose on heating but the way they decompose is complex and is not fully understood.
• may decompose in two different ways depending on temperature.
Where as on heating decomposes to .
Chlorates are much more soluble than bromates & iodates. Chlorates are used to makes firework and match boxes. Sodium chlorate is widely used as a powerful weedkiller. Sodium chlorate has been used by terrorists in making bombs.
iv) Perhalic acid (HXO4): Perchloric, periodic acids as well as their salts like perchlorates and periodates are known to exist :
The perhalates (MXO4) are prepared by the electrolytic oxidation of halates.
Pt (anode) gives high oxygen over potential & thus provent electrolysis of water.
Perchlorates could be prepared fro the disproportionation of chlorates.
The disproportionation of is unfavourable therefore per bromates are obtained only by oxidation of in basic solution.
Acidic character of oxoacids:
Case I: If same halogen is present then acidic strength of oxyacids increases on increasing the number of oxygen atoms i.e.
Case II: If different halogens are present then acidic strength of oxyacids decreases with increase in atomic number of halogen. i.e., HClO > HBrO > HIO
Now the charge stabilizationis minmum
Structural aspects of oxy acids
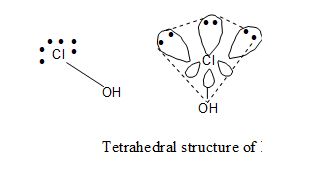
i) Hypo Chlorous acid (HClO): EC of Cl – 1s2 2s2 2p6 3s2 3p5.
`Chlorine’ undergoes hybridization. There fore its shape is tetrahedral with 3 lone pairs. It has no π bond:
ii) Chlorous acid (HClO2): Chlorine – undergoes There fore its shape is tetrahedral with 2 lone pair (or it is a angular molecule. It has one pπ-dπ bond.

iii) Chloric acid (HClO3):
Chlorine undergoes hybridization therefore, its shape is tetrahedral with one lone pair. It has two pπ-dπ bonds.
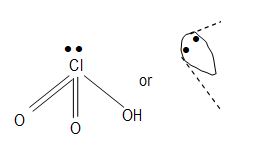
iv) Perchloric acid (HClO4): Chlorine– undergoes. Therefore, its shape is tetrahedral and it has no lone pair. It has three pπ-dπ bonds.
Oxoacids of Chlorine & their structural parameters:
|
SNO. |
Acid |
Anion formed |
Cl-O distance |
L O Cl O in anion |
Cl – O bond energy in kj mol-1 |
|
1. |
HClO |
OCl– |
1.70 Aº |
– |
209 |
|
2. |
HClO2 |
1.64 Aº |
111º |
245 |
|
|
3. |
HClO3 |
1.57 Aº |
106º |
244 |
|
|
4. |
HClO4 |
1.45 Aº |
109º |
364 |
Illustration 1: Which among the following pairs is stronger acid?
(A) HF or HCl (B) HIO or HBrO (C) HI or HCl (D)HClO2 or BrO2
Solution: (A) HCl (B) HBrO (C) HClO4 (D) HI
Illustration 2: Among hydrides of halogens predict the hydride having
i) lowest boiling point
ii) highest boiling point
iii) most acidic
iv) most stable
Solution: i) HCl ii) HF iii) HI iv) HF
Illustration 3: HBr and HI reduce sulphuric acid ; HCl can reduce KMnO4 and HF reduces.
(A) (B) (C) (D) none of these
Solution: (D) HF does not act as reducing agent.
Interhalogen compounds: The compounds of one halogen with other halogen are called inter halogens or inter halogen compounds. The main reason for their formation is the large electronegativity and the size differences between the different halogens. Taking A as the less electronegative and X as the more electronegative halogen, these are divided into four types. i.e., AX, AX3, AX5, AX7 type. Less electronegative halogen (A) is always written first.
Preparation:
These inter halogens can be prepared by direct combination or by the action of a halogen on a lower inter halogen. The product formed depend upon the conditions.
Eg. (Colourless gas)
(colourless gas)
(pale yellow liq)
(colourless liq)
(unstable)
(colourless liq)
(colourless gas)
(ruby red solid)
(bright yellow solid)
Some characteristics of inter halogen compounds:
i) They are covalent compounds.
ii) They are more reactive than the constituent halogens.
It is because A- X bond is relatively weaker than X-X bond. (except F)
iii) They are strong oxidizing agent and are diamagnetic in nature.
iv) Their melting (points) and boiling points increase with the increase in the difference of electronegativity.
(ClF is thermally more stable as compared to IBr)
v) They ionize in solution or in liq state.
vi) Hydrolysis of interhalogen compounds always produces a halide ion derived from smaller halogen and oxyhalide derivd from larger halogen.
ICl + HOH → HCl + HOI
vii) They are used to fluorinate many metal oxides, metal halides and metals.
viii) Largest number of inter halogens are formed by fluorine due to its smaller size and higher oxidizing power or electronegativity.
ix) Iodine mono Chloride (ICl) is used as Wij’s reagent in the estimation of the iodine number of fats and oils. The iodine number is a measure of the number of double bonds i.e. the degree of unsaturation of the fat.
(Brown colour disappears)
The Iodine number is simply the volume (ml) of a standard solution of ICl which reacts with a fixed weight of fat.
When ICl reacts with organic compounds if often iodinates them through chlorination may occur depending on the conditions.

x) Order of reactivity :
Structure of Inter halogen compounds:
i) Structure of AX type: Linear
ii) Structure of types : Have distorted trigonal bipyramidal shapes ( sp3d hybridization) or T-shaped due to 2 lone pairs in equatorial positions. ICl3 is dimeric () and has a planar structure
iii) Structure of types:
Have distorted octahedral shapes ( sp3d2 hybridization) or square pyramidal shape due to a lone pair in one of the axial positions.
iv) Structure of types :
has pentagonal bipyramidal structure ( sp3d3 hybridization)
Polyhalide ions:
Halide ions often ract with molecules of halogens or interhalogens and form polyhalide ions.
Eg. (Poly iodide or triiodide).
Iodine is only slightly soluble in water (0.34 gl-1). Its solubility is greatly increased if some iodide ions are present in the solution.
More complex ions such as pentaiodides I5– , hepta iodide I7– and ennea iode have also been prepared.
Many polyhalides are known which contain two or three different halogens.
Eg. , . These are prepared from inter halogens by the action of metal halides.
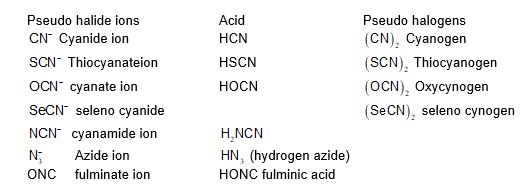
Pseudohalogens and Pseudo halide ions:
Certain uninegative ion which are made of two or more electronegative atoms containing at least one N atom, have properties similar to halide ions. They are called pseudo halide ions. They form salt similar to halide ions. The corresponding covalent dimmers of pseudo halide ions are called pseudo halogen eg:
Preparation, properties and uses of halogens:
Fluorine (Super halogen):
Occurrence: It does not occur in free state because of its high reactivity but in combined state it occurs as
i) fluor spar –
ii) Cryolite – .
iii) Fluorapatite –
Cause for late discovery of fluorine or Difficulties encountered during Isolation of fluorine: the Isolation of fluorine from its minerals was a huge challenge in chemistry.
Many unsuccessful attempt were made to isolate fluorine for over six decades the main obstacles in its isolation are
i) It attacks all the material of the apparatus used in its preparation, such as glass, platinum, carbon, water and other metals.
ii) It is strong oxidizing agent and hence no oxidizing agent can oxidize F– ions to F2 ( HF is very stable) and the reduction potential of fluorine is very high, hence, HF can not be radily oxidize to F2)
iii) It can not be prepared by electrolysis of aqueous solution of HF because electrolysis products are instead of the expected .
2HOH + 2F2 → 4HF + O2
This result is attributed to high reactivity of fluorine, therefore, it displaces O2 from water forming HF.
It also can not be prepared by electrolysis of anhydrous HF because it is not only poisonous, corrosive, and volatile but also is a bad conductor of electricity.
At last, Henary Moissan (1886) over came these difficulties and succeeded in preparation of fluorine by electrolysis of a mixture of anhydrous HF and KF in 1:12 proportions at in platinum – iridium U-tube, platinum – iridium alloy is inert to the action of fluorine.
Preparation: (i) Commercially several types of electrolytic cells are used for the preparation of fluorine. All these methods utilize the same Moissan’s principle in fluorine isolation.
ii) Dennis Method : By the electrolysis of fused sodium or potassium hydrogen fluoride (dry) using graphite electrodes.
At cathode :
At anode :
(KHF2 is known as Fremy salt)
i) Whytelaw Gray Method: It is a commonly used method.
In this method electrolysis of fused KHF2 (i.e. 1:2, KF : HF) is carried out in an electrically heated copper cell.
At cathode copper vessel :
At anode (graphide rod) :
Modern Method: In this method electrolysis of fused mixture of KF and KH is carried out in steel cell. Which surves the purpose of cathode & a graphite rod that the anode.
Properties of F2:
Physical properties:
i) It is a pale yellow gas with a pungent smell.
ii) It is heavier than air and is poisonous in nature
iii) It can be condensed to an yellow liquid (B.P = 86K) or yellow crystal (mp = 53k).
Chemical properties: Fluorine is a very reactive element and therefore reacts with a large number of substances.
i) Reaction with water: It decomposes water forming
HF being avolatile liquid fumes in air.
ii) Reaction with alkalies:
If excess of fluorine is passed through an alkali, and HF may be formed.
iii) Reaction with Metals:
Fluorine is highly reactive and directly combines with metals, including nobel metals to form fluorides. Eg. Silver burns in fluorine forming AgF. Copper and mercury form a protective layer of fluorides on metal surface. This layer of prevents further reaction of between Cu and F2, This property is taken advantage in whyt law Gray method where the electrolytic cell is made of Cu-metal.
iv) Reaction with non-metals:
Like metals, it also directly combines with non-metals (except ) to give binary compounds i.e. fluorides. Eg. Hydrogen reacts with fluorine even at very low temperature (21-23k) and in dark, giving HF.
- . This reaction shows that fluorine has a great affinity towards Hydrogen as well as the high reactivity of fluorine.
- Sulphur combines with fluorine to form SF6. (Sulphur hexafluoride)
- Halogens ( ) react with fluorine directly to form inter halogen compounds.
- Carbon on reaction with fluorine forms fluoro carbons.
- (Carbon tetrafluoride)
- The compound of carbone and fluorine are called fluoro carbons (FCs) and that of carbon, fluorine and chlorine are called Chloro fluoro carbons (CFCs) these compounds of carbon cause ozone depletion in the atmosphere :
A small number of chlorine radical make a very effective scavenger for ozone.
v) Reaction with hydrocarbons: It reacts with hydrocarbons violently.
Fluorination is carried out in presence of & catalyst copper gause.
Here is used to dilute
vi) Reaction with :
vii) Reaction with inert gases:
Heavier inert gases like and Xe are found to form several compounds with .
viii) Reaction with KHSO4 & :
ix) Reaction with metal halides:
We know that lighter halogen displaces a havier halogen. Fluorine being the lightest of all halogens, displaces all other halogens from their ionic halides.
Uses of fluorine:
Fluorine and its compounds are used in several ways
i) HF is used in etching of glass i.e. silicon present in glass reacts with HF and gives hydrofluoro silicic acid, (soluble)
ii) Fluorine is used in preparation of , chloro fluorocarbons, Teflon, cryolite and HF.
iii) is used as an electrical insulator.
iv) are used as insecticides.
v) Like Chlorocompound (i.e. DDT) a fluoro compound (i.e. DDFT) is used as fungicide.
vi) Freon is used as a refrigerant.
vii) Teflon, is used as a plastic.
viii) Fluorine is also used in separation of by forming from natural uranium.
ix) NaF is used for the fluorination of water (one part per million level fluoride in drinking water prevents tooth decay)
x) It is used in rocket fuels.
Abnormal behaviour of fluorine:
Fluorine differs considerably from halogens
Reason: Reasons are given below. It has
i) Small size
ii) Highest EN
iii) absence of d-orbitals in its valence shell.
iv) low dissociation energy for F-Fbond.
v) 2 electrons only in (n-1) shell while other halogens have 8 electrons.
Some points of difference are:
i) It exhibit only -1 oxidation state in its compounds because it is the most electronegative element of periodic table.
ii) It has lower EA as compared to chlorine.
iii) It forms hydrogen bonding with hydrogen in its compounds (i.e. hydrides). While other do not form hydrogen bonding.
iv) It combines directly with carbon while other halogens do not, even under drastic conditions.
v) Halides of fluorine have maximum ionic character than other halogens.
Eg. is ionic while is covalent compound.
Chlorine:
Discovery: It was discovered by scheele by heating HCl acid (muriatic acid) with MnO2.
Preparation:
Lab method: i) By oxidation of conc., HCl:
i) Scheele prepared chlorine in laboratory by the oxidation of HCl with MnO2
ii)
In place of HCl, mixture of NaCl and conc. can be used.
Manufacture:
i) Deacon’s process: In this process HCl (gaS) is oxidized by in presence of catalyst at 400C.
ii) Electrolytic process:
a) Down’s process: By electrolysis of fused NaCl.
b) Nelson cell process: By electrolysis of aqueous NaCl solution (Brine)
At anode: (oxidation) :
At cathode (Reduction):
If the products come into contact with one another, following reactions are likely to take place, in the electrolytic cell. Eg
Note: Nelson’s cell has such arrangement that NaOH & do not come in contact.
Properties of Chlorine:
1. Physical properties:
i) It is a greenish yellow, pungent smelling gas
ii) It is approximately 2 ½ times heavier than air.
iii) It is collected by upward displacement of air.
iv) It is poisonous in nature.
v) It is soluble in water. It’s aqueous solution is known as chlorine water which on careful cooling gives chlorine hydrate
vi) It effects the mucous membrane in the nose.
vii) It causes headache when inhaled in small quantities but it may prove fatal if taken in large quantities.
viii) It can be condensed to an yellow liquid and at 172K it solidified to a pale yellow solid.
ix) It boils at 239 k.
Chemical properties:
i) Reaction with water:
Chlorine is soluble in water the aqueous solution of chlorine is known as chlorine water. A fresh solution contains HCl and HOCl
The Chlorine water is a strong oxidizing agent because hypochlorous acid is unstable and decomposes into HCl and nascent oxygen.
HOCl → HCl + [ O]
Coloured matter + [O] → colourless metter
The bleaching action of chlorine is due to oxidation, hence it is permanent.
The water acts as an ink remover.
ii) Reaction of hydrogen:
They combine explosively but in presence of charcoal catalyst combination is smooth at room temperature.
iii) Reaction with alkalies:
iv) Reaction with Metals:
Active metals combine with to give the respective metal chlorides.
e.g.
How ever, a metal can form more than one Chloride, in such condition a metal chloride in which the metal possess highest stable oxidation state is produced by direct union of element.
e.g.,
Copper reacts with chlorine to give copper (II)
Chloride and does not copper (I) chloride.
Similarly Iron produces Iron (III) Chloride and does not Iron (II) Chloride.
v) Reaction with non – metals:
vi) Reaction with ammonia:
vii) Displacement Reactions:
the reverse reactions do not take part.x
viii) Reaction with and NO:
ix) Reaction with hydrocarbons:
Saturated hydrjocarbons undergo substitution reactions in sum light or U.V. light
Unsaturated hydrocarbons like alkenes, alkynes, readily undergo addition Leactions.
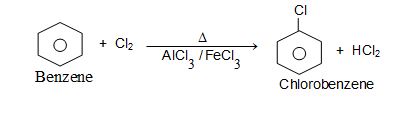
Benzene gives Chlorobenzene with Chlorine in presence of Lewis acid like etc.,
Oxidising properties:
It oxidizes:
It oxidizes to Sulphur
It oxidizes sodium sulphite to sodium sulphate
It also oxidizes thiosulphate to sodium sulphate.
Uses of Chlorine: It is used
i) As a bleaching agent for pulp, rayon, cotton or linen and Chlorine.
ii) As disinfectant for sterilization of water because it kills bacteria.
iii) In the presence of solvents (like) ,ethylene dichloride, refrigerants, insecticides (like DDT), plastics (PVC) and rubber etc.
iv) In extraction of metals like gold and platinum.
v) In the manufacture of poisonous gases like mustard gas , Phosgene (COCl2), teargas [CCl3 NO2]. These gases are used in Warfase.
vi) In the production of HCl, PCl3, PCl3, PCl5, NaOCl, CaOCl2 and enchlorine (Cl2+ClO2)
(2 KClO3+4HCl → 2KCl + Cl2 + ClO2 + 2H2O
Uses of Bromine: It is used
i) In the preparation of ethulene bromide which is used as an additive to leaded petrol.
ii) To make AgBr for photography
iii) In preparation of NaBr and KBr which are as
Bleaching powder: CaOCl2 (Calcium Chlorohypochlorite, Chloride of Lime):
Bleaching powder is the calcium salt of hypochlorous acid. It may be represented by the formula . Actually it is a mixture of and basic calcium chloride,
Manufacture: Bleaching powder is manufactured by slowly moving forward dry slaked lime through a series of shelves (provided with rotating rakes) against a current of chlorine (Bachmann or Modem method).
Properties:
A) Physical:
(i) Bleaching powder is a very light yellow coloured powder having a strong smell of chlorine.
ii) It is soluble in cold water; the part which is not dissolved is lime, . It aqueous solution confirms the presence of calcium, chloride and hypochloric ions.
B) Chemical: Reaction with insufficient dilute acids.
On account of evolution of nascent oxygen, it acts as an oxidizing and bleaching agent.
a) Oxidising reactions:
i)
ii)
iii)
iv)
v)
vi)
vii)
b) Bleaching action:
Coloured matter + [O] → Colourless oxidized product.
2. Reaction with excess of dilute acids carbon dioxide.
The amount of chlorine obtained from a sample of bleaching powder by treatment with excess of dilute acid or carbon dioxide is called available chlorine. More the available chlorine in bleaching powder better is its quality; a good bleaching powder contains 35-38% of available chlorine. The amount of available chlorine may be estimated volumetrically by arsenite and iodometric methods.
3. Decomposition: Although bleaching powder has 55.9% of chlorine (71/127 x 100 = 5.9%), whole of it is not available for reaction. This is because of the fact that bleaching powder, on standing, undergoes slow auto-oxidation to form a mixture of calcium chlorate and .
or
The so formed do not have available chlorine and hence are responsible for decreasing the percentage of available chlorine in bleaching powder*
Bleaching powder also decomposes in the presence of cobalt chloride (catalyst) to liberate oxygen.
Uses: Bleaching powder is used.
i) Mainly as bleaching agent for cotton, linen and wood pulp. However, it can’t be used for bleaching delicate articles like straw, silk and ivory because these are injured by chlorine.
ii) for sterilizing drinking water.
iii) in the manufacture of chloroform.
iv) for making wool unshrinkable.
v) as an oxidizing agent in chemical industries.
vi) Bleaching property:
Coloured matter + [0] → colourless matter:
Uses of bleaching powder: It is used
i) As a disinfectant and germicide especially in the sterilization of drinking water.
ii) As a bleaching agent
iii) In the preparation of Chloroform
iv) In making wool unshrinkable
v) As an oxidizing agent and chlorinating agent in industry.
Illustration 4: Iodine is more soluble in KI than in water why ?
Solution: Iodine combines with KI to form the soluble complex, .
Illustration 5: Chlorine bleaches a substance permanently but SO2 does it temporarily why ?
Solution: Cl2 bleaches by oxidation white SO2 does it by reduction. The reduced product gets oxidized again and the colour returns.
Illustration 6: ClF3 exists whereas FCl3 does not why?
Solution: EC of Cl = .
An electron from 3p can jump to 3d orbitals. So, it can show an oxidation state of 3 and combine with the more electronegativity fluorine.
EC of F =
In fluorine there is no d-orbital available for oxidation of electron.
Moreover it is most electronegative element So, it shows as oxidation state of -1 only.
Therefore ClF3, exists whereas CCl3 does not.
Illustration 7: Why do noble gases form compounds with fluorine and oxygen only ?
Solution: The EN of both F & O is very high. More over F is highly reactive element.
FORMULAE AND CONCEPTS AT A GLANCE
1. Tincture of iodine is a mixture of and KI dissolved in rectified spirit.
2. Iodex ointment contains iodoform which liberates slowly.
3. Freons are Chlorofluoro Carbons (CFCs). Eg. Freon-11 is CCl3F, Freon 12 is CCl2F2. These are marketed under the popular brand names such as Freon and Genetron and used as refrigerant.
Freon – 113, 114 and 115 are also useful in industry. All these compounds cause ozone depletion.
4. Chlorine gas is collected by upward displacement of air as it is heavier than air.
5. Iodine is purified by sublimation,
6. Halons – These are fluoro chloro bromo carbons used as fire fighting in tanks and armoured personal carriers eg. Halon -1211 is CF2ClBr, Halon – 1301 is CF3 Br, Halon -2402 is C2F4 Br2
7. Aqueous solution of NaOCl is called Javelle water and is used as a bleaching agent.
8. The dazaling fireworks and flares observed during Diwali or other festival time contain a mixture of KClO3 as the oxidizer and small quantities of salts of Sr to give red colour, Ba to give green colour and Cu to give blue colour as colour producing substances.
9. Hypohalites disproportionate in aqueous solution on heating to produce halates. This reaction is facilitated in the basic medium.
The rate of disproportionation increases in the order
10. In case of oxoacids of chlorine order of acidic strengths & thermal stability is
Order of oxidizing power is
Order of strengths of conjugate base of oxoacids of Chlorene is
Order of stability of these anions is
11. Order of oxidizing power of perhalates
12. HF is more reactive and corrosive than HF.
13. In case oxides of Chlorine order of oxidizing power is
Order of stability is
14. Incase of hydrogen halides (HX). Order of boiling point is HCl < HBr < HI < HF
Order of volatility is HCl > HBr > HI > HF
Order of thermal stability, dipole moment, & bond strength is HF > HCl > HBr > HI
Order of acidic strength & reducing character is HF < HCl < HBr < HI
Order of acidic strength & reducing character is HF < HCl < HBr < HI
15. HI is least stable of all hydrogen halides and decomposes to and . This is the reason that a bottle containing HI acquires brown colour due to after some time.
16. HF is not stored in glass vessels since it reacts with of glass. It is stored in copper, Wax, polythese or gutta-percha.
SOLVED PROBLEMS-1
Prob 1. HCl is not used to make the medium acidic in titrations involving potassium permanganate. Explain.
Sol: KMnO4 is a stronger oxidizing agent than Cl2 and in solutions containing HCl it oxides Cl– to Cl2 gas. Hence, it is not used for acidification of KMnO4.
Prob 2. Iodine does not form compounds with Xe. Explain.
Sol: Iodine is weakly electronegative and for, thus, reason does not form compounds with xenon.
Prob 3. Halogens are strong oxidizing agents. Explain.
Sol: Halogens have a great tendency to accept an electron in their valence-shells and form the halide ions. So, they are very strong oxidizing agent.
Prob 4. HF is a weak acid despite the fact that F is the most electronegative element. Explain.
Sol: HF is a weak acid due to the following reasons: (i) Association of HF molecules due to strong H-F bonding in HF (ii) The strength of H-F bond.
Prob 5. HF is stored in wax-coated bottles. Why?
Sol: It is stored in wax-coated bottles because it reacts with silica of glass to give water soluble acid H2SiF6.
SiO2 + 4HF SiF4 + 2H2O
SiF4 + 2HF H2SiF6
Prob 6. The brown colour of an acidified dilute solution of iodine in aqueous KI is intensified by the addition of a nitrite but is discharged by the addition of sulphite. Explain.
Sol: On the addition of a nitrite, I– is oxidized to I2, thus, the colour of the solution is intensified. The addition of sulphite reduces free I2 to I– and, hence, the colour is discharged.
Prob 7. HCl is covalent but its aqueous solution is conducting. Explain.
Sol: Even though HCl is covalent is nature, its aqueous soltuion conducts electricity. This is because HCl is a strong acid and on dissolution in water dissociate completely forming H+ and Cl– ions. These ions help in the electrical conduction.
Prob 8. IF5 has zero dipole moment. Explain.
Sol: IF5 has an octahedral structure with one position occupied by a lone pair. Thus, the individual dipole moments cancel each other out resulting is a zero dipole moment for the IF5 molecule.
Prob 9. Arrange the following in decreasing order of property indicated.
(i) F2, Br2, Cl2, I2 – bond energy and oxidizing power
(ii) HF. HCl. HBr. HI – acid strength
(iii) HCl. HBrO. HIO – acid strength
(iv) HClO4.HClO3.HClO2.HClO – acid strength
(v) M–F. M–Cl, M–Br, M–I – ionic character of the bond
(vi) CIO4–, BrO4– – oxidizing power
Sol: (i) (a) Decreasing bont energy : F2 < Cl2 > Br2 > I2
(b) Decreasing oxidizing power : F2 > Cl2 > Br2 > I2
(ii) Decreasing acid strength : HI > HBr > HCl > HF
(iii) Decreasing acid strength : HClO > HBrO > HIO
(iv) Decreasing acid strength : HClO > HBrO > HIO
(v) Decreasing ionic character of the ionic bond : M-F > M-Cl > M-Br > M-I
(vi) Decreasing Oxidising power :
Prob 10. (a) Why does fluorine not form polyfluorides?
(b) Give the geometry of the following molecules: ClF2, BrF5 and IF7.
Sol: (a) Fluorine does not form polyfluorides because its valence shell contains only one half filled orbital and fluorine is short of only one electron to complete its octet. Moreover, there is no provision for the d-orbitals in the second energy state. Thus, fluorine cannot expand its octet and it cannot form polyfluorides.
(b) (i) ClF3 : Trigonal bipyramidal with two positions occupied by lone pairs.
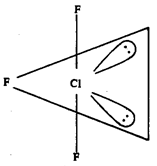
(ii) BrF5 : Octahedral with one position occupied by lone pair.
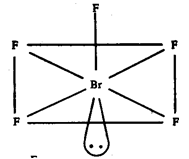
(iii) IF7 : Pentagonal bipyramidal structure
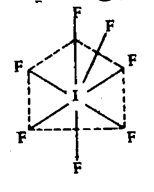
SOLVED PROBLEMS-2
Prob 1. The atomic number of astatine which belongs to six period is
(A) 53 (B) 54 (C) 85 (D) 86
Sol: The atomic number of astatine is 2 + 8 + 8 + 18 + 18 + (14 + 17) = 85
Prob 2. Which of the following orders of electron affinities of halogens is correct?
(A) F < Cl < Br (B) F > Cl > Br (C) F > Cl < Br (D) F < Cl > Br
Sol: Because of small size (electrons held in small volume), the electron affinity of F is smaller than Cl, which, in turn, has higher electron affinity than Br.
Prob 3. Which of the following orders of bond dissociation enthalpy of halogens is correct?
(A) F – F > Cl – Cl > Br – Br
(B) F – F < Cl – Cl < Br – Br
(C) F – F > Cl – Cl < Br – Br
(D) F – F < Cl – Cl > Br – Br
Sol: F-F bond is the strongest, then comes Cl – Cl and finally Br – Br.
Prob 4. Which of the following halogens does not exhibit positive oxidation state?
(A) F (B) Cl (C) Br (D) I
Sol: F is the most electronegative atom.
Prob 5. Which of the following orders of melting point of hydrides of halogens is correct?
(A) HF > HCl > HBr
(B) HF < HCl < HBr
(C) HF > HCl < HBr
(D) HF < HCl > HBr
Sol: Because of hydrogen bonding, HF has higher melting point than HCl, which, in turn, has smaller melting point than HBr.
Prob 6. Which of the following does not contain fluorine?
(A) Fluorspar (B) Cryolite (C) Fluoroapatite (D) Chile salt peter
Sol: Chile saltpeter is NaNO3.
Prob 7. Which of the following statements is not correct?
(A) Binary compounds of fluorine and oxygen are known as oxygen fluorides and not fluorine oxides.
(B) For a metal exhibiting more than covalent than the one in the higher state.
(C) Halogens do not occur freely in nature
(D) Most of binary compounds between oxygen and halogens are unstable
Sol: The halide in the lower oxidation state are less covalent than the one in the higher.
Prob 8. Which of the following statements is not correct?
(A) All halogens except fluorine form oxo-acids
(B) Hypohalous acids HOCl, HOBr and HOI are all strong acids
(C) Hypochlorous acid is the most stable among hypohalous acids
(D) Chlorous acid is the only halous acid known
Sol: Hypothalous acids are all weak acids.
Prob 9. The anhydride of HClO4 is
(A) ClO4 (B) Cl2O7 (C) ClO2 (D) ClO3
Sol:
Prob 10. Which of the following molecules is not paramagnetic?
(A) ClO2 (B) ClF3 (C) ClO3 (D) BrO2
Sol: ClF3 contains even number of electrons whereas rest of the three contain odd number of electrons.








