GROUP 15 ELEMENTS
Elements of group 15 are called members of N-family, consists of nitrogen (N), phosphorous (P), arsenic (As), antimony (Sb) and bismuth (Bi). Nitrogen and phosphorus are non-metals; arsenic and antimony are metalloids; bismuth is metallic. Beside nitrogen, the other important element of group 15 is phosphorous. These elements are also known as pnicogens and their compounds as pnicomides.
Electronic configuration: The general electronic configuration of pnicogens is ns2np3, where n = 2 to 6.
| Element | At. No. | Electronic configuration |
| N | 7 | [He] 2s22p3 |
| P | 15 | [Ne] 3s2 3p3 |
| As | 33 | [Ar] 3d10, 4s2 4p3 |
| Sb | 51 | [Kr] 4d10, 5s2 5p3 |
| Bi | 83 | [Xe] 4f14, 5d10, 6s26p3 |
Occurrence: Nitrogen occurs in free as well as in combined state. In atmospheric air percentage of nitrogen is 75% by mass and 78% by volume. In combined state it mostly found as nitrates (i.e., chile salt petre-NaNO3, saltpeter – KNO3), proteins and amino acids while, phosphorus is an active element. It does not occur free in nature. It is the 11th most abundant element in earth’s crust. It is essential for life of both plants and animals. It is widely distributed in nature in the combined state. The main minerals of phosphorus are
(i) Phosphite – Ca3 (PO4)2
(ii) Flourapatite – 3Ca3(PO4)2.CaF2
(iii) Chlorapatite – 3 Ca3 (PO4)2.CaCl2
(iv) Hydroxyapatite – Ca5 (PO4)3(OH)
An average person has 3.5 kg of calcium phosphate in his body. Phosphorous is also present in DNA, RNA, ATP, ADP etc.
As, Sb, Bi occur as sulphides or oxides.
Minerals of arsenic:
(i) Realgar – As4S4 (red-orange)
(ii) Orpiment – As2S3 (yellow)
Minerals of antimony:
(i) Stibinite – Sb2S3
(ii) Flue dust – Sb2O3
Minerals bismuth:
(i) Bismuth glance – Bi2S3
(ii) Bismuth ochre – Bi2O3
(iii) Bismuthite – BiO2CO3
Atomic and Physical Properties:
1. Atomicity and Physical state: At room temperature nitrogen is a diatomic gas after hydrogen whereas other member of this group are solids. The physical property of these elements gradually changes from gaseous state to solid state. Hence nitrogen is a covalent molecule, which is formed by
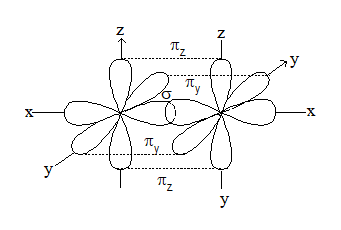
i) px – px overlap
ii) py – py overlap
iii) pz – pz overlap
Thus, in nitrogen molecule a triple bond is present between the two nitrogen atom. Therefore, one sigma bond and two pie bonds are formed by px – px, py – py and pz – pz overlapping respectively. It is inactive in normal chemical reactions due to its higher dissociation energy (i.e., 945.4 kJ). On the other hand, phosphorus is waxy solid at room temperature. It is a tetratomic molecule (P4). It has a regular tetrahedron structure having one P atom at each vertex of the tetrahedron. (∠PPP = 600). Whereas all the other elements of this group (VA) are normally solids.
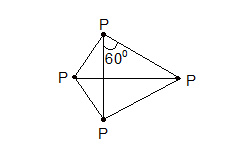
Tetrahedral structure of P4 molecule (As, Sb also)
2. Atomic radius: Atomic radii as well as covalent radii increase with increase in atomic number from Nitrogen to Bismuth.
Density increases down the group (d = m/v). Atomic No. & Atomic wt = Increases down the group (N → Bi).
3. Ionization enthalpy (kJ/mol): Ionization energy of nitrogen is very high due to small atomic radius. The ionization energy decreases down the group.
Order of
IE1 : N > P > As > Sb > Bi
IE2 : N > P > As > Bi < Sb
IE3 : N > P > As > Bi < Sb
4. Electronegativity: Electronegativity of nitrogen is 3.0 on Paulling scale. The electronegativity decreases down the group (N → Bi).
|
Element |
N |
P |
As |
Sb |
Bi |
|
EN |
3.0 |
2.1 |
2.0 |
1.9 |
1.9 |
5. Metallic character:
6. Melting points (K): Melting point of the elements of group 15 first increases then decreases down the group.
|
Element |
N |
P |
As |
Sb |
Bi |
|
MP(K) |
63 |
317 |
1089 |
904 |
544 |
7. Boiling points:
|
Element |
N |
P |
As |
Sb |
Bi |
|
BP(K) |
77 |
554 |
888 |
1860 |
1837 |
8. Catenation: All these elements show the property of catenation but to a much smaller extent than carbon, due to less M – M bond dissociation energy i.e. the catenation capacity depends on M-M bond energy. Thus, catenation capacity gradually decreases down the group.
|
Bond |
C – C |
N – N |
P – P |
As – As |
|
BE (kJ/mol) |
353.3 |
163.8 |
201.6 |
147.4 |
Thus, nitrogen forms longer chain as compared to phosphorus. e.g., hydrazine (NH2NH2) has two ‘N’ – atoms bonded together, hydrozoic acid (NH3H) has three N – atoms bonded together longer while diphosphine (P2H4) has two ‘P’ – atoms bonded together.
9. Allotropy : (Greek : allos = another, trops = shape): The property of an element to exist in two or more physical forms, having more or less similar chemical properties but different physical properties, is called as allotropy. The different form are called allotropes or allotropic modifications.
All elements of group 15 show allotropy (except Bi).
Nitrogen : α-Nitrogen, β-Nitrogen
Phosphorous : White P Red P, Scarlet P, violet P, α – black P, β – black P.
Arsenic : Grey, Yellow, Black.
Antimony : Yellow or silvery grey, explosive black.
10. Oxidation states: The elements of group 15 have five electrons (ns2np3) in their outermost shell (n)
These elements exhibit a maximum oxidation state of + 5 towards oxygen by using all the five valence electrons. Common oxidation states of these elements are + 3, + 5. Stability of + 3 oxidation state increases down the group because inert pair effect increases down the group while stability of + 5 oxidation state, decreases (down the group). Nitrogen exhibits a wide range of oxidation states.
|
Oxidation state |
Examples |
|
– 3 |
NH3, NH4+, NH2– |
|
– 2 |
NH2NH2 |
|
– 1 |
NH2OH, NH2F |
|
0 |
N2 |
|
+ 2 |
NO |
|
+ 3 |
HNO2, NO2–, NF3 |
|
+ 4 |
NO2, N2O4 |
|
+ 5 |
N2O5, HNO3, NO3 |
N and P generally exhibit -3 O.S. due to high electronegativity & small size. N forms nitride ion N3– with highly electropositive elements. P also forms phosphide ion P3– to some extent.
Illustration 1: Why does nitrogen in ammonia exhibit negative oxidation state?
Solution: The high ionization energy, high electronegativity and small size of N-atom is responsible for ‘-3’ state of ‘N’.
Illustration 2: What is the oxidation state of phosphorous in the following
(i) H3PO3 (ii) PCl3 (iii) Ca3P2 (iv) Na3PO4 (v) POF3
Solution: i) + 3 ii) + 3 iii) – 3 iv) + 5 v) + 5
Chemical properties:
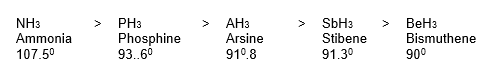
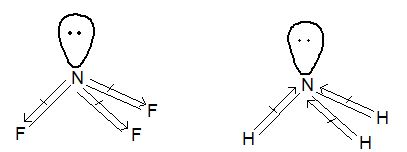
1. Formation of Hydrides: All the elements of group 15 form gaseous trihydrides of the general formula MH3, which are covalent and trigonal pyramidal in shapes.
Some properties, such as (i) ease of formation (ii) basic character (iii) solubility (iv) stability (v) dipole moment (vi) bond angle (vii) strength of M – H bond (viii) decomposition temperature, follow the order given below.
Some properties like (i) reducing character (ii) covalent character (iii) poisonous character (iv) rate of combustion, follow the order given below.
Order of MP :
Order of BP :
Methods of preparation of some hydrides:
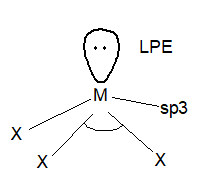
Structure of the hydrides: All these hydrides are covalent and have trigonal pyramidal structure and the hybridization is sp3.
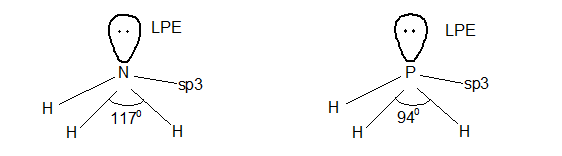
Structure of NH3 & PH3
In general, the force of repulsion between bonded pairs of electrons decreases as we move from NH3 to BiH3 and therefore, the bond angle also decreases from NH3 to BiH3.
Illustration 3: Ammonia is a good complexing agent. Explain.
Solution: Ammonia is good complexing agent because of the presence of lone pair of electrons on nitrogen. This lone pair can easily be donated to electron deficient compounds forming complexes.
Illustration 4: Among the hydrides of group 15, predict the hydride having
i) most basic character
ii) highest thermal stability
iii) lowest boiling point
iv) strongest reducing agent
Solution: i) NH3 ii) NH3 iii) PH3 iv) BiH3
2. Formation of halides: Elements of group 15 form two series of halides i.e., Trihalides (MX3) and penta halides (MX5).
Trihalides (MX3): These are formed by all elements of group 15.
These are predominantly covalent in nature (except BiF3 – ionic). Trihalides are formed by the direct reaction of elements with halogens.
Preparation: i)
ii) The trihalides are also formed when a suitable compound is treated with the halogen.
Structure: The trihalides have a pyramidal structure like hydrides and the hybridization is sp3.
Characteristics of trihalides: All the trihalides are stable except NCl3, NBr3, NI3 due to low polarity of N-x bond and a large difference in the size of nitrogen and halogen atoms. NF3 is a colourless, odourless gas and most stable trihalide. It has low reactivity.
NCl3 is a yellow oily liquid. It reacts with water to form ammonia and hypochlorous acid where as NI3 is shock sensitive and decomposes explosively when touched.
These act as a lewis base due to presence of one lone pair on the central atom. In case of trihalides of nitrogen basic character decreases from NI3 to NF3.
The trihalides of phosphorous antimony especially fluorides and chlorides act as Lewis acid also by using the vacant d-orbitals.
e.g.,
All the trihalides are hydrolysed with water except NF3 and PF3.
SbCl3 and BiCl3 are only partially hydrolysed to form oxychlorides.
Order of ease of hydrolyses: BiCl3 > SbCl3 > AsCl3 > PCl3 > NCl3.
Pentahalides (MX5):
All the elements of group 15 form pentahalides except nitrogen. Nitrogen does not form pentahalides due to the absence of vacant d-orbitals in its outer most shell. Bismuth also, does not form pentahalides because of inert pair effect.
Preparation: The pentahalides are formed by the reaction of these elements with an excess of halogens.
Structure: All the pentahalides have a trigonal bipyramidal shape with sp3d hybridization.
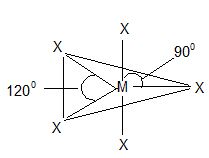
Trigonal bipyramidal shape of pentahalides.
Characteristics of pentahalides:
PF5 exists as molecule in both gaseous and solid states. PCl5 exists as molecule in the gas phase but exists as [PCl4]+ [PCl6]– in the crystalline state. PBr5 and PI5 are also exist in the ionic form as respectively in solid state.
PCl5 is used as a starting materials for a variety of organophosphorus compounds and fumes in air and acts as an effective chlorinating agent. These pentahalides completely hydrolysed and form corresponding acids.
These pentahalides are thermally less stable than trihalides, in gaseous and liquid states. i.e., In gaseous and liquid states these pentahalides are not very stable, hence they decompose into trihalides.
Illustration 5: NF3 does not have donor properties like animonia. Explain.
Solution: NF3 has a pyramidal shape with one lone pair on N-atom.
In NF3, lone pair on N – is in opposite direction to the N – F bond moments and therefore, it has very low dipole moment (μ = 0.234D). Thus, it does not show donor properties but ammonia has high dipolemoment because its lone pair is in the same direction as the N – H bond moments. Thus, it has donor properties.
Illustration 6: Compare the acidic nature of PCl3 and PCl5.
Solution: Both PCl3 and PCl5 are acidic. PCl5 is more acidic than PCl3 as the former gives phosphorous acid and later gives phosphoric acid on hydrolysis.
Oxides formation: All the elements of this group form two series of oxides i.e., trioxides (M2O3) and pentaoxides (M2O5).
Nitrogen forms several oxides in which the oxidation state varies from + 1 to + 5.
|
Oxides of N: |
||||
|
Oxides of P: |
|
P2O3 (or P4O6 |
P4O8 or (P2O4) |
P2O5 (or P4O10) more acidic |
|
Oxides of As: |
|
As2O3 |
As2O4 |
As2O5 |
|
Oxides of Sb: |
|
Sb2O3 |
Sb2O4 |
Sb2O5 |
|
Oxides of Bi: |
|
Bi2O3 |
Bi2O4 |
Bi2O5 |
Trioxides and pentaoxide are also represented as M4O6 and M4O10 respectively.
Acidic character of oxides ∝ EN ∝ O.S of element in same oxides.
In the oxides of particular elements, the acidic nature increases as the percentage of oxygen increases or as the oxidation state increases.
For example: N2O5 is most acidic while N2O3 is less acidic. Similarly, P2O5 is more acidic than P2O4 and P2O3.
Thermal stability of oxides of higher oxidation states decreases with increasing atomic number (down the group). i.e., Thermal stability, in each series decreases from N to Bi. e.g., N2O3 is most stable oxide and N2O5 is less stable.
However P2O5 is stable but As2O5, Sb2O5, Bi2O5 (least) are less stable. Pentaoxides act as oxidizing agent (except P2O5). Among these oxidise N2O5 is the strongest oxidizing agent. The oxides of N & P are chemically similar while their structures are – different.
Preparation, properties and structures of oxides of Nitrogen and phosphorus:
1) Nitrousoxide (N2O) : It is also known as dinitrogen oxide or nitrogen monoxide or laughing gas.
Prep: (i)
(ii)
Prop. It is a stable, relatively unreactive, colourless natural gas, with pleasing odour and sweet taste. It supports combustion.
Structure : N2O is a linear molecule.
Uses : Mixed with oxygen, it is used as anaesthetic i.e., usually N2O is administered to the patient put him to sleep.
2) Nitric oxide or nitrogen oxide (NO):
Prep : i)
ii)
Prop: Colourless, paramagnetic toxic gas. It is combustible and supports combustion.
i)
ii)
iii)
Structure:
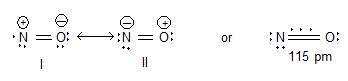
Uses:
i) As reducing as well as oxidizing agent.
ii) For manufacturing of HNO3 and H2SO4 (as catalyst in lead chamber process).
3) Nitrogen trioxide (N2O3) or nitrogen sesquioxide:
Prep : NO + NO2 → N2O3
Prop. It is anhydride of HNO2 a weak acid. It exists only as a pale blue solid, in pure state and melt to give a deep blue liquid.
Structure: 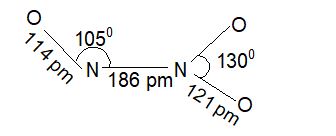
Uses: Uses are not known.
4) Nitrogen dioxide (NO2 or N2O4):
Prep.
i)
ii)
Prop. Highly toxic, paramagnetic, redish brown gas with choking odour and is very reactive. Its dimeric form N2O4 is diamagnetic in nature.
i) (It is mixed anhydride of HNO2 & HNO3)
ii)
iii) It is combustible and supports the combustion of burning P, Mg or charcoal. Burning S or candle is extinguished.
Structure : Trigonal planar.
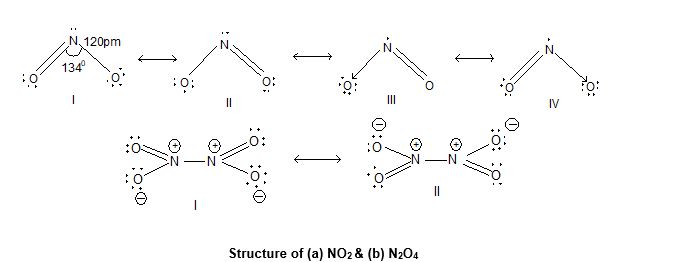
5) Nitrogen pentaoxide (N2O5):
Prep:
i) ii)
Prop:
i) It is a colourless crystalline solide and subslimes.
ii) (anhydride of HNO3)
iii)
Structure: N – O – N bonding is present.
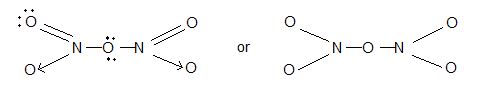
Uses : As powerful oxidizing agent.
6) Phosphorous trioxide (P2O3 or P4O6):
Prep:
Prop: White solid like wax, with garlic odour and highly poisonous.
Structure:
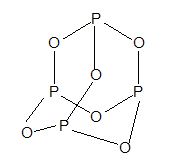
7) Phosphorous pentaoxide or flower of phosphorous (P2O5 or P4O10)
Prep:
Prop: White crystalline solid with garlic odour and sublimes.
Structure:
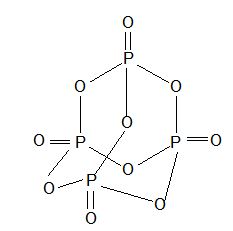
Uses : As powerful dehydrating agent.
Illustration 7: Which of the oxidizes N2O5 and P2O5 stronger dehydrating agent? Give an example for the same.
Solution: P2O5 is stronger dehydrating agent. e.g., acidic acid on heating with P2O5 gives acetic anhydride with the removal of water molecular.
4. Formation of oxyacids:
1) Oxyacids of N : Nitrogen forms, number of oxyacids which are given below :
|
S.No. |
Name |
Formula |
ON |
Nature |
|
i) |
Nitroxylic acid |
H4N2O4 |
+ 2 |
Highly explosive, difficult to get in pure state. |
|
ii) |
Nitrous acid |
HNO2 |
+ 3 |
Weak acid and unstable |
|
iii) |
Nitric acid |
HNO3 |
+ 5 |
Strong acid & stable |
|
iv) |
Peroxy nitric acid |
HNO4 |
+ 5 |
Unstable and explosive |
Nitrous acid (HNO2) and nitric acid (HNO3) are two important oxyacids of nitrogen & others are of less importance.
2) Oxyacids of P: Phosphorous form two series of oxiacids.
|
S.No. |
Name of oxyacid |
Formula |
Oxidation number |
Basicity |
|
1) |
Hypophosphorus acid series |
|
|
|
|
i) |
Hypophosphorus acid (phosphinic acid) |
H3PO2 |
+ 1 |
1 |
|
ii) |
Ortho Phosphorus acid (Phosphonic acid) |
H3PO3 |
+ 3 |
2 |
|
iii) |
Metaphosphorus acid |
HPO2 |
+ 3 |
1 |
|
2) |
Phosphoric series of acids. |
|
|
|
|
i) |
Orthophosphoric acid |
H3PO4 |
+ 5 |
3 |
|
ii) |
Metaphosphoric acid |
HPO3 |
+ 5 |
1 |
|
iii) |
Hypophosphoric acid |
H4P2O6 |
+ 4 |
4 |
|
iv) |
Pyrophosphoric acid |
H4P2O7 |
+ 5 |
4 |
|
v) |
Peroxomonophosphoric acid |
H3PO5 |
+ 7 |
3 |
The formula of oxyacids of P can be remembered as the prefix.
Meta acid – is used for the acid obtained by the less of one water molecule.
Hypo acid is used for the acid having lower oxygen content than the parent acid.
Pyroacid is used for the acid obtained by heating two molecules with lose of one water molecule.
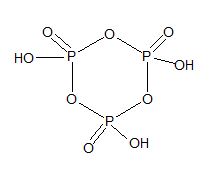 |
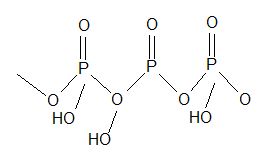 |
|
Trimetaphosphoric acid (HPO3)3 |
Polymetaphosphoric acid (HPO3)3 |
Arsenic form two oxyacids, arsenious acid (H3AsO3) and arsenic acid (H3AsO4).
Antimony forms one oxyacid H3SbO3, which exists in solutes.
Bismuth also forms one oxyacid, HBiO3, metabismuthic acid.
In same oxidation state of element, the strength and stability of oxyacid, gradually decreases with decrease in electronegativity of central atom.
Some important characteristis of oxyacids of P:
i) In general, in all these oxyacids, ‘P’ has tetrahedral shape.
ii) At least one – OH group is linked to the phosphorus atom. The hydrogen atoms of – OH groups are ionizable and responsible for acidic nature.
iii) The phosphorus series of oxyacids may have P – H bonds in addition of to P – OH bonds. The P – H bonds are responsible for the reducing properties of the acids.
iv) Phosphoric series of oxyacids do not have P – H bonds.
Dinitrogen (N2):
Prep:
(i)
(ii)
(iii)
(iv)
(v) Very pure N2 can be obtained by heating sodium or barium azides in vacuum.
Prop:
(i) It is a colourless, odourless, tasteless, slightly lighter than air, slightly soluble in water, non poisonous gas but animals do not survive in its atmosphere due to absence of oxygen.
(ii) It is incombustible and non-supporter of combustion.
(iii) It can be liquefied to a colourless liquid (BP = – 195.80C).
(iv) It is chemically inert under ordinary conditions. However it shows chemical activity under high temperatures.
a)
b)
c) It combines with metals and non-metals to form nitrides.
d)
Uses:
i) To decrease concentration of oxygen in air and make combustion less rapid.
ii) To create inert atmosphere in certain metallurgical operations.
iii) In the manufacture of NH3, HNO3, CaCN2 and other nitrogen compounds.
Active nitrogen: When an electric discharge is allowed to pass through nitrogen under very low pressure (about 2 mm) a brilliant luminiscence is observed which persists for some time after the stop page of the discharge. It is observed that nitrogen after the discharge is more active. This nitrogen is termed as active nitrogen.
Ammonia (NH3): It is the most important hydride of nitrogen.
Prep: i) By heating Ammonium salts with NaOH or Ca(OH)2 or any strong base (lab).
ii) By heating Ammonium compounds
iii) By the action of NaOH on urea
iv)
v) Manufacturing:
a) By Haber’s Process: (discovered by German chemist Fritz Haber)
This equation reveals that the reaction is a) a reversible reaction b) exothermic in forward direction and c) formation of NH3 is followed by decrease in volume. According to Le–Chatelier principle, optimum conditions required for the greater yield of ammonia are given below
i) Low temperature (450 – 5000C) and high pressure (200 atm).
ii) Catalyst: Fe is used to speed up the slow reaction and molybdenum (M0) is used as catalyst promoter.
Other catalysts employed are:
i) Finely divided Os or Cl.
ii) Finely divided Ni deposited over pumice stone.
iii) Fe(OH)3 with trace of SiO2 and K2O.
Impure gases poison the catalyst. Hydrogen and nitrogen used therefore, be free from CO, etc which can spoil the working of the catalyst.
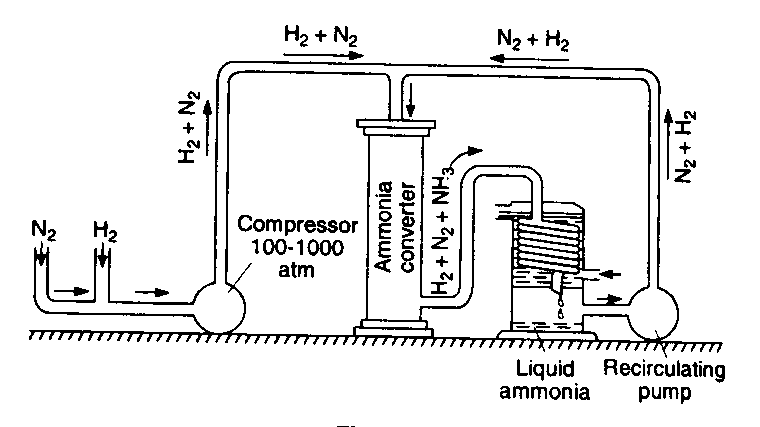
Method: Pure and dry N2 and H2 mixture in 1:3 ratio by volume is compressed to 200 to 300 atmospheres pressure. Then it passes into, a catalytic chamber. The gases, partially heated in the heat exchanger, react in the presence of the catalyst at 450 – 5000C in the chamber. Ammonia is formed to the extent of about 10% in the reaction.
b) Cynamide process:
Now a days low temperature (500 – 6000C) is used under 6 – 8 atmospheric pressure.
c) From ammonical liquor: Obtained during distillation of coal.
When coal is heated to a temperature of 1000 – 14000C in the absence of air iron retorts (destructive distillation) the following important products are formed.
i) Coal gas ii) Coaltar iii) Ammonical liquor and iv) Solid residue.
The ammoniacal liquor is treated with “milk of lime” and steam is blown through the solution. The mixture of steam and NH3 gas produced is absorbed in H2SO4. Ammonium sulphate is formed in the solution. (The salt is separated by crystallization and used directly as a fertilizer) alternately ammonia and steem mixture is passed through water under pressure to get a concentrated solution of ammonia.
Properties of NH3: i) It is a colourless gas, with a characteristic pungent odour. It brings tears into the eyes.
ii) It is lighter than air and collected by down displacement of air.
iii) It is a strong base and it is highly soluble in water due to H-bonding.
iv) It has higher M.P. and B.P. in comparison to other hydrides of V group due to H-bonding.
Chemical properties:
i) It is highly stable but decomposes on heating, into nitrogen and hydrogen.
ii) Ordinary, ammonia is neither combustible nor a supporter of combustion. However, it burn in the presence of oxygen to form nitrogen and water.
iii) It is a Lewis base in nature & forms salts with acids.
iv) It oxidise to nitrogen when passed over heated CuO or PbO.
Both chlorine and bromine oxidise ammonia.
Hypochlorites and hypobromides oxidise ammonia to nitrogen
Bleaching powder is also oxidise ammonia on warming.
Thus, ammonia acts as a reducing agent.
v) When dry ammonia is passed over heated sodium or potassium amides are formed with the liberation of hydration.
vi) Aqueous ammonia on reaction with metal salts, forms metal hydroxides and complex compounds.
vii)
Uses of NH3:
i) As refrigerant
ii) In the manufacture of sodium bicarbonate (solvay process), nitric acid (ostwald’s process) ammonium compounds e.g., – ammonium nitrate in used in certain explosives and ammonium sulphate, ammonium calcium phosphate, ammonium calciumnitrate urea etc are used as fertilizers. Urea is an excellent fertilizer of Nitrogen.
iii) As cleansing agent for removing grease.
iv) In the preparation of rayon and artificial silk.
v) As a good solvent (liq NH3) for both ionic as well as covalent compounds.
Drying of NH3: Moist NH3 can not be dried by usual drying agents like conc. H2SO4; fused CaCl2 is P2O5 as they react with NH3. Dry quick lime is useful agent to dry NH3 as it does not react with NH3.
Illustration 8: What is the function of CaCl2 in cyanamide process?
Solution: Function of CaCl2 in cyanamide process is as catalyst.
Illustration 9: How do you convert NH4NO3 into NH3?
Solution: By treating NH4NO3 with strong base like NaOH.
Illustration 10: Why conc. H2SO4 cannot be used to dry ammonia?
Solution: For drying of ammonia gas, the dehydrating agent sulphuric acid cannot be used as it reacts with ammonia as follows.
Ammonium chloride (NH4Cl): It is also known as salammoniac.
Prep.
Prop. It is white crystalline solid highly soluble in water and decomposes at a high temperature.
Uses:
1) In, soldering, tenning, making of drycells, dyeing, calicoprinting and in medicine.
2) As laboratory reagent.
Ammonium nitrate (NH4NO3):
Prep:
Prop. It is a colourless, deliquescent, explosive solid. It is highly soluble in water. On gentle heating it decomposes as
On rapid heating it explodes
Uses: For making explosives such as
Amatol:
Ammonal :
Nitrous acid (HNO2):
The free acid is unknown. It is known only in solution.
Prep:
i)
ii)
iii)
iv)
Prop: Its aqueous solution is pale blue in colour due to presence of N2O3 (anhydride).
Its aqueous solution is unstable and decomposes on heating.
It behaves as both oxidizing and reducing agent.
1) Oxidising properties:
i)
ii)
iii)
iv)
v)
2) Reducing properties:
i)
ii)
iii)
iv)
3) It reacts with anomonia to from nitrogen and water.
4) It decomposes urea and other aliphatic primary amines to nitrogen.
With aromatic primary amines, in presence of HCl at 0 to 50C, it forms diazonium salts.
Structure: Since HNO2 forms two types of organic compound, the nitrites (R – ONO) and nitro compounds (R – NO2), it is considered to be tautomeric mixture of two forms.
Uses:
i) In organic chemistry in the preparation of diazo compounds which are employed for making aniline dyes.
ii) As oxidizing and reducing agent in analytical chemistry.
iii) For the replacement of – NH2 group by – OH group in diphatic primary amines.
Nitric acid (HNO3): It is also known as aquafortis
Preparation:
1) Lab Method: By distilling nitre with conc. H2SO4.
2) Industrial Method:
a) Birk land – Eyde process – or Arc process (old):
Air free from CO2 and moisture is passed through arc at 30000C, ‘NO’ is formed; No oxidise to NO2 which is absorbed in water in the presence of air to give HNO3.
b) Ostwald’s process:
Concentration of HNO3:
Physical properties:
i) It is anhydrous colourless, syrupy, pungent liquid usually available as 68% and 15.7M.
ii) BP = 84.10C, FP = – 420C
iii) It’s aqueous solution is often yellow due to the presence of small concentration of NO2.
iv) HNO3 containing NO2, is called fuming nitric acid. It is brown (yellow) in colour and obtained by distilling conc. HNO3 with a little starch.
v) It has extremely corrosive action on skin and causes painful sores.
Chemical properties:
i) Acidic nature: It is a strong acid and in aqueous solution the ionization is virtually completed.
Thus, it reacts with basic oxides, hydroxides, carbonates, bicarbonates etc to form corresponding salts.
ii) Oxidising nature: (strong oxidizing agent)
i) Reaction with non metals: (oxidation of non-metals)
ii) Reaction with compounds: (oxidation of compounds):
iii) Reactions with metals: Armstrong’s theory: Most of the metals are attached by nitric acid except noble metals like gold and platinum. According to Aranstroug’s theory, the metals first displaces nascent hydrogen from acid which further reacts with the nitric acid to give secondary reactions.
Factors affecting the secondary reactions:
i) Nature of the metal
ii) Concentration of the acid
iii) Temperature
iv) presence of impurities
A) Reaction with metals which lies above hydrogen in electrochemical series:
i) Reactions with ‘Zn’ under different conditions:
a) With cold and very dil nitric acid.
b) With cold and dil HNO3
c) With cold and moderately conc HNO3
d) With cold & conc HNO3
ii) Reactions with iron under different conditions:
Iron is rendered passive by highly concentrated nitric acid (80%).
iii) Reactions with Tin:
iv) Reaction with lead:
v) Metals like Mg and Mn give hydrogen with dil HNO3:
Passivity: Metals like, Fe, Cr, Ni, Al or Co become inactive or passive by the action of conc. HNO3, due to stable oxide layer.
B) Reaction with metals lies below hydrogen in the electrochemical series:
i) Reaction with copper under different conditions:
a) With cold and dil HNO3;
b) With cold and moderately conc. HNO3
c) With cold and conc. HNO3
d) With hot and conc. HNO3
(Silver behaves like copper)
ii) Reactions with mercury:
a) With dil HNO3
b) With conc. HNO3
Summary:
|
Conc. of HNO3 |
Metals |
Main products |
|
Very dil. HNO3 |
Mg, Mn |
Metal nitrate + H2 |
|
Fe, Zn, Sn |
Metal nitrate + NH4NO3 |
|
|
Dil. HNO3 |
Pb, Cu, Ag, Hg |
Metal nitrate + NO |
|
Fe, Zn |
Metal nitrate + N2O |
|
|
Sn |
Metal nitrate + N2O |
|
|
Conc. HNO3 |
Zn, Fe, Pb, Cu, Ag |
Metal nitrate + NO2 |
|
Sn |
Metastanic acid (H2SnO3) + NO2 |
4) Reactions with metalloids: Forms highest oxyacids like non-metal.
5) Reaction with organic compounds:
i) Nitration:
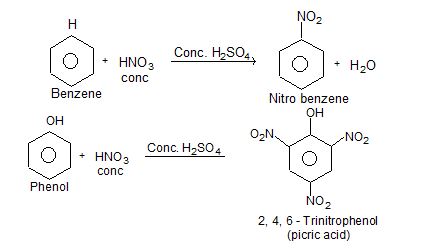
ii) Oxidation:
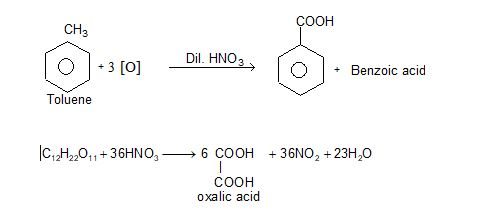
Structure:
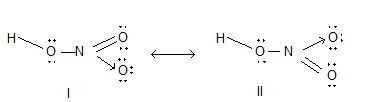
Uses:
i) In manufacturing of fertilizers & explosives like TNT, picric acid, nitroglycerine, dynamite etc.
ii) In the manufacture of artificial silk, dyes, drugs etc.
iii) In purification of silver and gold.
iv) In the preparation of aqua regia.
v) As laboratory regent.
vi) As oxidizing agent.
vii) As nitrating reagent etc.
Illustration 11: What are the anhydrides of nitrous and nitric acids?
Solution: N2O3 is the anhydride of nitrous acid and N2O5 is the anhydride of nitric acid.
Illustration 12: HNO3 acts only as an oxidizing agent while nitrous HNO2 acid can act as both an oxidising and a reducing agents. Explain.
Solution: In HNO3, N–exhibit + 5 oxidation state (maximum) whereas in HNO2, N-exhibits + 3 oxidation state, which can be raised as well as lowered. So, HNO3 can act only as an oxidizing agent while HNO2 can act as both an oxidising and a reducing agents.
Phosphorous: (P4): (Greek word, phos = light, phoro = I carry)
Extraction: Phosphorus is extracted either from phosphorite or bone ash by the application of following two processes.
i) Retort process (old process)
ii) Electrothermal process (Modern process)
i) Retort process: Bone ash or phosphorite mineral is digest with concentrated H2SO4 (60%) orthophosphoric acid is formed, which is changed into metaphosphoric acid. Metaphosphoric acid is mixed with powdered coke and distilled in fireclay retorts. The acid is reduced to phosphorus by carbon which comes in vaporized form the vapours are condensed below water.
ii) By electrothermal process (modern):
Bone-ash or powdered mineral phosphate is mixed with silica and coke and the mixture is heated in an electric furnace at 1400 to 15000C. Phosphorus vapours so evolved are passed through water then solid phosphorus is obtained
Purification: By melting under acidified solution of K2Cr2O7. The impurities are oxidized and redistilled.
Allotropic modification of phosphorus: It’s important allotropes are white and red.
White phosphorus (yellow): Freshly prepared phosphorus is colourless. On standing it acquires pale lemone colour due to formation of red variety. It is therefore called yellow phosphorus. Due to its poisonous nature the jaw bone start decay and diseases is known as “phossy Jaw”.
By heating in an inert atmosphere at 240 – 2500C, it changes into red phosphorus.
Structure:
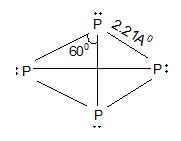
Since the hybridization of P in P4 is sp3, the % of p-character is 75%.
Uses : As rat poison.
Red phosphorus: It is an odourless, non-poisonous, dark red powder.
It is prepared by carefully heating yellow phosphorus in an inert atmosphere for about 8 days.
It changes to white (yellow) phosphorus when it is vaporized and vapours are condensed.
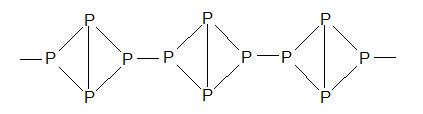
Structure: Polymeric structure.
Scarlet phosphorus: It is prepared by heating PBr3 with Hg at 2400C.
Black phosphorus: It is prepared by heating white P at 2000C. It is the most stable forms and good conductor of electricity.
Comparison between white and red phosphorus:
|
|
Property |
White P |
Red P |
|
1. |
Physical state |
Soft waxy solid |
Brittle powder |
|
2. |
Colour |
White in pure state and become yellow on standing |
Red |
|
3. |
Odour |
Garlic |
Odourless |
|
4. |
Specific gravity |
1.8 |
2.1 |
|
5. |
M.P. |
440C |
Sublimes in absence of air at 2900C |
|
6. |
Ignition temperature |
Low, 300C |
High, 2600C |
|
7. |
Solubility in water |
Insoluble |
Insoluble |
|
8. |
Solubility & in CS2, Cl4, benzene |
Soluble |
Insoluble |
|
9. |
Physiological action |
Poisonous |
Non-poisonous |
|
10. |
Chemical activity |
Very active |
Less active |
|
11. |
Phosphores cence |
Glows in dark |
Does not glow in dark |
|
12. |
Burning in air |
Forms P4O10 |
Forms P4O10 |
|
13. |
Reaction with NaOH |
Gives phosphine |
No reaction |
|
14. |
Reaction with Cl2 |
Form PCl3 and PCl5 (spontaneously) |
On heating forms PCl3 and PCl5 |
|
15. |
Reaction with hot HNO3 |
Forms H3PO4 |
Forms H3PO4 |
|
16. |
Stability |
Unstable |
Stable |
|
17. |
Conductivity |
Bad conductor |
Semiconductor |
|
18. |
Molecular formula |
P4 |
Complex polymer |
Uses of phosphorus:
i) In match industry. In match box.
side contains: Red P or P2S3 + Sand + Glue.
On tip: Red P + oxidizing agents like KClO3 or KNO3 or Pb3O4 + glass powder or chalk for friction.
ii) As rat poison i.e., yellow P4.
iii) In manufacture of fertilizers and alloys.
iv) In treatment of leukemia and other blood disorders ie., radioactive P32.
Illustration 13: Why white phosphorous is more reactive than red phosphrous?
Solution: In white ‘P’ atoms are tetrahedrally distributed with a bond angle 600 causing more strain to the molecule. In addition to it P – P covalent bonds are weak with a bond dissociation energy of 48k cal/mole. Red ‘P’ exists as polymerized P4 tetrahedral units.
Compounds of phosphorus:
Phosphine (PH3):
It was disocovered by Gengembre in 1783.
Prep:
i) By heating white P with 30 – 40% NaOH solution in an inert atmosphere. (Lab method).
ii) By the action of water or dil mineral acid on metallic phosphides:
iii) By heating phosphonium iodide with 30% KOH:
iv) By heating phosphorous acid (decomposition):
Physical properties: It is a colourless gas with the odour like rotten fish, highly poisonous slightly soluble in water.
Pure PH3 is not inflammable.
It liquefies at – 890C and solidifies at – 1340C.
Chemical properties:
i) Basic nature: It is neutral toward litmus. However it is a weak base, even weaker than ammonia, it reacts with HCl, HBr or HI to forms phosphonium compounds.
ii) Decomposition:
iii) Combustibility: Pure PH3, is not spontaneously inflammable but ordinary PH3 is spontaneously inflammable due to the pressure of P2H4.
iv) Reaction with metallic salts:
v) Reaction with chlorine: PH3 burn in the atmosphere of chlorine.
vi) Reaction with anhydrous AlCl3 and SnCl4.
Structure: It has pyramidal structure like ammonia.
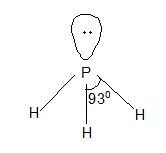
In PH3 ∠HPH = 930
In NH3 ∠HNH = 1070
Uses of pH3:
i) Holme signals: A mixture of CaC2 and Ca3P2, on reaction with water gives phosphine which catches fire and lights up acetylene. The burning gases serve the purpose of signals. These are used in ships.
ii) Smoke screen: Calcium phosphide is used in making smoke screens. PH3 obtained from it catches fire to give needed smoke.
iii) Rat poison: Zinc phosphide is used as rat poison, which gives PH3.
iv) Cellphos: Cellphos is a trade name of AlP (aluminium phosphide) and used as fumigant. In presence of moisture, it gives pH3, which kills insects and pests.
Orthophosphoric acid (H3PO4):
Prep:
i) By dissolving P4O10 in boiling water:
ii) By the hydrolysis of PCl5
iii) By heating red P with conc. HNO3 in presence of iodine catalyst:
iv) By heating calcium phosphate with 60% H2SO4:
Properties:
i) It is a transparent deliquescent crystalline solid.
ii) It melts at 42.30C
iii) It absorb water and forms colourless syrupy mass.
iv) It is highly soluble in water.
v) Heating effects:
vi) Acidic nature: It is a tribasic acid and ionise in three steps
(secondary salt)
(normal or tertiary salt)
vii) Reaction with ammonium molybdate, in presence of nitric acid : (test of
Uses of H3PO4:
i) In medicine, as a substitute for H2SO4 in the preparation of C2H4 from C2H5OH, HBr from KBr and HI from NaI.
ii) As a flowering agent in soft drinks.
iii) In making fertilizers and other phosphates.
iv) For preparing metaphosphoric acid.
v) As a stabilizer for H2O2
Orthophosphorus acid (H3PO3):
Prep:
i) By dissolving phosphorus trioxide in water.
ii) By hydrolysis of phosphorus trichloride
Prop:
i) It is colourless crystalline compound (HP = 730C)
ii) It is highly soluble in water.
iii) Acidic nature: It is a strong and dibasic acid.
It thus, forms two series of salts such as NaH2PO3 and Na2HPO3 known as primary phosphates and secondary phosphates respectively.
iv) Decomposition: (disproportionation reaction)
v) Reducing nature: It acts as a strong reducing agent the potential equation is
Uses: As reducing agent.
Illustration 14: H3PO3 is diprotic acid. Explain.
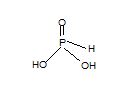
Solution: H3PO3 has three H-atoms and therefore, it is expected to be tribasic. However, in its structure, two hydrogen atoms are joined through oxygen atoms and are ionisable. The third H-atom is linked to P and is non-ionisable.
Fertilizers: These are chemical compounds prepared by artificial means which supply the three most essential elements – N, P and K to the soil.
Types of fertilizers:
Depending upon the nourishing elements N, P and K they are divided into
I) Nitrogenous fertilizers: These provides nitrogen to the plants. They are
a) Urea (NH2CONH2):
It is highly soluble in water and hence requires air tight packing. It does not alter the pH of the soil.
b) Ammonium sulphate (Sindrifertilizer):
Prep:
c) Basic calcium nitrate (nitrate of lime or Norwegian salt petre) (Ca(NO3)2.CaO):
Prep:
Being highly deliquescent, it is packed in water proof bags.
d) Calcium cyanamide (CaCN2):
Prep:
Prop: In soil, it is converted into urea which then decomposes into ammonia. Ammonia is finally converted into nitrates by nitrifying bacteria.
It is added to the soil before sowing and not when the plants are actually growing.
e) Calcium ammonium nitrate Ca (NO3)2. NH4NO3. CAN is known as Nangal fertilizer.
Prep:
Prop: It is hygroscopic; therefore, the pellets are coated with calcium silicates as to protect from moisture.
It is more soluble in water and does not make the soil acidic and therefore, is superior to ammonium sulphate.
Phosphatic fertilizers:
These provides phosphorus to the plants.
a) Superphosphate of lime or calcium superphosphate:
Prep:
Prop: It is a fine powder and has 16 – 20% of P2O5.
b) Phosphatic slag or Thomas slag:
It is a by product of steel industry.
Prep:
Prop: Fine powder is used as fertilizer. It has 14 – 18% of P2O5.
c) Triple phosphate or Triple super phosphate:
This is another form of superphosphate.
Prep:
Prop: It has about three times the amount of available P2O5 in comparison to superphosphate of lime (i.e., 42 – 46% of P2O5) and hence called triple super phosphate.
d) Nitrophosphate or Nitrophos:
It is also known as calcium superphosphate nitrate.
Prep:
Prop: This is a mixed fertilizer. The advantage of this fertilizer is that in addition to phosphorous, it contains nitrogen as well.
III. Potash fertilizers:
These provide potassium to plants. e.g., KCl, KNO3, K2SO4 are used as potash fertilizers.
N, P, K, Fertilizers:
Fertilizers containing N, P, K in suitable adjusted proportions are known as N, P, K fertilizers.
These are mixed fertilizers and are obtained by mixing nitrogenous, phosphatic and potash fertilizers in suitable proportions.
Ex: Expression live 4 – 8 – 2 used for a mixed fertilizer indicates that it contains 4% N2, 8% P2O5 and 2% K2O.
Illustration 15: Write the composition of ‘superphosphate’ of lime, is it variable?
Solution: Mixture of calcium hydrogen phosphate and gypsum is known as super-phosphate of lime . Composition of superphosphate is variable.
FORMULAE AND CONCEPTS AT A GLANCE
1. Nitrogen and phosphorus show both positive and negative oxidation states but the heavier elements show only positive oxidation states. Nitrogen show all the oxidation states from – 3 to + 5.
2. Nitrogen and phosphorus behave as non-metals, arsenic and antimony as metalloids and bismuth as a metal.
3. The reactivity of various allotropic forms of phosphorus, towards other substances, follows the following order White > Red > Black.
4. The basic nature, bond angle, thermal stability and dipole moments of hydrides (MH3) decreases in the following order.
5. The reducing power, poisonous nature and covalent nature of hydrides increase in the following order.
6. The correct order of M.P. of hydrides is
7. The correct order of B.P. of hydrides is
8. Stability of trihalides of N – decreases in the following order
9. Lewis base strength of trihalides of N – increases as follows.
10. The ease of hydrolysis of trihalides of group 15 elements decreases as follows.
donot hydrolysed.
11. Trihalides of P, As and Sb also behave as lewis acids and the acidic strength decreases down the group.
12. In case of trihalides of phosphorus lewis acid strength decreases from F → I.
and the bond angle increases as the electronegativity of the halogen decreases from F→ I.
13. In solid state PCl5 exist as [PCl4]+ [PCl6]– having tetrahedral and octahedral structures respectively; PBr5 exists in solid as [PBr4]+ [Br]– while PI5 exists as [PI4]+ [I–] in solution.
14. The normal oxides and hydroxides of nitrogen and phosphorus are strongly acidic; arsenic is weakly acidic, antimony is amphoteric and bismuth is largely basic.
15. The acidic strength of oxides of nitrogen increases in the order:
16. The acidic strength of trioxides decreases in the following order
17. The acidic strength of pentaoxides decreases in the following order
18. The stability of pentaoxides decreases in the following order
19. All oxides of P, As and Sb are dimeric. Thus, trioxides and pentaoxides written as M4O6 and M4O10 respectively.
20. The strength and solubility of oxyacids of group 15 elements decreases rapidly in the following order.
21. BiOCl is called pearl white.
22. In tooth past, CaHPO4.2H2O is added as mild abrasive and polishing agent.
23. Arsenic trioxide (As4O6) is called white arsenic and is a poison.
24. Phosphorus pentaoxide (P2O10) due to its appearance as a snowy powder is called flowers of phosphorus.
25. SbF3 is called swarts reagent, which is used as a fluorinating agent for various compounds of nonmetals.
26. Phosphine in combination with acetylene is used in preparing Holme’s signals for ship to know about the position of rocks or icebergs in the sea. Titanic sank in seawater on hitting the iceberg.
A mixture of CaC2 and Ca3P2 is taken in a vessel which is allowed to be in contact with water. Phosphine and acetylene are formed phosphine catches fire in air and lights up acetylene which acts as a signal for the approaching ship.
27. For drying of NH3 quicklime (CaO) is used. Other dehydrating agents like H2SO4, CaCl3, and P2O5 can not be used as they react with NH3.
SOLVED PROBLEMS-1
Prob 1. Explain, molecular nitrogen N2 is not particularly reactive.
Sol: In molecular nitrogen, there is a triple bond between two nitrogen atoms (N º N) and it is non-polar in character. Due to the presence of a triple bond, it has very high bond dissociation energy (945 kJ mol–1) and therefore it does not react with other elements under normal conditions and is very unreactive. However it may react at higher temperature.
Prob 2. NH3 acts as a ligand but NH4+ does not. Explain.
Sol: Ammonia molecule has a lone pair of electrons on the N-atom which it can donate to an electron acceptor. Therefore, it can act as a ligand. But, in NH4+, the lone pair of electrons on N-atom has already been donated to the proton and hence, is not available for donation to an electron acceptor. Hence, NH4+ ion can not act as a ligand.
Prob 3. NH3 is a strong base but NF3 does not show any basic property. Explain.
Sol: NF3 has a pyramidal shape with one lone pair on N-atom. The lone pair on N is in opposite direction to the N-F bond moments and therefore it has very low dipolemoment (about 0.234D). Thus it does not show donor properties. But ammonia has high dipolemoment because its lone pair is in the same direction as the N – H bond moments. Thus, NH3 has donor properties.
Prob 4. NF3 is an exothermic compound (DHf = – 109 kJ/mol), whereas NCl3 is an endothermic compound (DHf = + 230 kJ/mol) explain.
Sol: NF3 is an exothermic compound, whereas NCl3 is an endothermic compound because in case of NF3, N – F bond strength is greater than the F – F bond strength while in case of NCl3, ‘Cl – Cl’ bond strength is greater than N – Cl bond strength.
Prob 5. The first ionization energy of ‘NO’ is less than that of N2. Explain.
Sol: In N2, there is no unpaired electron in the molecular orbital. So its first ionization energy is high. But in NO, there is one unpaired electron in one of the p* antibonding orbital. Thus, this electron p* antibonding orbital is easily lost to give the nitrosonioum ion, NO+. Hence, first ionization energy of NO is less than that of N2.
Prob 6. Concentrated HNO3 turns yellow on exposure to sunlight. Why?
Sol: On exposure to sun light, HNO3 decomposes into NO2, O2 and H2O. The presence of NO2 in the partially decomposed HNO3 gives it yellow colour.
Prob 7. Phosphoric acid has high viscosity and high melting point. Why?
Sol: Phosphoric acid (H3PO4) has a tendency to form hydrogen bonding in concentrated solutions. Therefore, it has high viscosity and is a syrupy liquid and has high boiling point.
Prob 8. Give one example each of oxyacid of P having the oxidation state (i) + 4 (ii) + 3.
Sol: i) Hypophosphoric acid (H4P2O6) ii) Phosphorous acid (H3PO3)
Prob 9. Nitric oxide becomes brown when released in air. Why?
Sol: When nitric oxide (NO) is released in air, it becomes brown due to the formation of NO2 (nitrogen dioxide), which is a brown gas.
Reaction :
Prob 10. Nitrous oxide (NO) supports combustion more vigorously than air. Explain.
Sol: Nitrous oxide decomposes above 5500C yielding oxygen and nitrogen i.e., . Because one third of the gas liberated is oxygen, N2O supports combustion better than air.
SOLVED PROBLEMS-2
Prob 1. The compound which exists as a molecule in gas phase but ionic in solid state is
(A) PCl5 (B) PCl3 (C) PCl5 (D) CCl4
Sol: (A) PCl3, exists in nature in gas phase but ionic in solid state, x-ray studies have shown that PCl5 exists as in the crystalline state.
Prob 2. In diammonium phosphate (NH4)2HPO4, the percentage of P2O5 is
(A) 35.27 (B) 46.44 (C) 51.99 (D) 53.78
Sol: (D)
2 132 = 264g 142g
Prob 3. The ammonium compound which on heating does not give ammonia is
(A) NH4Cl (B) NH4NO2 (C) (NH4)2SO4 (D) (NH4)2CO3
Sol: (B) NH4 NO2 gives N2 and H2O instead of NH3
Prob 4. A colourless gas was slowly passed over heated copper. It gave rise to an equal volume of another colourless gas which was odourless, non combustible and a non supporter of combustion, the original gas was
(A) nitric oxide
(B) ammonia
(C) hydrogen chloride
(D) nitrous oxide
Sol: (D)
Prob 5. The well known chemical fertilizer nitrolim is made by passing nitrogen over heated
(A) limestone (B) quick lime (C) calcium carbide (D) gypsum
Sol: (C)
Prob 6. Ammonium carbonate is a smelling salt because
(A) it has pleasant smell
(B) it decomposes
(C) it is crystalline
(D) it gives the smell of ammonia
Sol: (D) (NH4)2CO3 is a smelling salt because it gives the smell of ammonia.
Prob 7. When equal weights of the two fertilizers urea and ammonium sulphate are taken, urea contains
(A) less nitrogen than ammonium sulphate
(B) as much nitrogen as ammonium sulphate
(C) twice the amount of nitrogen present in ammonium sulphate
(D) more than twice the amount of nitrogen present in ammonium sulphate
Sol: (D)
% of N in urea =
% of N in
Prob 8. When HNO3 is dropped into the palm and washed with water it turns into yellow. It shows the presence of
(A) NO2 (B) N2O (C) NO (D) N2O3
Sol: (A) Nitric acid usually acquires yellow colour due to its decomposition by sunlight into NO2.
The yellow colour of the acid can be removed by warming it to 60 – 800C and bubbling dry air through it.
Prob 9. Fixation of nitrogen means
(A) reaction of nitrogen with oxygen
(B) conversion of free atmospheric nitrogen into nitrogen compounds
(C) decomposition of nitrogenous compounds to yield free nitrogen
(D) the action of denitrifying bacteria on nitrogen compounds
Sol: (B) conversion of free atmospheric nitrogen into nitrogen compounds
Prob 10. Which of the following properties is not correct regarding red phosphorus?
(A) Soluble in CS2
(B) Does not undergo oxidation at room temperature
(C) Does not react with NaOH solution
(D) Non-poisonous
Sol: (A) Soluble in CS2








