SOME BASIC PRINCIPLES IN ORGANIC CHEMISTRY
Introduction:
The branch of chemistry which deals with the study of hydrocarbons and their derivatives is called organic chemistry. The elements in organic compound are usually bound to carbon by covalent bonds.
Like other chemical reactions, organic reactions are also a process of bond breaking and bond making.
A – B + C A – C + B
Every reaction follows a reaction mechanism. Reaction mechanism is a step by step description of the sequence of processes involved in a chemical (organic) reaction.
Substrate + Reagent Intermediate Products
(Transition state)
Covalent bond fission:
The breaking of covalent bond can take place in two different ways. They are
1. Homolytic cleavage: The symmetrical cleavage of bond in which electrons are shared equally by the atoms and free radicals are formed, is known as homolytic cleavage. It is also known as free radical cleavage or non-polar bond fission.
A – B A· + B·
The conditions favourable for homolytic cleavage are,
(i) high temperature
(ii) light of suitable wavelength
(iii) non-polar solvent
(iv) presence of peroxide or oxygen.
2. Heterolytic cleavage: The unsymmetrical cleavage of bond in which electrons are shared unequally between the fragments formed, is known as heterolytic cleavage. It is also known as ionic cleavage or polar bond fission.
[Electronegativity of A is greater than B] [Electronegativity of B is greater than A]
The conditions favourable for heterolytic cleavage are,
(i) low temperature
(ii) polar nature of the substrate and attacking reagent
(iii) polar solvent
(iv) presence of acid or base catalyst.
Electronic displacement in covalent bonds:
Inductive Effect:
The displacement of electron along a carbon chain due to presence of a substituent on the carbon atom is called inductive effect. It is a permanent effect and decreases rapidly as the length of carbon chain increases.
| Case – I | (- I effect) |
| (Z – electron withdrawing group) |
The electronegativity of Z is more than carbon atom, so the shared pair of electrons in the bond C1 – Z is more towards Z, which induces a positive charge on C1 (i.e. ). As we move along the chain from C1 to C3, the partial positive charge decreases.
| Case – II | (+I effect) |
| (Z – electron donating group) |
The electronegativity of Z is less than carbon atom, so the Z group releases electron and gets a partial positive charge (i.e. ) and induces a partial negative charge (i.e. ) on C1. As we move along the chain from C1 to C3, the partial negative charge decreases.
When more electronegative atom is attached to carbon atom (i.e. electron withdrawing group), the inductive effect is called – I effect. The examples of electron withdrawing group
etc.
When less electronegative atom is attached to carbon atom (i.e. electron donating group), the inductive effect is called + I effect. The examples of electron donating group are (CH3)3C, (CH3)2CH, CH3CH2, CH3 etc.
Applications of inductive effect:
1. Dipole moment: Inductive effect produces dipole moment in a molecule. As this effect increases (electronegativity difference increases) the dipole moment also increases.
2. Strength of fatty acids: As the number of alkyl groups attached to COOH group increases, the acidic strength decreases. The order of acidic strength is
(i) HCOOH > CH3COOH > CH3CH2COOH
(ii) CCl3COOH > Cl2CHCOOH > ClCH2COOH > CH3COOH
Illustration 1: Which is the strongest carboxylic acid among the following?
(A) Cl3CCO2H
(B) Br3CCO2H
(C) F3CCO2H
(D) Cl2CHCO2H
Solution: (C) It is because of – I effect of F which stabilizes the respective carboxylate ion
Illustration 2: The order of the basicity among the amines is
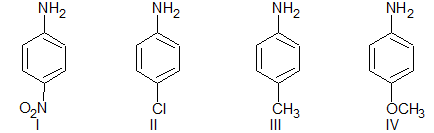
(A) I < II < III < IV
(B) II < I < III < IV
(C) I < II < IV < III
(D) II < I< IV < III
Solution: (A) It is because of + M of OCH3, then + I of CH3 then + R of Cl and – I of NO2.
Illustration 3: Which of the following order is correct regarding –I effect of substituents?
(A) NR2 < OR < F
(B) NR2 > OR > F
(C) NR2 > OR < F
(D) NR2 < OR > F
Solution: (A) F is strongly electronegative. In OR and NR2 because of high electronegativity of O the – I effect of O is higher then N.
Electromeric Effect:
Temporary polarization of the substrate molecule at the site of multiple bonds, by shift of an electron pair from one atom to the other under the influence of attacking reagents. It is a temporary effect.

There are two types of electromeric effect.
(i) Positive electromeric effect (+E): Displacement of electron pair towards the attacking reagent.
(ii) Negative electromeric effect (-E): Displacement of electron pair away from the attacking reagent.
Mesomeric or Resonance Effect:
The displacement of electron relayed through electrons of multiple bonds in the carbon chain causing permanent polarization. For resonance effect, it is essential that the atom should have a lone pair of electron or a group containing a bond. This lone pair of bond should be in conjugation with the multiple bond of the rest of the molecule. The conditions required for M or R effect:
1. Molecule should be unsaturated with conjugated system of double bond.
2. Negative charge is in conjugation with double (or multiple) bond.
3. Lone pair of electrons in conjugation with double bond.
The reactivity of compounds is affected by the presence of groups like
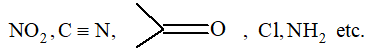
The movement of electrons from one end to the other end of the chain through a conjugated system of double bond is observed in resonance effect. It is a permanent effect.
Illustration 4: Resonance structures of a molecule does not have
(A) identical arrangement of atoms
(B) nearly the same energy content
(C) the same number of paired electrons
(D) identical bonding
Solution: (D) Resonating structure of a molecule has all the properties mentioned in(A),(B) & (C).
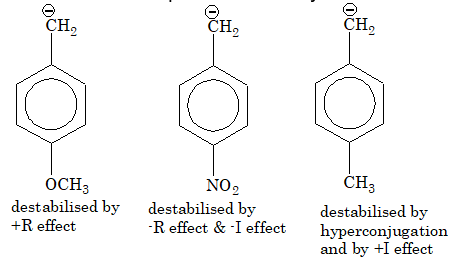
Illustration 5: Arrange the following in increasing order of resonance stabilization.
(A) I < II < III
(B) III < I < II
(C) II < I < III
(D) I < III < II
Solution: (C). Stability number of resonating structures.
Exercise 1; (i) Aniline is a weaker base than ethylamine. This is due to
(A) – I effect of NH2 in aniline
(B) -M effect of NH2 in aniline
(C) +I effect of NH2 in aniline
(D) + M effect of NH2 in aniline
(ii) Vinyl chloride is stabilized by resonance. The three resonating structures of vinyl chloride are
The decreasing order of stability of the resonating structures of vinyl chloride is
(A) A > B > C
(B) A > C > B
(C) B > A > C
(D) C > B > A
Hyperconjugation (Baker and Nathan Effect):
Shifting of s electrons induces resonance in rest of the molecule. The delocalization of s and p bond orbitals is called hyperconjugation.
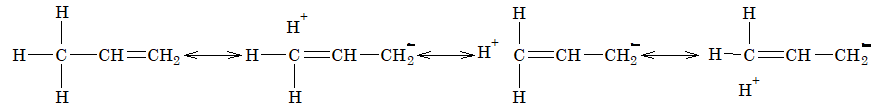
In the resonating structures, there is no definite bond between carbon atom and one of the hydrogen atoms, hence hyperconjugation is also known as no-bond resonance.
Applications of hyperconjugation effect:
1. Stability of alkenes: More number of methyl groups attached to double bonded carbon atom more would be the stability of alkene.
CH2 = CH2 < CH3 – CH = CH2 < (CH3)2 C = CH2
No hyperconjugation structures 3 hyperconjugation structures 6 hyperconjugation structures
2. Stability of carbonium ions: More number of hyperconjugation structures of the carbocation more will be its stability.
tert–butyl > isopropyl > ethyl
9 hyperconjugation 6 hyperconjugative 3 hyperconjugation
structures structures structures
3. Bond lengths: The bond length in a molecule changes if there is hyperconjugation. In , the C1-C2 bond length is found to be more than 1.34 (normal C = C bond length) while the C2-C3 bond distance is less than 1.54 (normal C – C bond length).
4. Directive influence of the group: +M effect of methyl group in toluene is due tohyperconjugation.
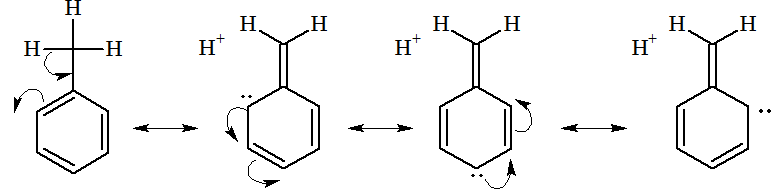
Due to hyperconjugation, there are nine different structures having negative charge at ortho and para positions. Hence, + M effect of alkyl group attached to benzene ring follows the order: .
In the same way, the meta directing influence and deactivating effect of –CCl3 group in benzotrichloride can be explained on the basis of hyperconjugation as follows,
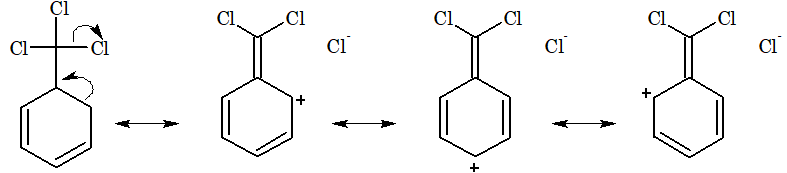
Due to low electron density at ortho and para positions, the meta position becomes point of high electron density, hence electrophilic substitution takes place in meta position.
Illustration 6: The molecule in which distance between the two adjacent carbon atoms is largest is
(A) ethane
(B) ethene
(C) ethyne
(D) benzene
Solution: (A) This is because in ethane bonding is in between sp3 – sp3 carbon.
Illustration 7: The compound with the most acidic hydrogen is
(A) CH3CHO
(B) CH3COCH3
(C)
(D) CH3-CO-CH2-CO2CH3
Solution: (C) It is because of – I effect group.
Exercise 2: (i) The compound having the most acidic proton is

(ii) Which one of the following is least acidic?

Reaction intermediates:
Free Radicals:
An atom or group of atoms possessing an odd or unpaired electron, are called free radicals. For example,
The reactions involving free radicals are
(i) catalysed by light, heat etc.
(ii) proceed in vapour phase or in non-polar solvents.
(iii) autocatalytic.
The order of stability of free radicals on the basis of resonance inductive effect is as follows:
Geometry of free radicals: They have trigonal shape and the hybridization is sp2. Free radicals are electrophilic in nature.
Carbonium Ions or Carbocations:
An organic species containing positively charged carbon atom is known as carbocation. The positively charged carbon atom contains six electrons in its valence shell. For example,
Formation of carbocations:
(i) (CH3)3CCl (CH3)3C+ + Cl– (Heterolytic fission)
(ii) CH3 – OH CH3 – OH2+ (Protonation)
(Abstraction of halide ion by Lewis acid)
(Removal of N2 from diazonium cation)
Rate of formation of carbocations:
More stable carbocation forms at faster rate as compared to less stable carbocation. For example,
(i) R – CH2 – Cl R – CH2+ + Cl–
(ii) R3C – Cl R3C+ + Cl–
A tertiary carbocation is more stable, the transition state of tertiary carbocation is lower in energy than transition state of primary carbocation. As a result, a tertiary carbocation will form more rapidly than primary carbocation.
Stability of carbocation: The stability of carbocation is similar to free radicals and it is based on resonance hyperconjugation and inductive effect. The order of stability is,
- Electron attractors (-I effect) increases the postive charge on carbon atom and thus reduces the stability of carbocation, e.g..]
- Carbocation are sp2 hybridized and have trigonal planar structure, so the attacking species can approach both from top and bottom.
Illustration 8: Which carbocation is most stable?

Solution: (D). Allyl carbocation is stable because of its resonating structures.
Exercise 3:
(i) The most stable free radical among the following is
(A)
(B)
(C)
(D)
(ii) Most stable carbonium ion is

(iii) Which is the decreasing order of stability of the ions?
(A) II>III>I
(B) I>II>III
(C) I>III>II
(D) III>I>II
Carbanion:
An organic species containing negatively charged carbon atom is called carbanion. There are eight electrons in the valence shell of the negatively charged carbon atom and two electrons remain as unshared pair. For example,
Formation of carbanion:
(i)
(ii)
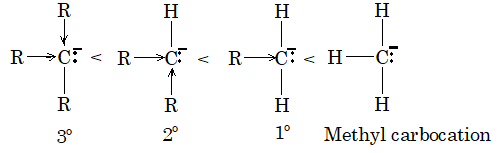
Stability of carbanion: The order of stability of carbanion is as follows:
Geometry of carbanion: Generally, alkyl carbanions are pyramidal in shape but in some cases it may be planar or linear.

Carbanions are nucleophilic in nature.
Carbene (Biradical):
A short lived divalent carbon atom with two unpaired electrons. It is electron deficient and contains six electrons in valence shell. It is a powerful electrophile due to its reactive nature. For example, :CH2 (methylene), :CCl2 (dichloro carbene) etc.
Carbenes exist in two possible forms
(i) Singlet
(ii) Triplet

Illustration 9: CCl2 forms by
(A) proton dissociation of CHCl3
(B) base abstraction of proton of CHCl3
(C) hydrolysis of CCl4
(D) none of the above
Solution: (B)
Reagents:
Reagents are broadly classified into two main categories, i.e.
(i) Electrophiles
(ii) Nucleophiles
Electrophiles:
Electrophiles are electron loving species having affinity for electron rich centres. They are of following types,
(i) Positively charged: The species having a positive charge, e.g.
.
(ii) Neutral: The molecules containing electron deficient atom (i.e. Lewis acids) e.g. :CH2, AlCl3, BF3, ZnCl2, FeCl3 etc.
(ii) Ambident: The molecules with two electron deficient centres, e.g. , – unsaturated carbonyl compounds.
Nucleophiles:
Nucleophiles are electron rich species having an affinity for electron deficient centres.
They are of following types
(i) Negatively charged: The species having a negative charge, e.g. Cl–, Br–, OH–, CN–, NO2– etc.
(ii) Neutral: The molecules having an unshared pair of electrons (i.e. Lewis base), e.g.
etc.
(iii) Ambident: The molecule with two electron rich centres, e.g.
etc.
Comparison between an Electrophile and a Nucleophile:
| Electrophile | Nucleophile |
| (i) Accepts the electron pair | (i) Releases the electron pair |
| (ii) Electron deficient species | (ii) Electron rich species |
| (iii) Attacks the points of high electron density | (iii) Attacks the point of low electron density |
| (iv) Acts as Lewis acid | (iv) Acts as Lewis base |
| (v) Possesses an empty orbital | (v) Possesses an electron pair which is loosely held and can be supplied easily |
| (vi) Usually positively charged species | (vi) Usually negatively charged species |
Illustration 10: What is the decreasing order of strength of the bases –OH–, NH2–, H-CC– and CH3-CH2–?
(A) CH3CH2– > NH2– > H-CC– > OH–
(B) H-CC–>CH3CH2– >NH2– >OH–
(C) OH–>NH2–>H-CC–>CH3-CH2–
(D) NH2– >H-CC– >OH– >CH3-CH2–
Solution: (A) This is because C is less electronegative than N and N is less than O.
Exercise 4: (i) Which of the following behaves both as a nucleophile and as an electrophile?
(A) CH3-CH3
(B) CH3-OH
(C) CH2 = CH-CH3
(D) CH3-NH2
(ii) Which of the following order of reactivity of acid derivatives towards a nucleophile is correct?
(A) anhydride > amide > ester
(B) anhydride > ester > amide
(C) amide > anhydride > ester
(D) amide > ester > anhydride
Types of organic reactions:
The organic reactions are classified into four categories.
(i) Substitution reaction
(ii) Addition reaction
(iii) Elimination reaction
(iv) Rearrangement reaction
Substitution Reaction:
The replacement of an atom or group from the organic molecule with another atom or group is known as substitution reaction.
A – B + X – Y A – X + B – Y
There are three types substitution reaction,
(i) Nucleophilic substitution
(ii) Free radical substitution
(iii) Electrophilic substitution
(i) Nucleophilic substitution reactions:
a) Unimolecular substitution reaction (SN1): Tertiary halides undergo substitution reaction by SN1 mechanism.
(CH3)3C-Cl + OH– (CH3)3C-OH + Cl–
Mechanism:
Step – 1 (CH3)3C-Cl (CH3)3C+ + Cl–
(intermediate)
Step – 2 (CH3)3C+ + OH– (CH3)3C-OH
(tert–butyl alcohol)
Rate reaction = k[tert–butyl chloride]
Order = 1, molecularity = 2
b) Biomolecular substitution reaction (SN2): Primary halides undergo substitution reaction by SN2 mechanism.
Mechanism:
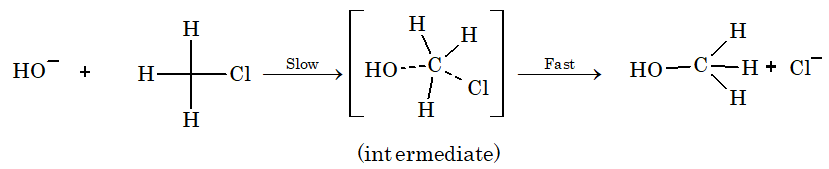
Rate of reaction = k[CH3Cl][OH–]
Order = 2, molecularity = 2
(ii) Free radical substitution: These reactions involve homolytic bond fission and the mechanism involves three steps. Chlorination of methane is the example of free radical substitution.
CH4 + Cl2 CH3 – Cl + HCl
Mechanism:
Step – 1 (chain initiation step)
Step – 2 (chain propagation step)
Step – 3 (chain termination step)
Illustration 11: Which of the following alkyl halides will under go SN1 reaction at a faster rate?
(A) Cl—CH2—CN
(B) Cl—CH2—NO2
(C) Cl—CH2—OMe
(D) Cl—CH2—CH3
Solution: (C) This is because of resonance stabilization of after elimination of Cl–.
Exercise 5: Which of the following is more reactive towards electrophilic substitution?
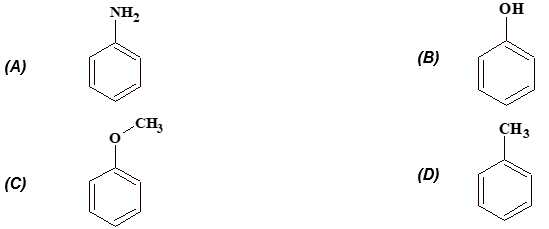
(iii) Electrophilic substitution reaction: It involves the attack by an electorophile. If the order of reaction is 1, it is written as SE1 (unimolecular) and if the order is 2, it is SE2 (Bimolecular).
a) SE1 Reaction Mechanism: Electrophilic substitution in aliphatic compounds (SE1) are very rate; some of the important examples are:
Replacement of the metal atom in an organometallic compound by hydrogen:
b) SE2 Reaction Mechanism: Electrophilic substitution (SE2) is very common in benzene nucleus.
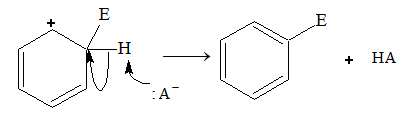
The electrophile displaces hydrogen atom and gets attached directly to the benzene ring. This is known as electrophilic aromatic substitution.
Ar – H + E+ Ar – E + H+
The electrophiles which take part in these substitution reactions are positively charged ions, electron deficient species with a partial positive charge and neutral molecules such as SO3. The five types of reactions, which comes under the category of electrophilic aromatic substitution are,
(i) Halogenation
(ii) Nitration
(iii) Sulphonation
(iv) Friedel–Crafts alkylation
(v) Friedel–Crafts acylation
General mechanism:
Benzene undergoes electrophilic substitution because of its exposed electrons. In case of electrophilic substitution, benzene resembles alkene as the site of electrophilic attack in alkene is bond only. But benzene also differs from alkene as the latter undergoes addition reaction and benzene prefers substitution rather than addition.
In step 1, the electrophile attacks the system of benzene to form a non-aromatic cyclohexadienyl carbocation or arenium ion.

Now, the carbon atom attached to electrophile E is sp3 hybridized, so it does not have p-orbital available for delocalization. As a result, aromatic character is lost. The four p electrons are delocalized over 5 p-orbitals (delocalization is there but aromaticity is not there). In step 2, a proton is removed from the carbon atom to which electrophile is attached. H+ leaves the ion leaving behind the shared pair of electron, which now forms a double bond and aromaticity is regained.
Halogenation:
Direct halogenation of benzene is not possible and it does not decolourize bromine solution as there is no reaction. Benzene readily reacts with Br2 or Cl2 in presence of Lewis acids such as FeCl3, FeBr3, anhydrous AlCl3 etc.
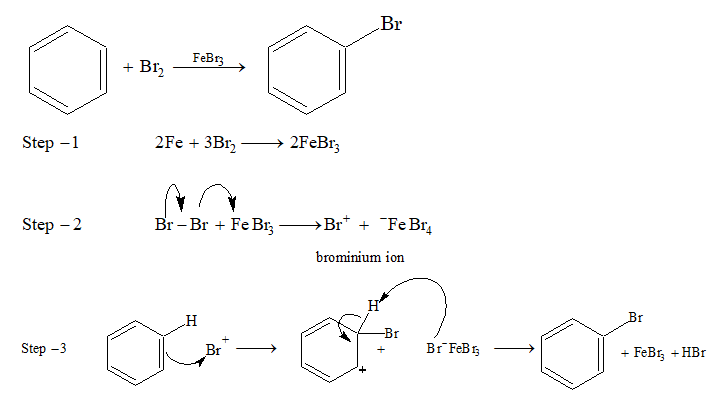
Fluorination takes place rapidly and always polyfluorinated benzene is formed rather than monofluorinated product. Iodine is unreactive towards halogenation of benzene.
Nitration:
Benzene reacts slowly with hot conc. HNO3 to yield nitrobenzene. The reaction is much faster if it is carried out in the presence of conc. H2SO4.
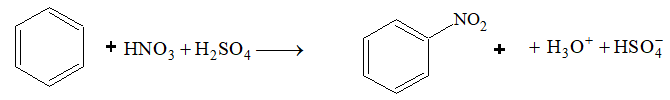
Here, H2SO4 acts as a an acid and HNO3 as a base.

Sulphonation:
Sulphonating agent is fuming H2SO4 + SO3 or conc. H2SO4.
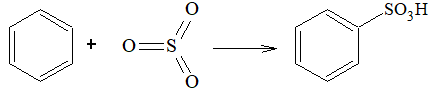
Step – 1 : (In fuming suphuric acid SO3 attacks directly)
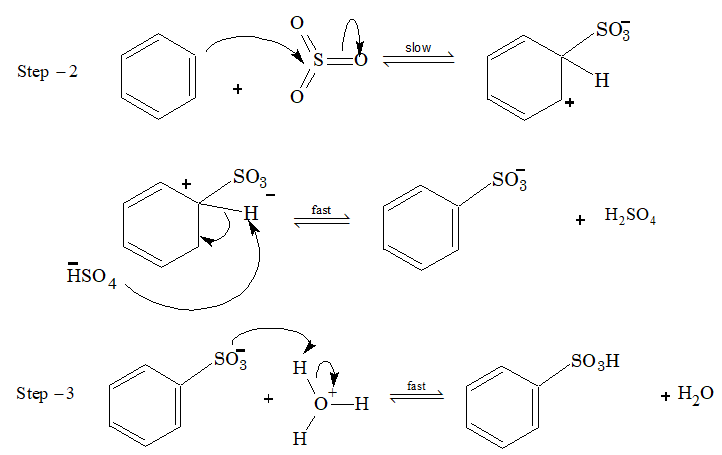
In sulphonation, all the steps are reversible.
To sulphonate benzene, concentrated H2SO4 acid is used (under these conditions position of equilibrium lies at the right).
To desulphonate benzene, dilute H2SO4 is used and steam is passed into the reaction mixture. Now the reaction equilibrium is shifted to left.
Exercise 6: Reactive species for sulphonation reaction is
(A)
(B) SO3
(C) Both (A) and (B)
(D) SO2
Friedel–crafts alkylation:
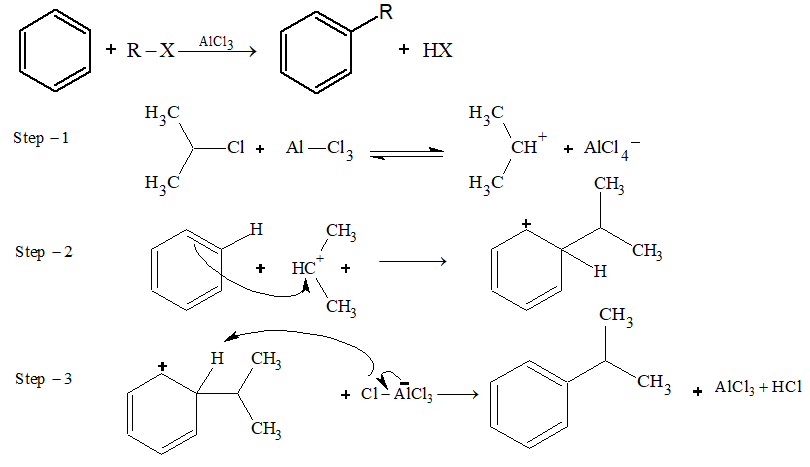
Addition Reaction:
Usually unsaturated molecules undergo addition reaction. If one p bond is broken then two s bonds are formed.
A = B + X–Y X–A–B–Y
There are three types of addition reaction.
Electrophilic addition: Generally, unsaturated compounds give electrophilic addition reaction.
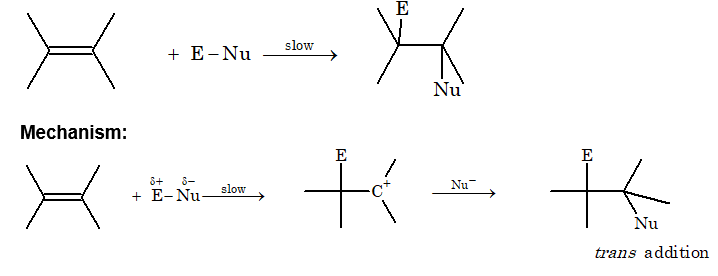
Nucleophilic addition reaction: Generally, aldehydes and ketones give nucleophilic addition reaction.
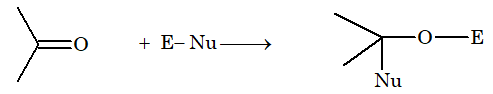
Mechanism:
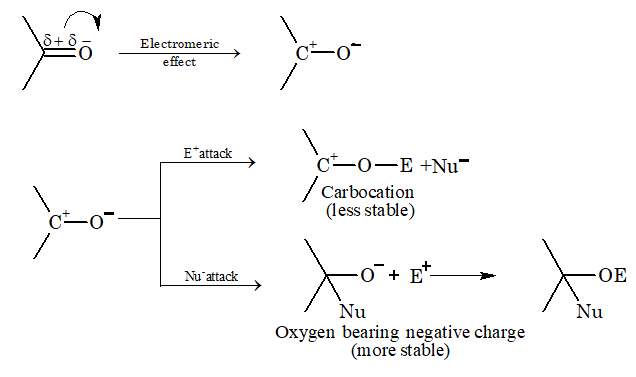
Free radical addition reaction: Addition of HBr to propene in presence of peroxide gives n-propyl bromide. This is anti-Markonikov or Kharasch effect.
CH3CH = CH2 + HBr CH3CH2CH2Br
Elimination Reaction:
The loss of atoms or group from adjacent carbon atoms (one in the form of a nucleophile and other in the form of an electrophile) resulting in the formation of an unsaturated compound is known as elimination reaction.
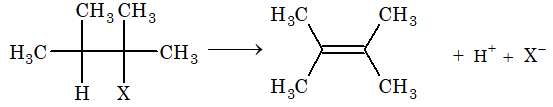
[Note: If these two groups or atoms are removed from adjacent carbon atoms, then it is known as – elimination reaction.]
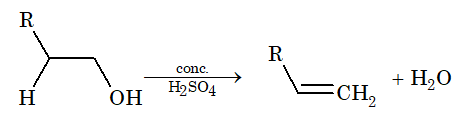
There are two types of elimination reaction.
Unimolecular elimination (E1): This reaction involves two steps.
Mechanism:
Step – 1: Heterolysis of substrate gives carbocation and halide ion.
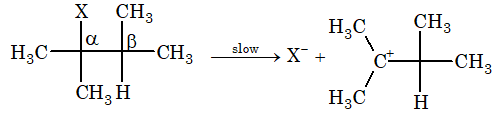
Step – 2: Carbocation gives up proton to a base immediately and alkene is formed.
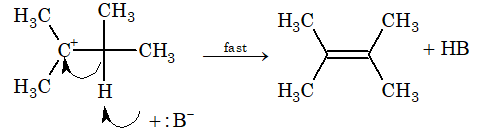
Rate of reaction = k [substrate]
Order = 1, molecularity = 1
Bimolecular elimination (E2): This reaction involves one step only. Base pulls out a proton from the -carbon atom and simultaneously a halide leaves and the double bond is formed.
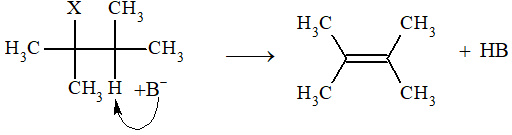
Rate of reaction = k [substrate] [base]
Order =2, molecularity = 2
Illustration 12: Which of the following is easily debrominated?

Solution: (B) This is because the intermediate will be stabilized due to conjugation with double bonds.
Exercise 7:

Rearrangement Reaction:
In these reactions, the substituents change their positions.
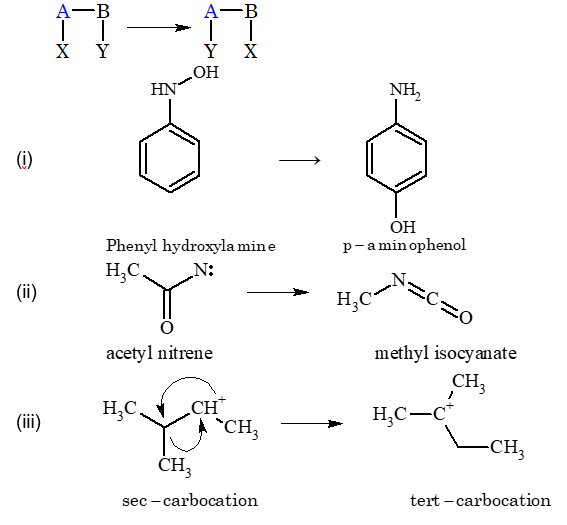
Answer to Exercises
Exercise 1. (i) D (ii) B
Exercise 2. (i) D (ii) A
Exercise 3. (i) C (ii) D (iii) C
Exercise 4. (i) C (ii) B
Exercise 5. C
Exercise 6. C
Exercise 7. C
FORMULAE CONCET AT A GLANCE
1. Application of inductive effect:
(a) Acidity of acid: If -I producing group are attached to carbon chain of carboxylic acid. e.g., Halogenated acids are much more strong acid than parent carboxylic acid.
(b) Basicity of Base: In general more alkylated base are more basic than parent base.
2. Application of mesomeric effect:
Mesomeric effect in which e–s are transferred from multiple bond to an atom or from a multiple bond to a single covalent bond or lone pair of electron from an atom to the adjacent single covalent bond (conjugation effect).
In case of aniline the electron density of unshared pair does not reside entirely on the nitrogen atom but is spread over the ring. Thus decrease the electron of nitrogen atom.
3. Stability of intermediate:
The order of stability can be explained on the basis of electron releasing inductive effect of alkyl groups. Greater the number of alkyl group on the carbon atom carrying positive charge, greater will be dispersal of the charge and hence more stable be the carbon atom.
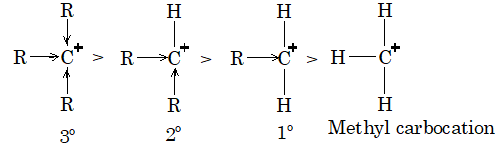
4. Stability of carbanions can be explained on the basis of +I effect of alkyl group. Greater the number of alkyl group on carbon atom carrying -ve charge greater will be intensity of -ve charge on carbon atom and hence less stable would be the carbanion.
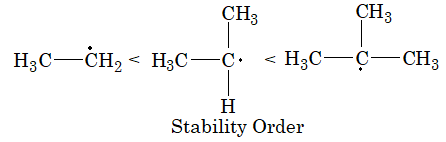
5. Stability of free radicals can be explained on the basis of hyperconjugation.
Greater the number of hyperconjugated structure greater will be stability.
6. Substitution reaction: It involves direct replacement (displacement or substitution) of an atom or group of atom in the organic molecule by another atom or group.
SOLVED PROBLEMS
Prob 1. Among the following which is the strongest base
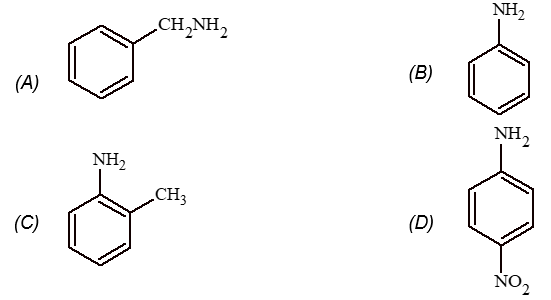
Sol: (A) Aliphatic amines are more basic than aromatic amines.
Prob 2. The least basic among aniline and methyl substituted anilines
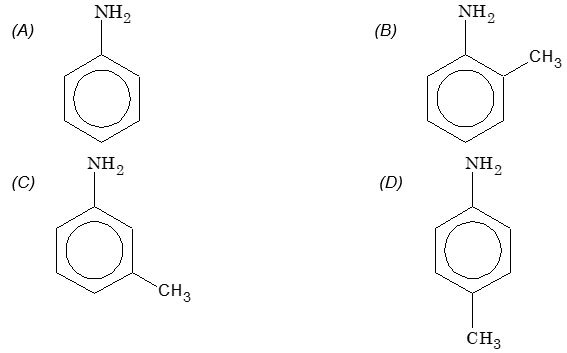
Sol: (B) Due to ortho effect, o-amino toluene is highly acidic, i.e. least basic.
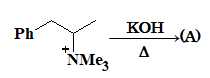
Prob 3.
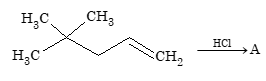
A is
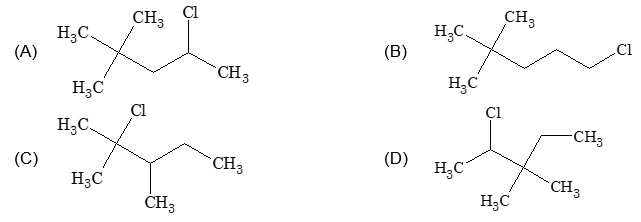
Sol:

Prob 4. The halogen compound likely to undergo nucleophilic substitution is
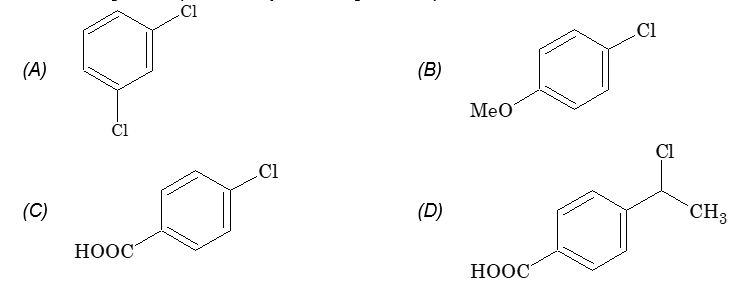
Sol: (D) Aliphatic halogen compound gives nucleophilic substitution reaction.
Prob 5. In the reaction sequence
A + B C6H5 – CH = CHO
(A) and (B) will be
(A) C6H5CHO and CH3CHO
(B) C6H5CH2CHO and CH3CHO
(C) C6H5CHO and CH3CH2CHO
(D) C6H5COCH3 and CH3CHO
Sol: (C) C6H5CHO + CH3 CH2COH C6H5 – CH = C(CH3)2
Prob 6. Examine the following two structures of anilinium ion and choose the correct statement from the ones given below:
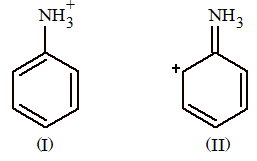
(A) II is not an acceptable canonical structure because carbonium ions are less stable than ammonium ions.
(B) II is not an acceptable canonical structure because it is non-aromatic.
(C) II is not an acceptable canonical structure because the nitrogen has 10 valence electrons.
(D) II is an acceptable canonical structure.
Sol: (C) Nitrogen can have only eight valence electrons.
Prob 7. Which one is the correct order of the increasing stability of the carbonium ions?
(A)
(B)
(C)
(D)
Sol: (A) Stability order is CH3+ < 10 < 20 < 30
Prob 8. The most stable carbonium ion is

Sol: (A) 30 carbocation with most extent of resonance.
Prob 9. Which one of the following compound will have the highest dipole moment?
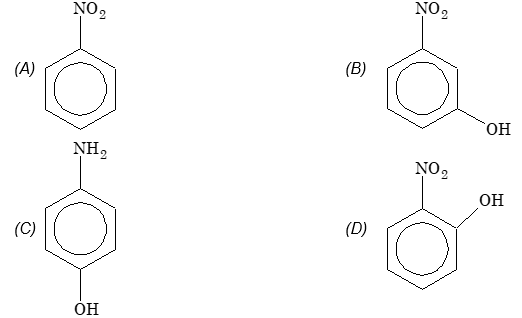
Sol: (D)
m cos
Prob 10. Which is not a planer compound?

Sol: (A) Lone pair of oxygen does not involves in delocalization, otherwise it will become antiaromatic compound hence hybridization O-atom is sp3.