THE CARBON FAMILY (Group 14)
Introduction:
Group IVA of Mendeleev’s periodic table or group 14 of long form of periodic table includes carbon, silicon, germanium, tin and lead. They constitute the second group of p-block elements, the first being group 13 – the boron family. Carbon, the first member of the group differs from the rest due to its smaller size, high electronegativity and non-availability of d-orbitals.
Occurrence:
Silicon is the second and carbon the seventeenth most abundant element (by mass) in earth’s crust. Tin and lead occur in small amounts while germanium in traces only.
Only carbon has been found to exist in elemental state (coal, graphite, and diamond) as well as combined state (CO2, CO32-, petroleum and biomolecules); all the rest exist in combined form only.
So, we can say carbon is the most versatile element in the world. It combines with H, O, N, Cl, S etc. and produces a variety of substances with amazing properties like drugs, food, living tissue and other consumables (plastic etc.).
Carbon exists in two stable isotopes in nature: C12 and C13; C14 – a radioisotope is also present which finds use in carbon-dating.
Silicon (27.2% in earth) second only to oxygen (45.5%) is present in the form of silica and silicates. Rocks and clays comprise of silicate minerals. Silicon is an important component of ceramics, glass and cement.
Germanium is found only in traces with some silver and zinc ores also in some type of coals. Ultrapure germanium and silicon are used for the semiconductor devices in electronics industry. Tin exists in the form of cassiterite ore (SiO2) and lead mainly as galena (PbS) and cerrusite (PbCO3).
Atomic Properties:
1) Electronic configuration:
The elements of group IVA or 14 have a general configuration of ns2 np2 with a total of 4 electrons in their valence shell.
| Period | Element | Symbol | At. No. | At. Mass | Electronic configuration |
| 2 | Carbon | C | 6 | 12.01 | [He] 2s22p2 |
| 3 | Silicon | Si | 14 | 28.09 | [Ne] 3s2 3p2 |
| 4 | Germanium | Ge | 32 | 72.60 | [Ar] 4s2 4p2 |
| 5 | Tin | Sn | 50 | 118.71 | [Kr] 5s2 5p2 |
| 6 | Lead | Pb | 82 | 207.2 | [Xe] 5s2 5p2 |
2) Atomic Radii:
Atomic radii increase from carbon to lead. The increase from carbon to silicon is abnormally high followed by a continuous small increase as usual.
| Element | C | Si | Ge | Sn | Pb |
| Atomic Radius (A0) | 0.77 | 1.11 | 1.22 | 1.41 | 1.44 |
Their radii are smaller in comparison to the corresponding group 13 elements. Increase in the size down the group is due to addition of a new shell with every new period. The decrease in comparison to group 13 is accounted for the increase in the effective nuclear charge.
- The abnormal increase from C to Si is due to presence of 8 penultimate, which produce greater shielding and thereby comparatively less attraction of the nucleus.
3) Ionization Enthalpy:
The first ionization enthalpy of group 14 decreases down the group from carbon to silicon. The decrease is much pronounced from carbon to silicon thereafter it decreases upto Sn followed by a slight increase for Pb.
The values of I.E., are greater than that of group 13.
On moving down, size increases and the screening effect of inner electrons decrease. However, the over all effect is that the electron can be pulled off more easily. The increased value of IE of Pb in comp. to Sn is due to poor shielding of 4f electrons making a slightly tightly bound e– which need just a bit more energy to pull them off.
| Element | C | Si | Ge | Sn | Pb |
| Ionization Enthalpy (kJmol-1) | 1086 | 786 | 761 | 708 | 715 |
The values for 2nd, 3rd and 4th I.E. are even higher and correspond to the order:
IE1 < IE2 < IE3 < IE4
The reason behind increasing value of IE is the increasing charge on atom from IE2 to IE4 which attracts the electron with more strength towards them (coulomb attraction); hence more energy will be required.
4) Electronegativity:
Electronegativity decreases from carbon to lead because of increasing size. Carbon has comparatively higher value i.e., 2.5; the rest have almost same value with very little decrease for further elements.
Small sized carbon has a control over its valence e– and can attract further e– to complete its octet. The capacity to attract an electron decreases down due to increasing size and shielding effect, which in turn gets almost forfeited by increasing nuclear charge.
| Element | C | Si | Ge | Sn | Pb |
| Electronegativity | 2.5 | 1.8 | 1.8 | 1.7 | 1.6 |
The value, is however greater than group 13 for the first 3 elements and almost equal for the last 2. The increase for the first three elements is due to smaller size and increasing nuclear charge, but this charge becomes very less effective when size increases. So the nucleus can attract an e– almost same extent.
5) Density: Initially there is gradual increase Cgraphite to Si followed by rapid increase for higher members.
|
Element |
C (Graphite) Diamond) |
Si |
Ge |
Sn ( – form) |
Pb |
|
|
Density (g cm-3) |
2.22 |
3.51 |
2.34 |
5.32 |
7.26 |
11.34 |
6) MP & BP: The mp and bp of this group are higher than group 13 and decrease down the group with only exception observed in mp of Pb (6000K) which is higher than that of Sn (503).
| Element | C | Si | Ge | Sn | Pb |
| mp (K) bp (K) |
4773 – |
1693 3550 |
1218 3123 |
505 2896 |
600 2024 |
The much higher values of mp & bp for C and Si are based on their strongly covalent network structure. Considerable decrease after germanium is due to weak metal-metal or metallic bonds.
7) Metallic character: Group 14 elements exhibit an increase in metallic character or decrease in non-metallic character on moving from carbon to lead, involving a metalloid exactly in between.
The increasing atomic size lets the e– to move out easily forming a +vely charged kernel – a characteristic metallic property, which is very less probable for Ge – exhibiting the properties of both the metal as well as non-metal and is almost impossible for C and Si – the non-metals.
Physical Properties:
All the elements are solid. Carbon being much harder (diamond and graphite) while tin and lead are soft metals.
Chemical Properties:
Valence and Oxidation state:
| Element | Common | Less Common |
| C | IV | II* |
| Si | IV | II |
| Ge | IV | II |
| Sn | IV | II |
| Pb | II | IV |
* Doubtful oxidation state
The number of valence electron is four; hence the maximum valence can be +4 and minimum – 4. Since the values of fourth I.E. are very much higher. So they normally do not form the compounds with either M4+ or M4–; however the compounds in which the Ox. St. is + 4 are covalent.
Such covalent compounds undergo sp3 hybridization. The stability of + 4 oxidation state decrease down the group while that of + 2 increases.
The heavier members tend to exhibit + 2 oxidation state rather than + 4 due to inert pair effect wherein ns2 electrons of valence shell don’t take part in a reaction and become chemically inert.
Order of stability of oxidation state
+ 4 C > Si > Ge > Sn > Pb
+ 2 Ge < Sn < Pb
Compounds of IV group elements:
C & Si form stable compounds in + 4 state, Ge in + 4 and only few in + 2, Sn forms in both + 2 as well as + 4, while lead in mostly + 2 and rarely in + 4.
Sn + 2 compounds are reducing agent, as they release 2e– and convert into + 4 state (+4 is more stable for Sn).
Pb + 4 compounds are strong oxidizing agent since they get converted into + 2, state by doing so (+2 is more stable for lead).
Illustration 1:
Which of the following orders regarding the melting points is correct
(A) C > Si > Ge > Sn
(B) C < Si < Ge < Sn
(C) C > Si < Ge < Sn
(D) C < Si > Ge > Sn
Solution:
(C) Because of catenation of carbon, its melting point is greater than silicon.
Illustration 2:
Which of the following orders regarding the boiling point is correct?
(A) Si > Ge > Sn
(B) Si < Ge < Sn
(C) Si > Ge < Sn
(D) Si < Ge > Sn
Solution:
(A) Boiling point decreases from Si to Pb.
Illustration 3:
Which of the following statements is not correct?
(A) The stability of the +4 oxidation state of elements of Group14 decreases down the group.
(B) The stability of the +2 oxidation state of elements of Group 14 increases down the group.
(C) Stannous fluoride is a covalent compound.
(D) A majority of the compounds of elements of Group 14 are four covalent and involve sp3 hybridization.
Solution:
(C) Stannous fluoride is an ionic compound.
Complex formation:
Since they are e– precise – with complete octet, hence they need neither to accept e– pair nor to donate. But if an electron donor is present, IV Group element can expand its octet and form complexes. Carbon because of non availability of d-orbitals can never do so, but the other members do accept e– pairs from ligands (e– pair donor species) and form complexes – involving their vacant d-orbitals.
These complex species involve sp3d2 hybridization at central atom and have an octahedral geometry.
Catenation (Catena = chain):
Tendency of forming long chain compounds having the covalent bonds with the same element is called catenation.
The property of catenation is present in group 14 and decreases from C to Sn. It is due to strong C–C bonds. Moving down from C to Sn the atomic size increases and electronegativity decreases, this leads to less strong ‘element-element’ (e.g., Si-Si) bonds hence the atoms cannot hold the electrons so tightly as they can be shared by other elements. The catenation power decreases for this very reason.
| Bond | Bond Energy( kJ mol-1) |
| C – C | 349 |
| Si – Si | 297 |
| Ge – Ge | 260 |
| Sn – Sn | 240 |
| Pb – Pb | No catenation |
The order of catenation is C > > Si > Ge Sn. There is no catenation at all in lead.
C has tendency to form double or triple bond due to possibility of formation of p-p bond with another carbon or any other atom of the same (i.e., 2nd) period. It is important to note that bond formation can effectively take place only with smaller molecules with increase of size, the extent of overlap of other orbitals decreases sharply.
Some compound with Si = Si, Ge = Ge, Sn = Sn and C = Si have been reported to exist.
C can also form bonds with hetero atoms (atoms other than C e.g. O, N, S) to give very long chain compounds. It forms even p-bonds with them.
Carbon can form as long chains as carbon atoms, but Si and Ge can’t extend their chain beyond 6 atoms. Sn doesn’t form the chain of more than 2 atoms.
Exercise 1: (i) Which of the following elements does not belong to Group 14?
(A) Carbon (B) Silicon (C) Germanium (D) Aresenic
(ii) Which of the following elements may be regarded as semi-metals?
(A) Carbon (B) Germanium (C) Tin (D) Lead
(iii) Which of the following elements behaves like a metal?
(A) Carbon (B) Silicon (C) Germanium (D) Tin
(iv) Which of the following statements is not correct?
(A) All the elements of Group 14 form covalent hydrides
(B) Carbon forms a very large number of compounds with hydrogen
(C) Silicon like carbon forms a large number of compounds with hydrogen. These are collectively known as silanes.
(D) The general formula of silanes is SinH2n+2 and these are strong reducing agents
Allotropy:
The occurrence of an element in more than one form – having different physical but almost same chemical properties, is allotropy.
[If a compound exists in two or more forms, it is polymorphism e.g. exists as quartz, tridymite and crisobalite; ZnS occurs as zinc blende and wurtzite.]
Allotropy arises either due to difference in number of atoms in a molecule (e.g., O2 and O3) or arrangement of atoms in a molecule (e.g., diamond and graphite).
All the group 14 elements (except Pb) exhibit allotropy.
Properties of diamond and graphite:
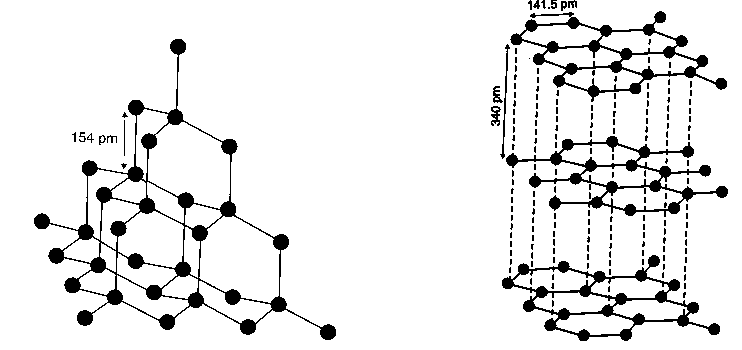
| Property | Diamond | Graphite |
| Crystallinity | Crystalline | Crystalline |
| Appearance | Transparent & high brilliance | Opaque & shining |
| Density | High (3.51 g/cm3) | Low (2.25 g/cm3) |
| Colour | Colorless | Grayish white |
| Hardness | Hard (hardest of all known substances) | Soft |
| Electrical conductivity | Bad conductor | Good conductor |
| Hybridization | sp3 | sp2 |
| Crystal structure | 3D – tetrahedral network | 2D – hexagonal sheets |
| C – C bond length | 1.54A0 | 1.415A0 |
| Bond angle | 109028’ | 1200 |
| H transformation | –0.5 kcal [diamond graphite] | – (16000C & 5.5×104 atm.) [Graphite diamond] |
| Solubility/reaction | Insoluble in all ordinary solvents | Insoluble in all ordinary solvents |
| Uses | cutting and grinding instruments, jewellary material | high temperature lubricant, electrodes in electrolysis & electric furnaces, lead pencils |
Fullerenes:
The third crystalline allotropic from of carbon, were discovered in 1985 by Kroto, Smalley and Curl, who shared Noble Prize in 1996 for chemistry.
Preparation:
Heating graphite in an electrical arc under inert environment (e.g., that of He or Ar) produces a sooty material which condenses into Cn type molecules which mainly consists of C60. Vaporization of graphite using pulsed laser technique is also employed for the preparation of fullerenes.
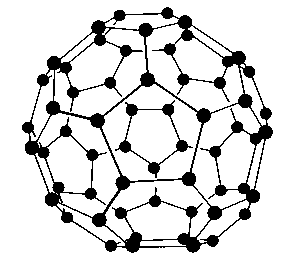
Structure:
Fullerenes are cage like molecules, having sp2 hybridization C60 molecules have a soccer ball like structure and are called buckminsterfullerenes in the honour of American architect R. Buckminster Fuller (1895–1983), whose geodesic dome structures incorporated patterns of hexagon with sufficient pentagons to give the close structure almost like those of these C60 molecules. Spherical fullerenes are also called Bucky balls in short.
C60 have 20 six–membered rings and 12 five–membered rings. A six-membered ring can fuse with either a five–membered ring or another six–membered ring, but a five-membered ring can only join with a six-membered ring.
The three e– of carbon are used in sp2 hybridized Carbon using which it forms 3 s bonds with three nearby carbons. The one unshared e– at each carbon becomes delocalized in the molecular orbitals, giving an aromatic* character to fullerenes. The carbon in fullerenes has both the single as well as double bonds like benzene, but the bond lengths being 1.43A0 for single bond and 1.38 for the double.
* Aromaticity is the presence of double bond in an organic molecule, but not giving its usual addition reactions – exhibiting somewhat more stability than otherwise expected. However, the aromaticity of fullerene is somewhat less than benzene; because the resonating structures are not favoured due to convexity of ring (for the resonating structures the molecule should be planar). Their aromatic nature decreases with the increase of non-planarity.
- Graphite is thermodynamically most stable form (allotrope) of carbon. Hence, the enthalpy of formation of graphite is assumed to be zero. The standard enthalpies of formation of diamond and fullerenes are 1.90 and 38.1 kJ mol-1
Cgraphite Cdiamond H0f = 1.90 kJ mol-1
Cgraphite C60 H0f = 38.1 kJ mol-1
Carbon nanotubes:
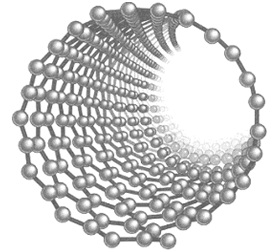
Nanotubes consist of cylindrical carbon chains arranged in hexagons. Depending on the arrangement of carbon atoms, the tubes act as electrical conductor or semiconductor and conduct current 10 times better than Cu. Their less density in comparison to metals makes them better option of electrical transmission if sufficiently long wires may be drawn.
Amorphous form of Carbon:
Coal: The formation of coal takes place in the nature by the slow carbonization of vegetable matter buried underneath the earth centuries ago under high temperature and pressure in absence of air.
Veg. Matter Peat Lignite Bituminous Anthracite
(Brown coal) (Common coal) (Purest form of coal)
(60%C) (70%C) (78%C) (90%C)
Common variety of coal is black, hard and burns with a smoky flame. In India large quantities are available in Jharia, Ranigunj, Bokaro etc.
Uses:
- As a fuel
- In manufacture of fuel gases – producer gas, water gas
- In manufacture of synthetic petrol
- In manufacture of coke, coal tar, ammonia liquor etc.
Coke:
When coal is heated strongly in the absence of air, it loses the volatile substances (e.g., coal gas, benzene ammonia, phenol etc.,) and a residue is left over. This is the coke, even purer than anthracite coal (80 – 95% carbon).
Uses:
– Reducing agent in iron & steel industry
– Preparation of water gas and graphite
– As fuel (no smoke is produced)
Wood charcoal:
When wood is heated strongly in the limited supply of oxygen (destructive distillation), wood charcoal is obtained.
It is black, porous, brittle solid. Charcoal in the powdered form is a good adsorbent. It adsorbs colouring matter from solution and several gases from air, including poisonous gases. It is a good reducing agent. It is heavier than water (density 1.5 g ml–3) but floats over it due to absorbed air in its pores. On heating upto redness, air expels out, now it sinks, if thrown in water.
The volume of gas adsorbed on charcoal depends on:
a) Temperature of charcoal:
More gas is adsorbed at low temperature. Increase of temperature increases kinetic energy of gas molecules. This helps them leave away the surface of adsorbent more easily.
b) Nature of gas:
Gases with higher boiling points are adsorbed upto more extent than others. Poisonous gases are easily condensed and more readily adsorbed.
c) Nature of charcoal:
Activated charcoal is the coconut charcoal treated with steam to increase the ability to adsorb more amount of gases. Activated charcoal adsorbs the more volume of a gas in comparison to ordinary charcoal.
Uses:
– As a fuel
– In gunpowder
– Deodorant and decolorizing
– In gas masks
Animal Charcoal (bone charcoal or bone black):
It is the residue of destructive distillation of bones, comprising of 10–12% amorphous carbon deposited over a porous framework of calcium phosphate [Ca3(PO4)2], which constitutes bones. In the process, bone oil and pyridine are also obtained as by-products.
This is more easily wetted by liquids, hence more suitable adsorbent than wood charcoal and thereby used in decolorization of crude sugar solution from which colourless sugar is crystallized later on.
If burnt it gives calcium phosphate finally.
Lamp black: Lamp black is prepared by burning tar and vegetable oils in insufficient supply of air and making the soot adsorbed on the wet blankets hung over there in the room. It is velvety black powder and finds its use in inks, printer inks, black paints, varnishes and carbon papers.
Carbon black: When natural gas is burnt in limited supply of air, carbon black is formed. It is not as greasy as lampblack. It is deposited on the lower surface of a revolving disk which is kept over the burning gas. It is used in the place of lamp black and as a ‘rubber mix’ in automobile tyres.
Gas carbon and petroleum coke: While destructive distillation of coal, the carbon that deposits over the wall of retort is gas carbon. The petroleum coke deposits when crude petroleum is distilled. Both the forms are good conductor of electricity hence used as the electrodes when pressed into sticks.
Chemical properties:
1. Formation of multiple d – p bonding:
Si and other heavier elements of the group 14 form multiple bonding (d-p) using their d-orbitals and can extend their covalence beyond 4. For the same reason halides of the heavier members undergo hydrolysis and have a tendency to form complexes e.g., [SiF5]–, [SiF6]2-, [GeCl6]2- [Sn(OH)6]2-.
The tendency of forming d – p bond is maximum with silicon which is bonded with N or O; e.g., geometry of N(CH3)3 is pyramidal while that of N(SiH3)3 is planar.
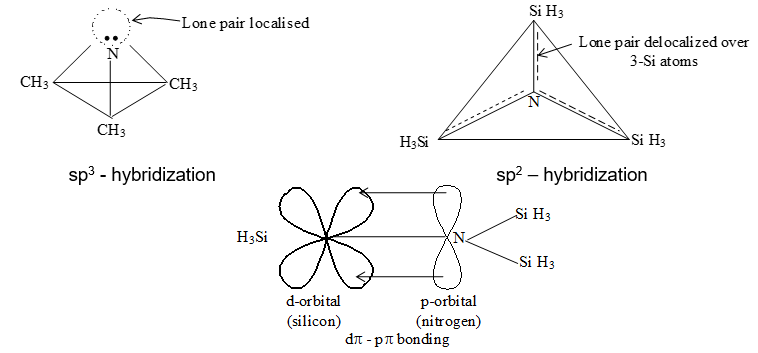
Here silicon, because of presence of empty d orbital accepts the lone pair from N–atom and makes a d-p bond rendering the N-atom sp2 hybridized. These sp2 hybridized Si atom remaining in the same plane hence the molecule is planer. In N(CH3)3 there is no d-p bonding possible since C has no d orbital, the N atom has a lone pair in one of the hybrid orbitals ‘plus’ 3 more s bonds correspond to a sp3 hybridization, which results into pyramidal geometry.
Hydrides:
All the elements (except Pb) form tetravalent hydrides of EH4 type which are covalent in nature. Tendency of hydride formation decrease down the group so that carbon forms a large no. of acyclic and cyclic hydrides called hydrocarbons. Silicon and germanium form fewer hydrides of general formula EnH2n+2 and are called silanes and germanes respectively. Tin forms only two hydrides while lead is supposed to form one hydride only in traces and in very dilute solutions.
| CH4 (Methane) | SiH4 (Silane) | GeH4 (Germane) | SuH4 (Stannane) | PbH4 (Plumbane) |
| C2H6 (Ethane) | Si2M6 (Disilane) | Ge2H6 (Digermane) | Sn2 H6 (Distannane) | |
|
: : |
: : |
Ge5 – H12 (Pentagermane) | ||
| C10H22 (Decane) | Si8 H18 (Octasilane) | |||
| (Alkanes) |
All the hydrides are volatile and volatility decreases with increase in atomic number. Thermal stability decrease down the group due to difference in electronegativity for alkanes and silanes (C = 2.5, H = 2.1, Si = 1.8), while for the germanes, stannanes and plumbane the weakening of M – H bond (Poor overlapping).
| Hydride | CH4 | SiH4 | GeH4 | SnH4 | PbH4 |
| Decomp. temp (0C) | 800 | 450 | 285 | 150 | 0 |
The reducing nature of hydride increases down the group. So, silanes are strong reducing agent unlike alkanes and get hydrolyse in alkaline solution liberating H2.
SiH4 + 2NaOH + H2O Na2SiO3 + 4H2
Monosilane is a colourless, spontaneously flammable gas, prepared by the reduction of SiCl4 with LiAlH4. Pyrolysis (thermal decomposition in absence of air) gives hydrogen and pure silicon, the method is employed to prepare semiconductor grade silicon commercially.
Oxides:
All elements give mainly two types of oxides, MO & MO2 when heated in oxygen and are called monoxide and dioxide respectively.
i) Monoxides (MO): All members of IVA form monoxides at ambient temp except Si which forms SiO only at high temperature and its existence at room temperature is in doubt.
CO SiO GeO SnO PbO
Neutral …. (? Distinctly acidic) amphoteric
ii) Dioxide (MO2):
All members of group 14 form dioxides, of which CO2 is gas and the rest are crystalline solid having high mp.
Acidic nature decreases with increase of atomic number.
CO2 SiO2 GeO2 SnO2 PbO2
acidic weakly acidic amphoteric
All these oxides dissolve in alkali to give carbonate, silicate, germanate, stannate and plumbate respectively.
EO2 + 2NaOH Na2EO3 + H2O
(E = C, Si, Ge, Sn & Pb)
The heavier oxides are soluble in acids also
MO2 + 4HCl MCl4 + 2H2O
(M = Ge & Sn )
PbO2 with ox. no. (+ 4) works as strong oxidizing agent because +4 state is less stable than + 2 due to inert pair effect, hence it gets converted into Pb2+ for the sake of its stability & meanwhile works as oxidant.
PbO2 + 4HCl PbCl2 + 2H2O + Cl2
2PbO2 + 4HNO3 2Pb(NO3)2 + 2H2O + O2
Halides:
Group 14 elements form MX4 type halides having tetrahedral geometry [PbBr4 & PbI4 do not exist because Pb4+ is strong oxidizing agent & Br– & I– are strong reducing agents]. Except carbon, all other elements also form dihalides, but there stability increases for heavier elements. Stability of tetrahalides:
CX4 > SiX4 > GeX4 > SnX4 > Pb X4
Stability of Dihalides:
SiCl2 < GeCl2 < SnCl2 < PbCl2 [Inert pair effect]
Of the two types of halides, dihalides are more ionic & hence have higher bp & mp than tetrahalides. For a given element, the stability decreases with increase in the size of halogen [less effective overlapping]
EF4 > ECl4 > EBr4 > EI4 C – F bond energy is higher than C – I.
Relative Stability of di and tetrahelides:
EX2 < EX4 (E=C, Si, Ge, Sn)
PbX2 > PbX4 (Inert pair effect)
All tetrahalides (except that of C) undergo hydrolysis and the extent of hydrolysis decrease in the group from Si to Pb. SiCl4 is hydrolysed even by moist air liberating fumes of HCl.
SiCl4 + 4H2O Si (OH)4 + 4HCl
Silicic acid
The hydrolysis proceeds via accepting lone pair of H2O by the d–orbitals of Si as follows:
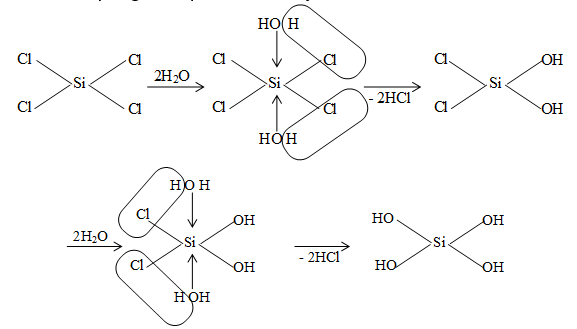
However, the empty orbitals with higher energy are always available with an atom. These orbitals can be utilized if sufficient energy is provided for the reaction to occur. CCl4 does not hydrolyse under ordinary conditions, but when treated with superheated steam in the presence of iron or copper, it is hydrolysed into phosgene. CCl4 + H2O (steam) COCl2 + 2HCl
Complex formation: Tetrahadides of higher elements having d-orbital form hexahalo complexes of [MX6]2- type. During the process they extend their coordination no. from 4 to 6.
Exercise 2:
(i) Silanes are compounds which contain
(A) C, H and Si (B) C and Si (C) O and Si (D) H and Si
(ii) Which of the following oxides is highly poisonous?
(A) CO (B) CO2 (C) C3O2 (D) C5O2
Anomalous bahaviour of carbon:
Difference between carbon and other elements of group 14:
| CARBON | OTHER ELEMENTS |
| Hard, high mp & bp | Relatively soft, low mp & bp |
| Maximum catenation | Very less or no catenation |
| p-p multiple bonds with carbon as well as other elements (N, O etc.) | No p – p bond instead d – p bond |
| CCl4 doesn’t undergo hydrolysis. | EX4 undergo hydrolysis. |
| No complex ions are formed. | Complex ions are formed. |
Reason of abnormal behaviour of carbon:
Smaller size and high electronegativity of carbon along with absence of d-orbital and high catenation power make the carbon atom to behave in way that is different from other elements of the group.
Chemical properties of carbon:
Carbon is relatively unreactive, hence reacts only under drastic conditions.
1) Reaction with H2O:
Carbon doesn’t react with water either in cold or hot. However, if steam is passed over red hot coke a mixture of CO & H2 (water gas) is obtained.
2) Reaction with acids:
Carbon is not attacked by dil. acids but conc. HNO3 and conc. H2SO4 oxidize it to carbon dioxide. Diamond remains unaffected by these acids. Graphite reacts with hot conc. HNO3 and gives mellitic acid [C6 (COOH)6].
3) With Halogens:
Diamond doesn’t react with halogens but graphite reacts with fluorine at 5000C and forms intercalation compounds, graphite fluoride (CF)n.
Compounds of carbon:
Carbon monoxide (CO):
Combustion or direct oxidation of carbon in limited supply of O2 produces carbon monoxide.
For small scale and laboratory use pure CO is obtained by dehydration of formic acid.
Other methods of CO preparation involve either reduction of CO2 (by Zn, C etc) or incomplete oxidation of C (by CO2, ZnO, Fe2O3 etc).
Properties:
CO is colourless, water insoluble and poisonous gas. Monoxide has strong tendency to bind with haemoglobin of RBCs of blood and forms carboxyhaemoglobin. This carboxyhaemoglobin is very stable and doesn’t let oxygen combine with it so that the supply of O2 to the body is cut off, which is the role of haemoglobin in blood circulation. This results into suffocation and finally death, if not treated.
Combustibility:
CO doesn’t support combustion but burns itself in air with pale blue flame and gives CO2 exothermically.
2CO + O2 2CO2 + Heat
Reducing nature:
CO reduces all metallic oxides other than that of alkali & alkaline metals.
ZnO + CO Zn + CO2
Fe2O3 + 3CO 2Fe + 3CO2
It reduces even I2O5 into I2.
I2O5 + 5CO I2 + 5CO2
Reaction with H2:
Under high temperature and pressure and in presence of catalyst CO gets reduced to CH3OH.
Reaction with Cl2:
When CO reacts with chlorine in the presence of sunlight, phosgene is formed.
(Phosgene or carbonyl chloride)
Reaction with NaOH:
The reaction takes place under high pressure to form sodium formate.
Formation of metal carbonyls:
Because of comparatively low nuclear charge over carbon it cannot hold its lone pair as strongly as by other elements (like N, O etc).

So CO is able to work as e– donor (Lewis base) and reacts with certain metals at high temperature and forms metal carbonyls.
Ni + 4CO [Ni(CO)4] (Nickel carbonyl)
Similarly, [Fe(CO)5] and [Cr (CO)6] are also formed. These complexes because of direct metal–carbon bond may be regarded organometallic compounds although they are coordination compound or complexes since they have coordinate linkage.
This is why heamoglobin having Fe2+ bonds with CO more strongly (300 times) than O2, which is not as good Lewis donor as CO.
These carbonyls decompose on heating at higher temperature and pure metal is obtained.
[Principle of Mond’s processes – extraction of nickel]
Uses:
– As fuel
– Production of water gas and producer gas
– Metallurgy of nickel (Mond’s process)
– Reducing agent at high temperature (in metallurgy)
Carbon dioxide (CO2):
Complete combustion of carbon, carbon monoxide or hydrocarbon produces carbon dioxide.
C + O2 CO2 + Heat
C + O2 CO2 + Heat
CH4 + 2O2 CO2 + 2H2O + Heat
Bicarbonates and carbonates are the other sources of CO2.
M = alkali metals
M = alkaline earths & Cu, Hg.
Carbonates when treated with mineral acids also give CO.
CO32– + acid Salt + H2O + CO2
CO2 is also obtained as by-product in some reactions like fermentation e.g.,
C6H12O6 2C2H5OH + 2CO2
Properties:
It is colourless gas with faint pungent smell, 1.5 times heavier than air, fairly soluble in water and non-poisonous, but it doesn’t support life because animals die in it due to lack of oxygen. In atmosphere it is approx 0.03% (by volume). It is taken by green plants where it is converted into carbohydrates (e.g., glucose) in the presence of chlorophyll and sunlight – the process called photosynthesis.
[*radio – labelled oxygen]
Excessive production of CO2 from increased fossil fuel, combustion & excessive decomposition of lime stone (in cement industry) is increasing the CO2 content of our globe. This layer of CO2 works like a green house wherein the light can enter but not escape, thereby the heat content of earth is increasing. This increase of temperature of our earth is called green house effect, which has many serious consequences.
CO2 can be solidified by converting it into liquid CO2 under high pressure (50–60 atm.), followed by expanding it through a nozzle. Excessive cooling takes place which makes it to solidify and deposit over the walls of the vessel like ice. Hence, it is called dry ice and used as refrigerant in food industry. Since it doesn’t support combustion, it is also used as fire extinguisher.
Acidic Nature:
CO2 dissolves in water and gives carbonic acid, hence also be called as carbonic anhydride.
H2O + CO2 H2CO3
Carbonic acid is very weak dibasic acid and forms two types of salts i.e., HCO3– (bicarbonate) and CO32- (carbonate).
Reaction with alkalis:
Being acidic CO2 reacts with alkalis and produces bicarbonates and carbonates.
CO2 + NaOH NaHCO3
Sodium bicarbonate
NaHCO3 + NaOH Na2CO3 + H2O
Sodium carbonate
Reaction with lime water produces a milky solution, which becomes clear (colourless) when CO2 is bubbled for more time. However, the milkiness reappears if the solution is heated.
lime water calcium carbonate calcium bicarbonate
(colourless) (milky) (clear)
The reaction is used in the detection of CO32- and HCO3– ions.
Reaction with Metals: Although CO2 neither burns itself nor supports burning but very active metals like Na, K, Ca etc. continue burning in the jar of CO2 and reduce it to elemental carbon.
CO2 + 4Na 2Na2O + C
Na2O + CO2 Na2CO3
CO2 + 2Mg 2MgO + C
Uses:
– In soft drinks
– As a fire extinguisher
– In manufacture of Na2CO3 & NaHCO3
– As refrigerant in the form of dry ice
– In artificial respiration as carbogen (O2 having 5–10% CO2) for the victims of CO poisoning
Halides of carbon:
Carbon combines with all the halogens to from tetrahalides (e.g., CF4, CCl4, CBr4 & CI4) and mixed halides (e.g., CFCl3, CF2Cl2, CCl4Br) along with other halides which have H as well (e.g., CHX3 – CHCl3, CHBr3, CHI3).
Properties:
All halides are covalent with tetrahedral geometry. The mixed halides of fluorine and chlorine are chemically inert, non-flammable and exist either as gas or liquid. They find use in various industries as follows:
CCl4 common solvent for plastic, resins etc.
CCl4 (pyrene) fire extinguisher (esp. for metal fires)
CF2Cl2 (freon) as refrigerant
Carbides:
Carbon when combined with more electropositive element than itself, gives carbides. The carbides are of three types
– Ionic or salt–like
– Covalent
– Interstitial
Ionic carbides:
Elements of group 1, 2, 10, 11, 12, 13 & some lanthanides form this type of carbide having units as carbide ions (-CC – )2-. They react with water and give hydrocarbons e.g., CaC2, BeC2, Al4C3 etc.
Covalent carbides:
Elements with slight difference in electronegativity i.e., B & Si form this type of carbides e.g., B4C – harder than SiC and used as abrasive and radioactive shields. SiC (carborundum) – abrasive & refracting material.
Interstitial carbides:
Transition metals form this type of carbides. The carbon atom occupies the interstitial space in these structures. They are very hard with high mp.
e.g., WC & carbides of Cr, Mn & Fe group metals
(WC – used for making, cutting & trilling tools).
Sulphides of carbon:
Carbon forms three types of sulphides CS, CS2 & C3S2 like oxides of these, CS2 is most stable and important. Passing the vapors of S over red hot coke produces carbon disulphide (Zahn’s process)
Now–a– days natural gas has become the source of CS2.
It is colourless, volatile liquid (bp 460C) highly inflammable & ignites spontaneously at 1000C. CS2 is highly poisonous and affects the brain and other parts of nervous system. Main uses of CS2 include solvent for S, P, I, Br, resin, wax and in manufacture of rayon. CS2 also finds its used in various insecticides & fungicides.
Fuel gases:
Any combustible material which may supply heat when burnt, without producing any objectionable by-products, is a fuel. A good fuel should
i) be cheap
ii) yield little ash
iii) have a high heat of combustion
iv) not give any undesirable by-product.
Out of solid, liquid & gaseous fuels, the gaseous fuel have advantage in high heat content, easy flow & no left-over (ash) after combustion, hence widely used in domestic and industrial applications. Some common gases fuels:
Coal gas:
The volatile products obtained from destructive distillation of coal have many combustible gases. When soluble impurities are removed from it coal gas is obtained.
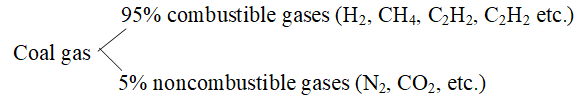
Manufacture:
Coal, placed in vertical retorts, is heated to 1275–1675K. The volatile products (heavy brownish smoke) start to evolve are passed through hydraulic mains and condensers where water soluble and tarry constituents are removed by dissolving and condensing respectively. The uncondensed gas is passed through a scrubber, where cold water is flowing; it removes NH3 as ammonical liquor ( 20%).
Now, the gas is passed through purifier where hydrated ferric oxide and slaked limes remove volatile sulphur compounds. The purified gas is collected in gas holders.
Uses:
– A gaseous fuel
– As illuminant
– For producing an inert or reducing atmosphere in various metallurgical & other process.
By-products: During manufacturing of coal tar by products obtained are as follows:
Coal tar: Thick black liquid, collected at condenser, has various aromatic hydrocarbons e.g., benzene toluene, naphthalene etc.
Ammonical liquor: Aqueous layer over water and from scrubber (commercial source of NH3).
Coke: Non volatile residue left in retort, used as a fuel & reducing agent.
Gas carbon: Finely divided carbon scrapped from the walls of retort.
Water gas:
A gas produced by passing steam over white hot coke and consisting of carbon monoxide & hydrogen is called water gas.
H = – 1215 k
Water gas
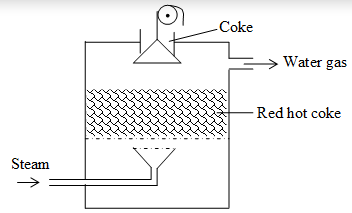
The reaction is endothermic however, blast of steam lowers the temperature of glowing coke. So, the production of water gas becomes discontinuous. Therefore, a blast of air is passed through the coke to raise the temperature.
C + O2 CO2 H = – 406 kJ
So, during “steam-run” water gas is produced & during “air-run” nitrogen, CO & CO2 are produced, making it an intermittent process.
Uses:
– As a gaseous fuel
– Source of industrial hydrogen
– For lightening purpose using Welsbach mantles
– For manufacturing of carbureted water gas
Carbureted water gas:
When water gas is mixed with some hydrocarbons, from the cracking of petroleum oils to enhance its calorific value is called carbureting & the gas as carbureted water gas.
Composition:
Hydrogen 30 – 40%
Saturated hydrocarbons 15 – 20%
Unsaturated hydrocarbons 10-15%
Carbon monoxide 20-28%
Carbon dioxide 0.2%
Nitrogen 2.5 – 5%
Producer gas:
When coke is burnt in the limited supply of air, a mixture of CO and N2 is formed, called producer gas. At bottom, carbon burns to CO2 which gets reduced to CO at red hot temperature of coke when comes above the coke bed. As a result the mixture of CO, N2 and traces of CO2, along with some hydrocarbons comes out.
C + O2 CO2 H = – 406kJ [in lower part]
CO2 + C 2CO H = 163kJ [in upper part]
Composition:
Carbon monoxide 30 – 40%
Nitrogen 50%
Hydrogen 2 – 5%
Some amount of carbon dioxide.
Uses:
– As a gaseous fuel in various metallurgical processes
– As substitute of petrol (as in World War–II]
Semi water gas:
Water gas can not be produced continuously because of lowering of temperature of coal as the reaction of steam over hot carbon is endothermic. At the same time the combustion of carbon being exothermic – the heat evolved in the reaction is lost upto 30% if not used just after its production. Since blowing of air to rise the temperature takes some time which is enough to loose the energy if the gas is stored. So, the mixture of air and steam in appropriate proportion is passed through red hot carbon to get a continuous source of fuel gas, called semi water and thus the whole heat can be utilized.
2C + O2 2CO H = – 226kJ (In producer gas)
C + H2O CO + H2 H = 117 kJ (In water gas)
The mixture in outlet in fact contains water gas and producer gas.
Composition:
H2 10 – 12% CO2 4 – 5%
CO 25 – 28% N2 50 – 55%
CH4 etc. 1-1.5%
Uses:
- Fuel in steel industry
- In internal combustion engines
Oil gas:
It is obtained by thermal decomposition of the kerosene in an iron retort, heated by furnace in the absence of air. The long chain hydrocarbons break up into simpler hydrocarbons, called cracking, which is an irreversible process.
Uses – Commonly used in laboratories
Natural gas:
Putrefaction of vegetable material under water produces methane which is actually the so called marsh gas. CH4 is the main constituent of natural gas, which is obtained above the layer of petroleum in the petroleum wells. It’s a low cost excellent fuel. When compressed under high pressure, it converts into the liquid, which is called compressed natural gas (CNG), a subtituent of diesel & petrol in the selected cities.
Exercise 3:
(i) Which of the following is not hydrolysed?
(A) CCl4 (B) SiCl4 (C) GeCl4 (D) SnCl4
(ii) Which of the following carbides is an ionic carbide?
(A) SiC (B) B4C (C) TiC (D) CaC2
(iii) Which of the following statements is not correct regarding graphite crystal?
(A) The carbon atoms are arranged in planar layers
(B) Each carbon in graphite is sp2 hybridised
(C) The atoms in each layer is not tightly bonded together
(D) The binding force between layers is weak allowing the layers to slip over each other
(iv) Which of the following compounds of carbon may be considered a resonance hybrid?
(A) C2H5OH (B) C6H6 (C) C4H10 (D) C2H2
Silicon:
Silicon is obtained by the reduction of silicon compounds by suitable methods e.g., that of sand (SiO2) by coke, of silica by Mg etc.
S and
Silicon exists in two allotropic forms: amorphous and crystalline. The former is more reactive dark brown powder; mp 1693 K, mp 1793K. Crystalline Si is similar to graphite in structure & properties. Silicon is semiconductor, having its electrical conductivity equal to graphite at normal temperature. Chemically it is unreactive, and reacts only with fluorine at room temperature. At higher temperature Si forms oxides, halides, carbides and silicides etc.
Compound of silicon:
Silicon dioxide :
95% of earth crust is made up of silica & silicates. Silica is and exists in 12 crystalline forms, of which the main are: quartz, tridymite and cristobalite. -Quartz is most common and main constituent of granite & sandstone.
Quartz (rock-crystal) – Purest form
Sand – Crushed form of quartz
Sandstone – Sand particles bound with iron oxide
Flint – Amorphous silica, associated with quartz
Kieselguhr – Siliceous rock with remains of minute sea animals
[Used as adsorbent & polishing powder]
In living beings it is present upto considerable extent (for example) in:
Plants: Stem and exterior coatings of straw and bamboo, rice husk etc.
Animals: quills of feathers, claws of animals & finger nails etc.
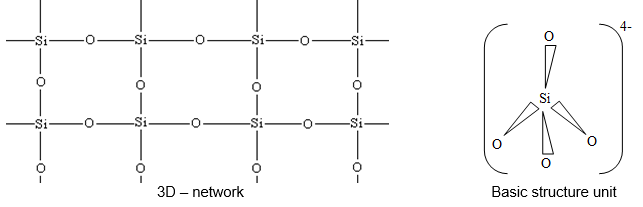
is covalent, 3–dimensional network solid, wherein each silicon atom is covalently bonded tetrahedrally to four oxygen atoms, and in turn every oxygen is bound with two silicon atoms.
Silica is highly unreactive because of strong Si – O bonds. It is not attacked by halogens acids, metals and hydrogen even at high temperature However it reacts with HF and NaOH.
At elevated temperature the bonds of polymeric chain become weaker and it converts into like structure, hence is behaves like powerful acid anhydride and reacts with bases and many basic compounds (e.g., metal oxides, metal carbonates etc.) to form salts (cf, slag formation in metallurgy of Fe & Cu).
Uses:
- Manufacture of chemical apparatus and optical instruments (“vitreosil”).
- Coloured varieties as gems (opal, amethyst, jaspar)
- Silica gel as dessicant and adsorbent in chromatography
- Silica – refractory bricks for furnace linings
- Sand – glass, mortar and porcelain
- Sandstone – building material
- Kieselguhr – in filtration plants and as adsorbent for nitroglycerine(DYNAMITE)
- Quartz – highly elastic and tenacious threads for electrical instruments.
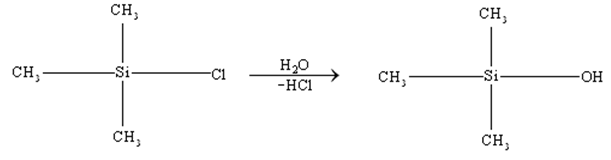
Silicones:
Silicones are the synthetic organosilicon polymers having repeating unit with chains. Silicones are so called because of their similarity with ketones.

Silicones are prepared from dialkyl or diaryl substituents of . These on hydrolysis followed by condensation give silicones.
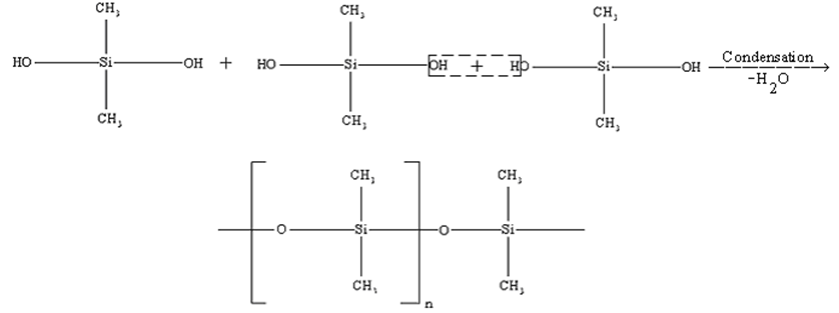
The length of the polymer chain is controlled by adding, which blocks the ends of the polymer and leaves no possibility of further condensation.
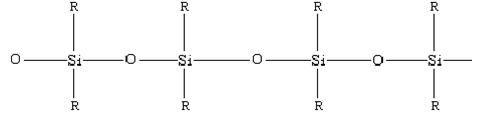
Silicones can be classified into:
Linear Silicones:
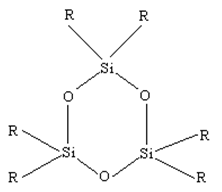
Cyclic Silicones:
Cross linked Silicones:
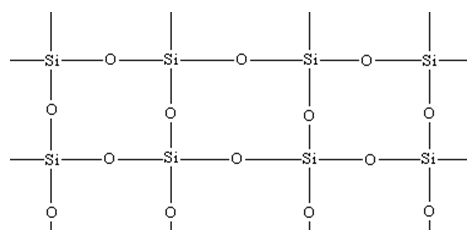
The silicones having methyl group are known as silicone oil. These can withstand more temperature than mineral oils (petroleum or paraffin oils) and do not thicken (increase of viscosity) much at lower temperature.
Silicone rubbers are linear silicones bridged with ethylene or similar group.
Uses:
- High temperature oil baths
- Lubricants over a wide range of temperature
- Water proof cloth and paper
- Insulating material
- Paints and enamels (to make them temperatures, light and chemical resistant)
Silicates:
A large % of silicates are present in the earth’s crust. It exists as in the form of silica, sand, quartz and flint. Other important silicate materials present in the earth crust are asbestos (calcium magnesium silicate, ), feldspar , zeolite and diopside . Silicates are the metal derivatives of silicic acid and have as their basic building unit with hybridized Si atom in tetrahedral shape. They are less closely packed than the structure of .
In silicates either there are discrete units or such units are present in a fashion that they share their corners with 1, 2, 3 or 4 oxygen atoms per silicate unit. Depending on the linking pattern, silicates are divided into 6 types:
| Type of Silicate | Structural unit | Sharing of O | Example |
| a) Orthosilicates | discrete units | — | |
| b) Pyrosilicates | simplest condensed silicates – units two tetrahedra joined through 1 O-atom | Two SiO4 units join through one O-atom |
Hemimorphite |
| c) Chain silicates | 2 O per unit | Spodumene | |
| two chains : some O share 2 & the other 3 | Tremolite | ||
| d) Cyclic silicates | 2 – oxygen per unit | Beryl | |
| e) Sheet silicates | 3O per units | Talc | |
| f) 3 – dimensional silicates | 4 oxygen per units | Zeolites (ZSM-5) |
Zeolites:
Zeolites are aluminosilicates with cations like having three dimensional structures wherein few Si atoms are replaced by & other ions.
The general formula,
x, y & z = any integer other than zero
n = charge on the cation
Some common zeolites:
Erionite
Gemelinite
Chabazite
ZSM – 5
Because of open structure of zeolites; they have channels & cavities of different sizes, having diameter upto 2 – 11 A0. The voids can be upto 50% of the volume of the zeolite. A variety of molecule like can be trapped into these cavities, depending on the reaction & selectivity of zeolites. For that, very reason zeolites act as ion-exchangers and molecular sieves. They trap from hard water to make it soft. ZSM–5 is used to convert alcohols directly into gasoline (petrol) by dehydration and giving a mixture of wide variety of hydrocarbons. Ultramarines:
Ultramarines are alumino silicates having no water of crystallization but some extra anions (like etc.). They are used as pigments ranging in colour from deep blue shade upto deep green.
Glass:
Ordinary glass, a transparent & translucent amorphous substance obtained by fusion of sodium carbonates and lime with sand, is a super cooled liquid (the properties don’t respond to that of a solid). Actually, glass is a solution of silica in a mixture of uncrystallized metal silicates. Because of much increase in viscosity, it acquires some apparent characters of a solid on cooling upto room temperature.
Since it is a solution, it has not any specific formula.
Glass remains unaffected by water, alkali and acids (except HF, which dissolves it)
Raw materials for manufacture of glass:
1) Silica (Ordinary sand) – should be free from Fe & organic impurities which provide colour to the glass
2) Alkaline earths – Lime, limestone or
3) Alkali metals – Carbonates and nitrates of sodium and potassium
4) Heavy metals – Litharge or red lead
5) Colouring material – Coloured glasses are obtained by adding certain substances into glass while in fused mixture or molten state.
| Colour | Colouring material |
| Yellow | (sodium uranate) |
| Green | |
| Purple | |
| Blue | CoO |
| Ruby | |
| Red | |
| Lemon Yellow | CdS |
| Amber | Organic matter (C) |
| Peacock Blue | CuO |
| Black | NiO & MnO |
Manufacture of Glass:
1) Fusion of raw materials
Some broken glass pieces (cullet) are added to the raw material and heated and until it melts. After fusion, undecomposed impurities (glass gall) are removed from surface.
2) Annealing:
If the fused mixture, as such or the glassware fabricated from it, is cooled at its own pace, the glass being brittle cracks or breaks up. Hence, the cooling is to be monitored precisely by making them travel on slow moving belt from higher to room temperature through a narrow chamber (lehr). It takes several days for an article to get annealed.
| Variety of glass | Composition (constituents) | Characteristic properties | Uses |
| Soft glass | Na & Ca silicates | Easily fusible | Window glass, common glass wares. |
| Hard glass | K & Ca silicates | Fusion difficult; more resistant to water & acid | Hard glass apparatus |
| Flint glass | K, Pb & Ca silicates | Lustrous, high refractive index | Bulbs, lenses prisms & optical devices |
| Bottle glass | Na & Ca silicate | Cheapest variety | Bottles |
| Jena glass | Zn & Ba borosilicates | Low coefficient of expansion; high resistance to heat, shock & common reagents | Round bottom flasks, condensers etc, beakers |
| Pyrex glass | Na & Al borosilicates | Low coefficient of expansion | Test tubes & heating glass wares |
| Crooke’s glass | Na & Ca silicates + Cerium oxide | Cuts off (absorbs) UV rays | Shields for UV rays Optical glasses for lenses. |
| Ground glass | Soft glass (ground by emery and turpentine) | The rough surface prevents smooth refraction | Window glasses |
| Quartz glass (silica glass) | Pure silica | Doesn’t break even when plunged in water while red hot | Chemical apparatus, Sheath of heating filaments |
| Reinforced glass | A network of wires is embedded | doesn’t shatter easily by the mechanical shock | Safety purposes |
| Safety glass or Shatter proof glass | A transparent layer of butyral plastic between two layers of glass | withstands high pressure and mechanical shock | Automobile wind shield, goggles. |
| Water glass |
Water soluble; solution alkaline due to hydrolysis |
…. |
Etching of glass:
The property of glass being attacked by HF is exploited in etching or engraving glass objects. The surface is coated with wax and the design to be engraved is marked by any pointed object. When the aqueous solution of HF is poured on it, the acid attacks only the exposed surface & not that under the wax. Washing away the wax and acid after few minutes gives an impression on it.
Silica garden (Chemical garden):
When a coloured salt like, etc., is added to the solution of sodium silicate (having density 1.1), hollow tubes of metallic silicate gel shoots up within few hours, giving a tree like structure, which are called silica or chemical gardens.
Illustration 4: Which of the following statements is not correct?
(A) All the elements of Group 14 form tetrahalides with the exception of PbI4.
(B) All tetrahalides of elements of Group 14 are tetrahedral.
(C) The stability of the halides of elements of Group 14 increases down the group.
(D) SiCl4 exhibits the phenomenon of hydrolysis.
Solution: (C)
The stability of the halides of elements of Group 14 decreases down the group.
Illustration 5: Which of the following statements is not correct?
(A) Carbon dioxide is a linear molecule with no dipole moment.
(B) Carbon dioxide and silicon dioxide have the same structures.
(C) Carbon dioxide is a gas whereas silicon dioxide is solid.
(D) In carbon dioxide, a double bond exists in carbon and oxygen whereas in silicon dioxide each silicon-oxygen bond is linked through a single sigma bond.
Solution: (B)
Carbon dioxide exists as individual molecule whereas silicon dioxide exists as giant molecule containing a network of S and O bonds.
Illustration 6: Which of the following statements is not correct?
(A) Organosilicon polymers are known as silicones
(B) Silicones have the general formula (R2SiO)n where R = – CH3, – C2H5, – C6H5, etc.
(C) Hydrolysis of dialkyldichlorosilane produces cross-linked silicon polymer.
(D) Hydrolysis of alkyltrichlorosilane produces cross-linked silicon polymer.
Solution: (C)
The hydrolysis of dialkyldichlorosilane produces linear silicon
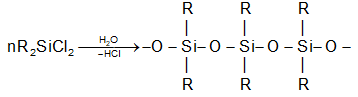
Exercise 4:
(i) Which of the following statements is not correct?
(A) The durability and inertness of silicones is due to the high bond enthalpy of Si–O bond.
(B) Silicones are used in water-proofing textiles.
(C) Silicon rubbers are excellent electrical insulators.
(D) The silicones always involve cross-linked between Si and O atoms.
(ii) Silicones contain
(A) C, O and Si
(B) C and Si
(C) O and Si
(D) C, N, O and Si
(iii) Synthetic zeolites have been prepared for use as
(A) lubricants
(B) molecular sieves
(C) semiconductors
(D) plastics
(iv) Carborundum is
(A) CaC2 (B) Fe3C (C) CaCO3 (D) SiC
TIN:
Occurrence:
Principal ore of tin is cassiterite or tinstone , which is called “black tin” by the miners to distinguish it from tin metal, the “white tin”. In India Sn is available as tinstone in Bihar and Orrisa.
Extraction:
Sn is extracted from tinstone, the impurities are siliceous matter, Mn, Fe, Cu etc. Following steps are involved in the extraction from its ore:
1) Crushing & Concentration:
Crushing by mall mills: The ore is converted into finely divided powder.
Concentration by gravity separation: Washing in running current of water removes the impurities that constitute lighter gangue particle. Impurities of iron and manganese tungstate are removed by the magnetic separation.
2) Roasting:
Heating in a revolving furnace volatilizes S & As as oxides and converts Cu & Fe into CuO and FeO.
3) Washing:
It removes soluble impurities giving 60-70% called “BLACK TIN”
4) Smelting:
The black tin is mixed with anthracite and smelted in reverberatory furnace. A little lime or fluorspar is added as flux to remove . 96.5% pure metallic tin is obtained.
5) Purification:
The tin obtained above has impurities of Fe, Pb, S & As and they are removed by:
a) Liquation: Metallic tin is heated upto its mp on sloping hearth. Tin melts and flows down leaving behind the impurities.
b) Poling: The metal is now stirred with green poles, while in molten state. This oxidizes the remaining impurities, which collect on the surface as ‘dross’ and are skimmed off.
c) Electrolytic purification: Electrolysis of impure tin is carried out with some hydrofluorosilisic acid as electrolyte in an electrolytic cell using black tin as anode and pure tin as a cathode. When current is passed, the tin dissolves from anode into the solution and then deposits onto the cathode in very pure form.
Uses of Tin:
1) Tinning: Containers and utensils (made of iron, steel, brass, copper etc) used for food stuffs are attacked by organic substances (mainly acids) present in them. Consumption of such food products leads to food poisoning due to these soluble metal salts or their hydrolysis products. To avoid such consequences, they are covered by a thin layer of tin, which is unattacked by organic acids-the process involved – is called tinning, achieved either by dipping the utensils in molten tin or by electroplating.
2) Tin Plating: Covering the sheets of iron or steel by a layer of tin plate is called tinplating which prevents metal to be attacked by air, moisture etc and employed for containers of tea, cigarettes, kerosene etc.
3) Mordent in dyeing and calico-printing
4) Tin-amalgam in making mirrors
5) Preparation of various alloys for different uses:
| Alloy | % Composition | Uses |
| Bronze | Sn 10% , Cu 90% | Utensils, coins, statues |
| Bell metal | Sn 20% | Bells |
| Gun metal | Sn10% , Cu86% , Zn 4% | Guns, gears |
| Type metal | Sn 5% , Sb 20% , Pb 75% | ‘Types’ for printing |
| Solder | Sn 50-60% , Pb 40-50% | Soldering wire |
| Pewter | Sn 75% , Pb 25% | Utensils |
| Antifriction alloy | Sn 82% , Sb 14% , Cu 4% | Bearings for machinery |
| Babbit metal | Sn 90% , Sb 7% , Cu 3% | Machinery |
| Britannia metal | Sn 93% , Sb 5% , Cu 20% | Table wares |
Lead:
Occurrence:
In nature, lead occurs as carbonate (cerrusite-) and as sulphate (anglesite-), but the principle ore is the sulphide (galena-PbS), which exists as grayish black crystals. Metal Corporation of India is locating the lead deposits in India besides the depositions at Udaipur & Jaipur (Rajasthan)
Extraction: The processes involved in the extraction are:
1) Concentration – by froth floatation (concentrated ore 50 – 60%)
2) Roasting – in excess of air and in reverberatory furnace
3) Self reduction -supply of air is cut off and more galena is added
Uses:
- Lead pipes and containers for corrosive gases and liquids
- Protective covering of underground cables
- Screens or shields for radioactive radiation
- Lead chambers in of
- Storage batteries
- Antiknocking material, TEL i.e., [Obsolete]
- of pigments (white lead, litharge, read lead, lithopone etc.,)
- In many alloys
Alloys of Lead:
| Name of Alloy | Composition | Uses |
| Type metal Solder Pewter | Discussed earlier | |
| Fusible alloys | ||
|
Wood’s metal Lipowitz alloy Rose’s metal Newton’s metal |
Pb 25% , Bi 50% , Su 12.5% , Cd 12.5% Pb 27% , Bi 50% , Su 13% , Cd 10% Pb 28% , Bi 50% , Su 22% Pb 31% , Bi 50% , Su 19% |
mp 333K electric fuse, soft solder, safety plug, automatic water sprinkler |
Plumbosolvency: The solvent action of water on Pb, which dissolves in it in presence of air forming water soluble lead hydroxide is called plumbosolvancy. The solubility is increased in the presence of salts and organic matter, whereas decreased in the presence of . All the lead salts are poisonous and accumulate in the body, which lead into colic fits followed by paralysis and ultimately death (lead poisoning).
Compounds of Sn:
Tin exists in three allotropic forms:
Rhombic – Crystalline
Tetragonal – Crystalline
Grey – Amorphous
Ordinary form of tin is the white tin, a silver-white, softer than zinc, but harder than lead. Conversion of white tin into grey is accompanied by the increase of volume. Grey tin is very brittle and at low temperature, it crumbles down into powder. In very cold climate, this much is most probable to occur and is called tin disease or tin pest or tin plague.
If a tin rod is bent, a crackling sound is heard, called tin cry which is due to rubbing of the crystals against one another.
Compounds of tin:
Stannic oxide ():
Air and water don’t attack tin under normal temperature. But when heated strongly (1775-1875K) it burns in air with a bright flame forming .
is the amphoteric oxide and reacts with conc. to give sulphate as well as with NaOH to give stannate.
Uses:
1) As polishing powder
2) Glazing of pottery
Stannous oxide (SnO):
SnO (Stannous oxide) is however, prepared by heating tin oxalate
The presence of CO prevents oxidation of SnO into . SnO is also amphoteric in nature.
Stannous chloride (SnCl2):
SnCl2 is prepared by dissolving tin in conc. HCl. On cooling, the crystals of SnCl2.2H2O separate out. In order to prepare anhydrous SnCl2, tin is heated in the current of HCl vapours.
In acidic solution acts as strong reducing agent.
a)
b)
c)
d)
e)
Stannic chloride (SnCl4):
It is prepared by passing Cl2 into molten tin in a retort.
Distillation of tin with excess of mercuric chloride also produces stannic chloride
SnCl4 is colourless fuming liquid having unpleasant odour. It’s a covalent compound soluble in benzene & the solution being non-conductor of electricity.
Illustration 7: The dissolution of stannous hydroxide in excess base produces
(A) Sn(OH)4 (B) Sn(Oh)62– (C) SnO32– (D) Sn(OH)3–
Solution: (D) Sn(OH)2 + OH– Sn(OH)3–
Lead:
Lead is soft, bluish grey, heavy, highly malleable but less ductile metal with poor electrical conductivity. It marks the paper black.
Compounds of lead:
Lead gives two types of salts:
1) 2)
Generally lead salt means salt e.g., lead chloride means as salts are more stable than .
Dry air has no action on lead but moist air forms a layer of basic lead carbonate due to absorption of by the base formed.
Lead monoxide (PbO):
PbO exist as:
1) Red (tetragonal form) LITHARGE – stable at RT
2) Yellow (Orthorhombic form) MASSICOT – stable above
Molten lead reacts with air or above to give PbO (litharge)
Heating Lead nitrate or Carbonate also gives lead monoxide. It is also a byproduct of cupellation process in the silver-metallurgy.
PbO is insoluble in water and amphoteric in nature.
Uses:
- Glass industry
- Paint and varnish industry
- Glazing of pottery
- Cement for glass and stones (Massicot + Glycerine)
Red Lead (Minium ):
It is prepared by heating Lead (II) oxide in air at in a reverberatory furnace.
It is red powder, insoluble in water and decomposes into PbO on heating above . It’s mild oxidizing agent and oxidizes HCl to & to .
is in fact a mixture of PbO and
Uses:
- Protective paint for iron & steel
- Glass and match industry
- Oxidizing agent
- Cement for joining iron pipes
Lead dioxide (Lead Peroxide ):
is obtained as chocolate brown powder when red lead is treated with nitric acid.
Oxidation of Lead (II) salt with bleaching powder also gives .
It is powerful oxidizing agent. On heating alone or with conc. it gives .
Cold HCl and hot NaOH react with to give Pb (IV) salts.
Exercise 5: (i) Which of the following is red lead?
(A) PbO (B) Pb3O4 (C) Pb2O (D) PbO2
(ii) Which of the following is white lead?
(A) Pb(OH)2.2PbCO3
(B) Pb(OH)2.Pb(CH3COO)2
(C) Pb(OH)2
(D) PbCO3
(iii) The dissolution of stannous hydroxide in excess base produces
(A) Sn(OH)4
(B) Sn(OH)62–
(C) SnO32–
(D) Sn(OH)3–
(iv) Iron may be rendered passive by application of
(A) PbO
(B) Pb(OH)2
(C) PbCO3
D) Pb3O4
Answer to Exercises
Exercise 1: (i) D (ii) B (iii) D (iv) C
Exercise 2: (i) D (ii) A
Exercise 3: (i) A (ii) D (iii) C (iv) B
Exercise 4: (i) D (ii) A (iii) B (iv) D
Exercise 5: (i) B (ii) A (iii) D (iv) D
FORMULAE AND CONCEPTS AT A GLANCE
1. Group IVA or 14 of the periodic table consists of five elements-carbon, silicon, germanium, tin and lead.
2. Carbon is seventeenth and silicon is second most abundant element by mass in earth’s crust. Carbon is found both in native and combined state. Carbon is an essential constituent of all living matter as proteins, carbohydrates and fats.
3. Silicon occurs very widely as silica and a wide variety of silicate minerals and clays.
4. Tin and lead are comparatively low but are found as concentrated ores.
5. Non-metallite nature decreases from carbon to lead.
6. All the elements show tetravalency due to sp3 hybridization. Last three members show inert pair effect and show the tendency to form M2+ ions. The stability of M2+ state increases as the atomic number increases.
7. The tendency of formation of long open or closed chains by the combination of same atoms in themselves is known as catenation. The property is maximum in carbon and decreases down the group.
8. Carbon exists as diamond, graphite, coal, charcoal, lampblack and fullerene. Silicon exists in two forms, crystalline and amorphous; germanium exists in two crystalline forms and tin exists in three forms; grey, white and rhombic form.
9. Carbon has three amorphous forms. These are coal, charcoal and lampblack.
10. The elements of group 14 react with oxygen on strong heating to form oxides of the type MO2. The acidic nature decreases with increase in atomic number. CO2 and SiO2 are acidic while GeO2, SnO2 and PbO2 are amphoteric in nature.
11. Except carbon, all other members of group 14 dissolve in aqueous NaOH solution evolved hydrogen.
12. All the members of group 14 form covalent hydrides of type MH4. Besides CH4, carbon forms a large number of hydrides, saturated and unsaturated.
13. All the member of group 14 form tetrahalides of the type MX4 except PbBr4 and PbI4. The halides are covalent and formed by sp3 hybridization.
14. The carbides are binary compounds in which carbon combines with elements of lower or about equal electronegativity.
15. (i) Water gas or synthetic gas is a mixture of CO and H2.
(ii) Producer gas is a mixture of CO and N2.
(iii) Coal gas is a mixture of hydrogen, methane, carbon monoxide, acetylene, etc.
16. Sodium silicate is commercially called water glass. It is chemically sodium metasilicate containing an excess of silica.
Its composition varies from Na2SiO3.SiO2 to Na2SiO3.3SiO2. It is soluble in water and its solution is alkaline.
17. Silicones are organosilicon polymers containing Si-O-Si linkages. These are formed by the hydrolysis of alkyl or aryl substituted chlorosilanes and their subsequent polymerization.
18. Glass is a transparent or translucent super cooled solid solution of silicates and borates.
19. The solution of lead acetate is sweet in taste and is known as sugar of lead but it is poisonous in nature.
20. Lead is mainly extracted from galena (PbS) ore. The ore is concentrated by froth floatation process. It is extracted either by air reduction process or carbon reduction process.
SOLVED PROBLEMS
Prob 1. In silicon dioxide
(A) each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bonded to two silicon atoms
(B) each silicon atom is surrounded by two oxygen atoms and each oxygen atom is bonded to two silicon atoms
(C) silicon atom is bonded to two oxygen atoms
(D) there are double bonds between silicon and oxygen atoms
Sol: (A) Each silicon atom is surrounded tetrahedrally by four oxygen atoms.
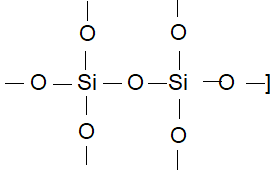
Prob 2. Silica reacts with magnesium to form a magnesium compound (X). (X) reacts with dilute HCl and forms (Y). (Y) is
(A) MgO
(B) MgCl
(C) MgSiO
(D) SiCl4
Sol: (B)
Prob 3. Silica is reacted with sodium carbonate. What is the gas liberated?
(A) CO
(B) O2
(C) CO2
(D) O3
Sol: (C) SiO2 + Na2CO3 Na2SiO3 + CO2
Prob 4. Buckminsterfullerene is
(A) graphite
(B) diamond
(C) C–60
(D) bone charcoal
Sol: (C) Fullerene, a carbon allotrope, consists of spherical C-60 molecules with the extra ordinary shape of a soccer ball. It has 12 pentagonal and 20 hexagonal faces with each atom sp2 hybridized and bonded to three other atoms.
Prob 5. Pb and Sn are extracted from their chief ores by
(A) carbon reduction and self reduction
(B) self reduction and carbon reduction
(C) electrolysis and self reduction
(D) self reduction and electrolysis
Sol: (B) PbS + 2PbO 3Pb + SO2 (self reduction);
SnO2 + C Sn + CO2 (carbon reduction)
Prob 6. . The compound (C) is
(A) SiO2
(B) Si
(C) SiC
(D) Na2SiO3
Sol: (D)
Prob 7. Which tetrahalide does not act as Lewis acid?
(A) CCl4
(B) SiF4
(C) GeCl4
(D) SnCl4
Sol: (A) CCl4 does not form hexachloro complex as d-orbitals are not present in carbon but rest of the tetrahalides can form hexahalo complexes.
Prob 8. Lead oxide (PbO) can be dissolved in
(A) HNO3
(B) HCl
(C) H2SO4
(D) H2O
Sol: (A) PbO dissolves in HNO3 as it forms lead nitrate which is soluble.
PbO + 2HNO3 Pb(NO3)2 + H2O
HCl forms insoluble PbCl2 and H2SO4 forms insoluble PbSO4.PbO is insoluble in water also.
Prob 9.
The compound (E) (g) is
(A) CO
(B) CO2
(C) CH4
(D) C2H2
Sol: (D)
Prob 10. An inorganic compound (A) made of two most occurring elements in the earth’s crust and used in building construction when made to react with carbon, forms a poisonous gas (B) which is most stable diatomic molecule. Compounds (A) and (B) are
(A) SiO2, CO2
(B) SiO2, CO
(C) SiO2, N2
(D) CaO, CO2
Sol: (B) SiO2 is a compound of oxygen and silicon, the two most abundant elements of earth’s crust and is used in building construction.
SiO2 + 2C Si + 2CO (poisonous gas and a stable diatomic molecule).








