IONIC EQUILIBRIUM (ACIDS & BASES)
Like chemical equilibrium that exists between two or more chemical species of a reversible chemical reaction, an ionic equilibrium exists between an electrolyte and its ions:
Some useful terms regarding ionic equilibrium:
1) Electrolyte: The substances which produce ions when dissolved in suitable solvents or when in molten state; so called, as they disintegrate when electricity is passed through their solution. [electro = electric current, lysis = disintegration] electrolytes can be divided into
a) Strong electrolytes: The substances which dissociate almost completely into ions in liquid state (molten or solution) are strong. Since the charged particles are responsible for the flow of electric current, the solutions of strong electrolytes are good conductor of electricity. Degree of dissociation or ionization of these substances is high i.e.,
e.g., HCl, NaOH, NaCl, CH3COONH4 etc.
b) Weak electrolytes : The substances which dissociate upto small extent in their liquid state are weak electrolytes. Since there are very few ion which can carry current, these are poor conductor of electricity. We say that their degree dissociation is less i.e.,
e.g., CH3COOH, NH4OH etc.
Since strong electrolytes are almost completely ionized, they do not form an equilibrium between unionized and ionized forms, but weak electrolytes do form an equilibrium between its ions and unionized form called ionic equilibrium.
Alternatively electrolytes can also be categorized as:
a) True electrolytes: In true electrolytes positively charged cation and negatively charged anion exists in pure state, e.g., crystals of CuSO4, MgS, NaCl etc. When ionic crystal is dissolved in a solvent, ions break off from the crystal and get surrounded by a sheath of solvent molecules called solvated ions break and the process, called salvation. In cases when water is a solvent, hydrated ions are produced and the process is called hydration.
All the hydrated species are represented by the term (aq) following the name of the chemical species.
True electrolytes are good conductor of electricity in pure liquid state (i.e. when no solvent is used).
b) Potential electrolytes: In pure state these are uncharged molecules but when dissolved in a solvent, they react upto some extent with solvent to furnish ions. e.g., CH3CO2H, HCl etc.
These are poor conductor of electricity in pure liquid state.
2) Non-electrolytes: A chemical species whose melt or solution doesn’t furnish any ion, is called non-electrolyte. e.g., urea, sugar etc.
3) Polyelectrolytes: The electrolytes which are polymeric in nature are called polyelectrolytes. e.g., RNAs and DNAs, which have ionizable acidic (PO43–) groups and proteins which have ionizable acidic monomers
Depending on their chemical nature, electrolytes can also be divided into acids, bases and salts, which requires an account of the theories/definitions put forth by variations workers to explain the acids and bases.
Various concepts of acids and basis:
1. Classical concept: This concept is based on the experiences of various early workers & there is no any explanation of their such properties:
| Acids | Bases |
|
|
Acidus (Lat.) = Sour
The above operational definitions can be explained on the basis of conceptual definitions of acids and bases given by various chemists like Arrhenius, Bronsted-Lowry and Lewis.
2. Arrhenius concept of Acids & Bases:
A substance that dissociates to give H+ in aqueous solution is called an acid, like HCl, CH3CO2H etc.
In general,
And, bases are the substances that furnish OH– in its aqueous solution e.g., NaOH, NH4OH etc
In general,
Existence of H+ and OH–:
High dielectric constant of water () lowers the attractive forces of between positive and negative ions, which results into dissociation of the electrolyte.
Because of salvation of H+, It exists as H3O+ (hydronium ion); some other species present in protonated aq. solutions are
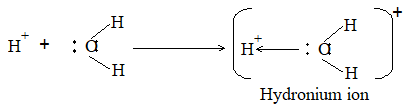
Similarly, the hydration of OH– results into the formation of etc.
Such that we can write:
Actually a H+ is surrounded by four H2O molecules tetrahedrally.
Oxides of many non-metals dissolve in H2O to give acids, these are called acidic oxides or acid anhydrides.
Similarly, oxides of many metals dissolve in H2O to form bases are called basic oxides.
NH3 and N2H4 are also base as they produce OH– when in aq. solution.
Strength of acid/base: More the number of H+/OH– (ions) furnished by an acid or a base, greater is the strength of that acid/base (electrolyte).
Arrhenius described neutralization as the process in which H+ ion & OH– ions combine to form unionized molecule of water.
e.g.,v
As the result of this process, the characteristic properties of acids and bases are destroyed.
Limitations of Arrhenius Theory:
1) Presence of water as a solvent is a necessary condition to display acidic & basic property according to the theory. So, HCl would not be an acid neither it will display acidic properties in any other solvents like acetone.
2) A supposition that H2O reacts first with a substance and produces H+ or OH– thus behaves like an acid (in case of oxide of non-metals) or base (in case of NH3 & metallic oxides).
3) Acidic nature of AlCl3, BF3, CO2 couldn’t be explained.
4) Acidic & basic characters of substances in non-aqueous solvents can’t be explained.
5) Neutralization process of acids & bases that don’t occur in aq. or any other solution, can’t be explained.
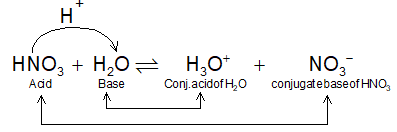
3. Bronsted–Lowry concept: (The proton – Donor – Acceptor concept)
In 1923, Bronsted & Lowry independently proposed a theory depending on the behaviour of a substance towards the proton. So that,
An acid is the chemical species that can donate a proton (H+).
And, the base is the chemical entity that can accept a proton, e.g.,
When an acid loses a proton it has tendency to regain its H+ and behave like a base called conjugate base. The same is true about a base which after accepting a proton turns into an acid called conjugate acid.
Conjugate acids and bases:
A pair of Bronsted acid and a bse that differs by one proton is known as conjugate acid – base pair. A strong acid has a weak conjugate base and a strong base has a weak conjugate acid.
Conjugate acids and bases are also called Bronsted-Lowry (or generally Bronsted) acids and bases.
The above reaction can be understood by breaking it into 2 parts, called conjugate pairs.
So we can write acid1 changes into base1 and base2 changes into acid2 after transfer of H+:
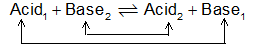
Examples:
Evidently, according to this theory a substance can behave like an acid only when another substance, capable of accepting the H+ is present. For example, HNO3 or acetic acid can donate their H+ to H2O molecules, so they are acidic in aqueous solutions. But if they are present in benzene solution, they won’t work as acid since benzene molecules do not accept the proton.
Acetic acid is weak acid in presence of water but in presence of stronger acid HF it has to accept H+ from it & thereby behaves like a base.
More interesting is the example of the strong acid, HNO3 which in (absence of water and) presence of H2SO4 can’t donate its proton to any other species, rather it being weaker than H2SO4, accepts H+ from the latter and becomes a base. [Refer to the nitration of benzene, where NO2+ (nitronium ions – the electrophiles) are generated as above and therein the main role of anhydrous H2SO4 is to produce NO2+ along with absorption of H2O formed during the reaction]
Any chemical species can be an acid as well as a base depending upon its ability to accept and/or lose the proton.
Above reactions called autoionization explain that within a solvent some molecules of same substance can behave like an acid & the other as a base.
etc are amphiprotic or amphoteric due to their ability to accept or donate H+.
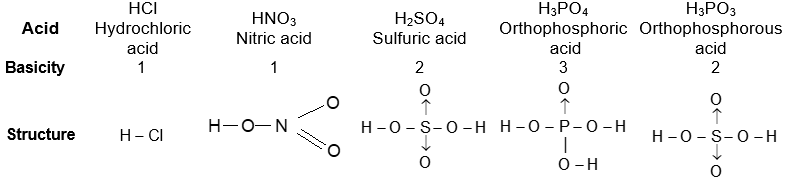
Basicity or Protocity of a Bronsted Acid:
The number of H+ furnished (ionizable protons) by a molecule of an acid is known as basicity of acid. e.g.,
Acidity or Hydroxicity of Bronsted Based
The number of OH– furnished by one molecule of a base is its acidity.e.g.,
| Base | NaOH | Ca(OH)2 | Al(OH)3 |
| Acidity | 1 | 2 | 3 |
Since H+ are transferred from an acid to a base, there is no free existence of H+. Either they become part of the conjugate acid else combine with H2O molecule to give H3O+ (called hydronium ion due to similarly with ammonium ion) which itself is a conjugate acid.
Relative Strength of Bronsted Acid-Base Pairs:
More the tendency of an acid to lose a H+ more easily it will dissociate, i.e., greater the ionization or dissociation stronger the acid.
HCl can give its proton more easily to water in comparison to CH3COOH, so HCl is stronger than CH3COOH.
ionization or dissociation constant
HCl is a stronger acid while is weaker and H2O is a stronger base compared to Cl–. This shows that Cl has little tendency to take up protons.
As already seen it can be inferred that always a stronger acid has a weaker conjugate base and a stronger base has weaker conjugate acid.
Therefore greater the Ka, more the concentration of H+, more the tendency to give up H+, stronger will be the acid.
Morever, stronger the acid, the weaker will be its conjugate base and vice versa. e.g.,
Solvent effect or Leveling effect:
Nature of solvent play a key role in the dissociation and hence in the relative strengths. HCl, HNO3, H2SO4, HBr & HClO4 all are strong acids and can donate protons to the solvents.
If their comparative strength is to be measured, water as a solvent can’t serve the purpose, because it is stronger enough base so as to accept or distract protons from the above acids in almost same intensity. So, if their solutions of equal normalities are taken, each of them will have equal number of H+. That’s why they are almost equally strong in presence of water as a solvent. This effect of water to treat all the above acids at same level of strength is called leveling or solvent effect.
If CH3COOH is taken as a solvent instead of water, the former being an acid will not easily accept proton from an acidic species. It can accept easily only from them which are stronger enough, and this ease of acceptance of H+ will be in the proportion of their strength.
Such solvent which differentiate the acidic/basic nature of the acids/bases having approximately equal strengths are called differentiating solvents.
Using conductivity methods the strength of above mentioned acids in acetic acid solution can be studied, which give the order of strength, as:
HClO4 > HBr > H2SO4 > HCl > HNO3
Ammonia, stronger base than water can easily accept protons from strong as well as weak acids. So that even weak acid like CH3COOH behaves a strong acid approximately equal in strength to the HCl, HNO3 H2SO4 & HClO4.
Classification of solvents:
| Protophilic | Protogenic | Amphiprotic | Aprotic |
| · Accept protons | · Produce protons | · Can accept as well as produce H+ | · Do not involve H+ |
| · Water, liq. ammonia, alcohol | · Water, liq. HF/HCl, glacial CH3COOH | · Water, ammonia, ethanol | · Benzene, CCl4, N2O4, liq. SO2 |
Advantage of Bronsted – Lowry concept:
i) This concept explains the behaviour of acids and bases in both aqueous and non-aqeuous solvents.
ii) It explains the behaviour of NH3.CaO etc, as bases and CO2.SO2 etc as acids.
iii) This is a more general theory encompassing larger number of acids and bases than Arrhenius theory.
Draw backs of Bronsted – Lowry concept:
i) Proton donation or acceptance happens only in the presence of other substances.
ii) It does not explain the behaviour of electron deficient molecule like AlCl3, BCl3 etc are acids.
4. Lewis Theory/Concept:
Limitation of Bronsted theory to transfer of only H+ (proton) was extended by Lewis incorporating the concept of transfer (or more accurately donation forming a dative bond) of lone pair. Since proton & electron are of opposite nature; the proton donor was acid in Bronsted theory, the electron (pair) donor will be a base in Lewis theory. Similarly the proton acceptor in Bronsted was base; the electron pair acceptor will be base in Lewis Concept. So that,
i) “A substance that can accept an electron pair to form a co-ordinate covalent bond with the donor”. Is called Lewis acid. g.,
ii) “A substance that can donate a lone pair of electrons to form a coordinate covalent bond with the acceptor” is called Lewis base. g., , ligands in complex compounds.
An acid base neutralization reaction is simply a co-ordination reaction involving the sharing of electron pair between acid & base. The product so formed is a co-ordination compound, co-ordination complex or adduct.
Examples of Lewis acids:
i) All cations : e.g., etc
ii) Compounds whose central atom has an incomplete octet and possessing an empty orbital. e.g., etc.
iii) Compounds in which the central atom has available d-orbitals and may expand its octet. e.g., etc.
iv) Molecules having multiple bonds between atoms of dissimilar eelectronegativities. e.g. etc.
v) Elements with an electron sextet. g., : S, O
Examples of Lewis Bases:
a) Neutral molecules with lone pairs: Compounds with N, P, O & S as a central atom behaves generally as Lewis base if they have at least one of their electron pair non-bonded. e.g., , amines (), , alcohols (), ethers (), thiols () etc.
Order of Basic Strength
b) Simple anions: etc.,
Order of Basic Strength:
i)
ii) [discussed earlier]
c) Molecules with carbon-carbon multiple bond: In some compounds there is partial charge transfer from alkene, alkyne or even benzene molecules to a molecule having vacant p or (in most cases) d-orbitals.
molecule in Charge transfer complex
d) Ligend species in complexes: All ligands [in co-ordination chemistry] donate electron pairs. So, they are Lewis bases. e.g., CO in [Ni(CO)4]; CN– in K4[Fe(CN)6] etc.
Example of Acid Base (neutralization) reactions:
(i)
(ii)
(iii)
Note :
All Bronsted bases are Lewis bases, but all Bronsted acid are not Lewis acids.
| Acids | Bases | |||||
| Bronsted | Lewis | Bronsted | Lewis | |||
| HCl | ✓ | ✗ | NH3 | ✓ | ✓ | |
| CH3COOH | ✓ | ✗ | H2O | ✓ | ✓ | |
| H2CO3 | ✓ | ✗ | Cl– | ✓ | ✓ | |
Limitations of Lewis Theory:
i) One of the serious defects in the theory is that it cannot explain the strengths of acids and bases.
ii) Acids like HCl : H2SO4 react with bases such as NaOH or KOH but do not form coordinate covalent bond.
iii) Generally acid-base interactions i.e., neutralization reactions are instantaneous reactions but some Lewis acid – base reactions go on very slowly.
iv) All the acid – base reactions do not involve coordinate bond formation.
v) H+ ion, as catalyst, cannot be explained by this theory.
Illustration 1:
Using Lewis concept, determine the order & acidic strength of HClO4, HClO3 and HClO2.
Solution.
In general, the greater the number of oxygen atom around a central atom in a oxyacid, greater the acidity. Since O is more electronegative than Cl, the former tends to pull away the electron from Cl atom, which in turn pulls electron pair from O-H bond. This leads into easier removal of H+ from the acid making it more acidic.
MORE THEORIES FOR ACID–BASE CONCEPT:
5) Lux Flood Theory (concept): The theory has been proposed to explain the high temperature acid-base reactions:
Molecules or compounds which can accept an anion (like Cl–, O2 –, S2– etc. are called acids. e.g. CO2, SiO2, NO2 etc. Molecules/compounds that donate their anions during a reaction are bases. e.g. Na2O, CaO, BaO, FeO etc.
Acid–Base Neutralization reaction :
Such reactions generally take place at high temperature in absence of any solvent. These reactions are the part of ceramic industry and metallurgy of elements (formation of slag).
Relative Strengths of Some Acids and Bases
Acids:
i)
ii)
iii)
iv) or
v)
vi)
Bases:
i)
ii)
iii)
iv)
v)
vi)
vii) R = alkyl; Ar = aryl
viii)
ix)
x)
6) Usanovich (Solvent) concept:
Acid base reactions of aqueous & non-aqueous solvents are well explained by this theory. Autoionization of a solvent gives two ions – a cation characteristic ion and the other anion characteristic ion. If any substance after being dissolved in a particle solvent produces cation characteristic ions of the solvent, the substance will behave like an acid and if anion characteristic, it will be a base in the solvent.
In water (solvent)
The substance producing in water are acids and those producing OH– are bases.
Since HCl when present in H2O, gives cation characteristic of solvent i.e., H3O+, it will be an acid.
Ammonia and NaOH give anion characteristic of H2O i.e., OH–, so they are bases in it.
Other solvents like liq. NH3, liq. SO2 also represent the same type of reactions.
In liq. NH3 substance furnishing will behave like acid and those giving will work as a base e.g.,
Liq. SO2 [non-aqueous as well as non-protonic (aprotic) solvent]
In liq. SO2, the compounds giving SO2+ will be acids and those giving will be bases e.g.,
Pearson Theory of Hard & Soft Acids & Bases:
Stability & ease of complex are salt formation between Lewis acids & Lewis bases was suggested by Pearson.
Hard Acids : Acceptor atoms have smaller atoms, high nuclear charge, low polarizibility and noble gas configuration. e.g., H+, Li+, Be2+, Al3+ Fe3+ etc.
Soft Acids : Acceptor atoms have larger size, low charge density high polarizability and donot have noble gas configuration. e.g., Cu+, Ag+, Hg+, Br+, I2 etc.
Hard Bases: Donor atoms have high electronegativity, low polarisability and smaller size. e.g. H2O, Cl–, F–, OH–, NH3, NO3–, CO32– etc.
Soft Bases : Donor atoms have low electronegativity and high polarizability e.g., R-SH, I– CN–, CO, H– etc.
A complex formed between soft acid & soft base as well as between hard acid and hard base is more stable in comparison to hard-soft or soft-hard combination.
Modern Theory of Acid and Bases: Its is nothing but integration of all previous theories.
Acids are the substances, which
i) furnish H+ (protons) in the solution
ii) can accept electron are electron pair
iii) accept anions (like O2–, S2–, etc.)
iv) give cation characteristic of the solvent, when dissolved in it
Bases are the substances which
i) furnish OH– in the solution
ii) can accept protons
iii) donate done pairs
iv) give anions (like O2–, S2– etc)
v) produce anion characteristic of the solvent
Salient Features of Acids & Bases:
Acids : i) Mineral acids are strong acids (HCl, HNO3, H2SO4 etc).
ii) In oxyacids, the ionizable (replaceable) H atoms are those which are attached directly to O atom e.g.,
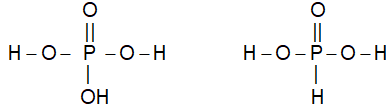
An acidic salt has some replaceable H atoms e.g., NaHCO3, NaH2PO4, NaH2PO3 etc.
Note : Na2HPO3 is not acidic since H is attached to P atom.
Bases :
Hydroxides and oxides of IA & IIA group (alkali & alkaline earth metals) are strong base.
Rest of the hydroxides & oxides are weak base.
Oxides of metals are basic (few of them being amphoteric) while those of non-metals are acidic (some are neutral).
| Basic | Amphoteric | Acidic | Neutral |
| BeO, Al2O3 | CO2 | CO | |
| CuO, Fe2O3 | ZnO, SnO2 |
N2O3, NO2, N2O5 SO2, SO3, P2O5, SiO2 |
N2O, NO |
Acid anhydrides:
If some acids are dehydrated they will leave behind an oxide, with same oxidation number of central atom; alternatively, if this oxide is dissolved in water it gives the corresponding acid. This oxide is called acid anhydride.
This list follows:
| Anhydride | CO2 | N2O5 | N2O3 | SO3 | SO2 | ||
| Acid | H2CO3 | HNO3 | HNO2 | H2SO4 | H2SO3 | ||
| Anhydride | P2O5 | P2O3 | CrO3 | CrO3 | Mn2O7 | ||
| Acid | H3PO4 | H3PO3 | H2CrO4 | H2CrO4 | HMnO4 | ||
Basic anhydrides:
| Oxide | Na2O | CaO | Al2O3 | ||
| Base | NaOH | Ca(OH)2 | Al(OH)3 |
Degree of Dissociation (α): It is the fraction of the molecules of a weak electrolyte which dissociates into ions in its solution.
Factors governing α :
1) Nature of solute : For strong electrolytes α 1
For weak electrolytes α <<1
2) Nature of solvents: Solvents with high dielectric constant (like H2O; = 86.6) make the strong electrolytes highly ionizable (i.e., α 1) in comparison to solvents with low dielectric constant (like CH3OH)
3) Temperature: α increases with increase in temperature.
4) Dilution: α increases with dilution of solution.
5) Presence of other species : If some ions present in solution are common to the electrolyte’s and the electrolyte is weaker, its α decreases [ common ion effect]. If such an ion is present in the solution that reacts with either of ions of electrolyte to give some product the α of electrolyte increases.
Ostwald Dilution Law (Ionisation of weak electrolytes):
Let an electrolyte AB dissociate into ions A+ & B–. If its degree of dissociation is a, for the condition of equilibrium,
AB +
Initial concentration C O O
Conc. at equilibrium C(1-α) Cα Cα
Equilibrium constant, . . . . (i)
. . . . (ii)
For weak electrolytes α << 1
So, (1-α) 1
. . . . (iii)
If V is the volume of solution having 1 mole of electrolyte, then
Thence, . . . . (iv)
or
“Degree of dissociation (α) of weak electrolytes is directly proportional to the square root of volume or dilution”.
Equations (iii & iv) describe that for the given volume or concentration the degree of dissociation (a) is proportional to K i.e., if K is greater, a will also be higher.
If deg. of disso. (α) for a weak electrolyte is greater than 0.05, (1-α) can’t be treated equal to unity. In that very case, eq. (ii) must be followed. For calculation of α, the quadric equation should then be used
i.e.,
Illustration 2 :
A monobasic acid has Ka = 1.8 ⨯ 10–5 at RT. Calculate the degree of dissociation and concentration of H+ furnished by the dissociation of acid having concentration 0.2 M.
Solution.
Assuming a to the very small
{or, [H+] = a ⨯ C}
= 0.001897
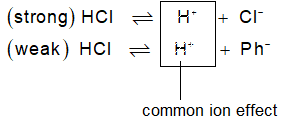
Common Ion effect: When a weak electrolyte is present in a solution, already having any of its ion common to that of another strong electrolyte, the degree of dissociation of weak electrolyte is further decreased. This is called common ion effect. For example if CH3COOH (weak) is present in a solution along with HCl (strong), then
Strong electrolyte HCl H+ + Cl– (greater dissociation)
Weak electrolyte CH3COOH H+ + CH3COO– (less dissociation)
Common ions
Dissociation of acetic acid will further decrease.
Explanation from Le Chatelier : HCl being strong will almost completely ionize to give H+ and Cl–. An equilibrium between undissociated HCl and dissociated (H+ & Cl–) will be established. Now, if we add CH3COOH, a weaker acid to the solution it will produce H+ along with CH3COO–. These new H+ from CH3COOH can disturb the equilibrium towards left i.e., to minimize the a of HCl which is not possible as HCl is strong acid. So, CH3COOH acid will become much weaker acid in presence of a strong acid like HCl.
However the dissociation of CH3COOH can be increased by an electrolyte producing OH–. Consider the addition of NH4OH to the CH3COOH:
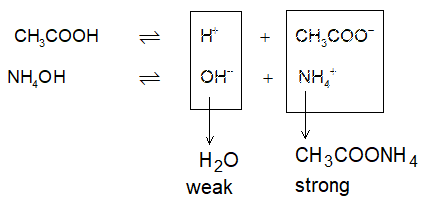
H+ are removed from the reaction mixture, so as the equilibrium gets disturbed and shifts toward right hand side to produce more and more H+, thereby increasing a of CH3COOH. Now, since H2O formed by union of H+ (from CH3COOH) and OH– (from NH4OH) is a weak electrolyte, it will not ionize back to produce H+ & OH– in the solution.
Relative strength of Acids & Bases:
For acids :
Relative strength =
Ka = Dissociation constant for an acid
Similarly,
For bases Kb = Dissociation constant. for a base
Ionic product of water (Kw):
Water is a weak electrolyte. It dissociates feebly as follows:
K = Dissociation constant
Since, the concentration of water remains practically unchanged, [H2O] will be constant.
K[H2O] = [H+] [OH–]
Kw = [H+] [OH–] Kw = Ionic product of water = K [H2O]
Since Kw is the product of two constants, it will also be a constant.
“The product of concentration of H+ (or H3O+) and OH– in pure water, remains constant for the given temperature, called ionic product of water”.
At RT (250C) its value is found to be 10–14 mole2/lit2
Kw increase with increase in temperature.
At 250C, in 1 lit of H2O having 55.5 moles only 10–7 moles are dissociated.
|
Temp |
Kw |
|
00 C |
n10–15 |
|
250C |
1 ⨯ 10–14 |
|
600C |
N10–13 |
Concept of pH:
S.P.L. Sorenson devised a more sophisticated method to express the [H+] of dilute solutions. The letter ‘small p’ stands for power raised to (Fr. puissance; Ger. Potenz) and ‘capital H’ for the hydrogen ion.
pH is the negative power raised to the base 10 to express [H+] or
pH is the inverse to the log [H+]
[H+] = 10–pH
or pH = – log[H+]
So, pH is the measurement of [H+] or acidic nature of the solution.
In normal water,
Since
So, we conclude that neutral solutions have pH = 7.
Suppose, a drop of a HCl is added to pure water, considering the equilibrium,
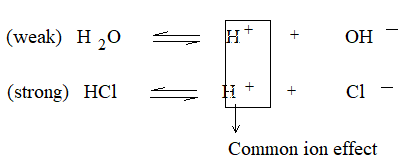
Due to H+ from HCl dissociation of H2O is decreased, so that the concentration of H+ & OH– from water falls appreciably. But due to HCl itself, extra H+ are introduced to the solution. The result is [H+] increases while [OH–] decreases because the product of [H+] and [OH–] is constant. One can say that since Kw is constant; if conc of OH– is decreased (due to common ion effect on H2O], the concentration of H+ must increased so as to make its Kw fixed at 1 ⨯ 10–14.
Consider an aq. solution of HCl. Let the concentration of H+ from HCl in the solution be 10–4 mole/lit.
The overall concentration of H+ in the solution will be due to the H+ from water as well as from HCl i.e., .
= (from water) + (from HCl)
= 10-4 (10-4+1)
= 1.001 ⨯ 10–4 or 1 ⨯ 10–4
Since, Kw = [H+] [OH–]
10–14 = 10–4 ⨯ [OH–]
[OH] = 10–10
i.e., concentration of [H+] is increased & those of OH– is decreased.
Considering its pH,
pH = – log [H+] = – log 10–4 = + 4 log 10
= 4
Acidic solutions have their pH < 7.
Similarly, if 10–4M NaOH is added to water and it contributes 10–4 OH–. The concentration of OH– in solution,
[OH–] = 10–4
[H+] = 10–10 [Kw = [H+] [OH–]
pH = – log 10–10 = 10
We conclude that basic solutions have their pH > 7
| Acidic solution e.g., HCl, CH3COOH, AlCl3 (aq) | Neutral solution e.g., pure water, NaCl (aq), urea (aq) | Basic solution e.g., NH4OH, NaOH, NaHCO3 etc | ||
|
[H+] > [OH–] (> 10–7) (<10–7) pH < 7 |
[H+] = [OH–] = 10–7 pH = 7 |
[H+] < [OH–] (< 10–7) (> 10–7) pH > 7 |
Evidently, [H+]
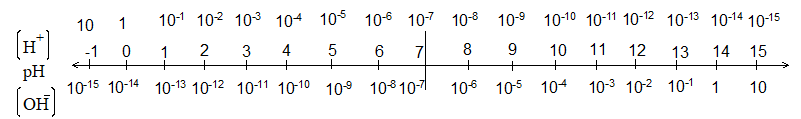
Neutral solutions at 600C have pH 6.5. Human body, at an average temperature of 370C has the neutral point at pH 6.8 (Kw < 10–14)
pH range of some common solutions.
Chemical Biological
| System | pH | System | pH | |
| Soft drinks | 2.0 – 4.0 | Gastric juice | 1.0 – 3.0 | |
| Vinegar | 2.4 – 3.4 | Hunan urine | 4.8 – 8.4 | |
| Sea water | 8.5 | Saliva | 6.5 – 7.5 | |
|
Milk of magnesia (Antacid) |
10.5 |
Blood Tears |
7.36 – 7.42 7.4 |
pH of strong acids & bases
pH = – log [H+]
For acids [H+] = Normality
For bases [H+] =
pH of weak acids & bases
For acids [H+] = Normality ´ a
For bases [H+] =
pKa & pKb
Like pH & pOH for strength of solutions of acids & bases, the relative strength of acid & bases can be compared using their pK values.
pKa = – log Ka (for acids)
pKb = – log Kb (for bases)
Higher the K value, lower the pK value, stronger the acid/base.
pH of very low concentration of an acid/base (e.g. 10–8 MHCl)
Since water also give H+ in the solution i.e., [H+] = 10–7. While for normally dilute solutions we ignore this very low concentration of H+ from water since it has practically no effect on pH. But for very dilute solution where there are only 10–8 ions given by the electrolyte, the ions from H2O must be considered to calculate the total concentration.
So that Net [H+] = 10–7 (from H2O) + 10–8 (from HCl)
= 10–7 (1 + 10–1) = 1.1 ⨯ 10–)
pH = + 7 log 10 – log 1.1
= 6.958
Kw doesn’t change by adding a salt, acid or base to a solution or neutral water. It changes with temperature.
Calculation of pH or pOH of a solution:
Case i): If H– ion or OH– ion concentration is given then
similarly
Case ii): When the acid or the base is completely ionized in aqueous solution (i.e., for strong acid or strong base).
[H+] = normality of the acid
[OH–] = normality of the base.
Case iii): When a mixture of strong acids is present in the aqueous solution. Then the normality of the mixture, N where V1 and N1 are the volume and normality of the first acid and V2 and N2 are those of the second acid.
in the mixed solution = normality of the mixed solution = N
Case iv): When strong alkalies are mixed o calculate the pH or pOH of the mixed solution. The normality of the mixture of the strong bases is given by
where V1, N1 are volume and normality of the first solution and V2, N2 are the values for the second solution.
Then [OH–] in the mixture – Normality of the mixed solution = N.
at room temperature, pH + pOH = 14. pH = (14 – pOH)
Case v): When a strong acid and a strong base are mixed, to calculate the pH of a mixed solution then normality of the mixture of the strong acid (A) and a strong base (B) is given by
(If the )
or (if VBNB > VANA)
[H+] = (N) = OH
Similarly
but, pH + pOH = 14
pH = (14 – pOH)
Illustration 3:
What is the pH of a solution having a [H+] = 2.512 ⨯ 10–6 M?
Solution:
Illustration 4:
Find the hydrogen ion concentration in a solution of pH = 3.75?
Solution:
Illustration 5:
What is the Kw value in an aqueous solution of pKw = 13.725?
Solution:
Illustration 6:
What is the pH of a solution containing 0.63g of HNO3 in 100 ml of solution?
Solution:
Concentation of
Illustration 7:
Ba(OH)2 is a strong base. Then
Solution:
= normality of the base.
Illustration 8:
Calculate the pH of an aqeusous solution having 2g of NaOH per 500 ml it.
Solution:
Molarity (M) of the solution=
NaOH is a strong base. Hence
= Normality of the base = 10–1 N
(here Molarity = Normality)
But,
Illustration 9:
150ml of 0.5N HCl and 100 ml of 0.2N HCl are mixed. Find the pH of the resulting solution.
Solution:
Normality of the mixed solution is given by
= 0.38N
pH = – log 0.38 = 0.42
Illustration 10:
Equal volumes of 0.5N NaOH and 0.3N KOH are mixed in a experiment. Find the pOH and pH of the resulting solution.
Solution:
Normality of the mixed solution
pH = 14 – 0.398 = 13.6
Illustration 11:
50ml of 0.2M HCl is added to 30ml of 0.1M KOH solution. Find the pH of the solution.
Solution:
In the mixed solution
Illustration 12:
40ml of 0.2N HNO3 when reacted with 60ml of 0.3M NaOH, gave a mixed solution. What is the pH of the solution?
Solution:
N = Normality of the mixed solution =
=
pOH = 1
pH = 14-1 = 13
Exercise 1:
(i) The Ka of a weak acid is 10–5. The value of pKb of its conjugate base will be
(A) 5 (B) 6 (C) 9 (D) 10–9
(ii) Ka of a weak monobasic acid in aq. soln. is 8.0 ⨯ 10–5. The degree of dissociation of a 0.05 M solution of the acid is
(A) 3.52% (B) 4.0% (C) 4.52% (D) 8.5%
(iii) The pH of an aq. solution is 4. It [OH–] will be
(A) 10 (B) 14 (C) 10–4 (D) 10–10
(iv) The pH of an aqueous solution having [H+] = 3.0 ⨯ 10–3M is
(A) 2.471 (B) 2.523 (C) 3.0 (D) 6.433
(v) The pH of a 5 ⨯ 10–3 M aq. solution of a weak monobasic acid with dissociation constant 1.8 ⨯ 10–5 will be
(A) 4 (B) 2.52 (C) 3.523 (D) 5.53
BUFFER SOLUTIONS:
If one drop of concentration acid is added to 1 lit H2O (pH = 7), its pH falls rapidly; had a drop of base been added it would have increased abruptly.
But there are some mixtures or solutions whose pH doesn’t vary so easily (i.e, by a single drop of an acid or a base); they actually resist any change in pH. Such solutions are called the buffer solutions.
Types of Buffer Solutions:
I) Neutral Buffers:
a) Strong acids and bases are buffers at higher concentrations
b) Proteins and amino acid solutions
c) Salts of weak acids and weak bases (CH3COONH4)
d) Mixture of a normal and an acidic salt of polybasic acids. (Na3PO4 + Na2HPO4)
II) Acidic Buffers:
Weak acids with their conjugate bases
III) Basic Buffers:
Weak bases with their conjugate acids (NH4OH + NH4+; glycine + glycine hydrochloride)
Buffer Action:
Consider a simple buffer – an aq. solution of CH3COONH4:
Excess of CH3COO– & NH4+ are present in the solution. If a drop or so of an acid is added, the following changes will take place.
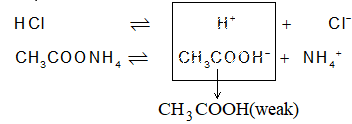
Dissociation of HCl adds H+ to the solution to increase (H+). But these H+ ions react promptly with acetate ions to form CH3COOH. Acetic acid is weak acid and doesn’t dissociate back to H+ & CH3COO–. So there is no effect on pH of the solution.
If a base is added,
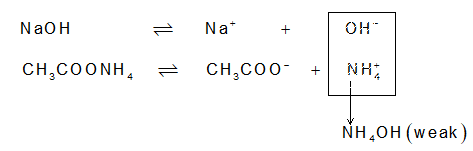
This time, extra OH–, which could have disturbed the pH of solution, react with NH4+ of ammonium acetate to give NH4OH. The pH of the solution remains unaffected.
pH of a Buffer solution (Handerson Equation):
For an Acidic Buffer
Let HA be the weak acid and the salt of its conjugate base be NaA,
. . . . (i)
. . . . (ii)
For eq. (i)
or . . . . (iii)
Since HA is weak acid, [A–] in solution is mainly due to the salt NaA. i.e., [A–] = [salt, NaA]
. . . . (iv)
Similarly, for a basic Buffer . . . . (v)
Eq. (iv) & (v) are Handerson equations.
Illustration 13:
What will be the pH of a solution obtained by the mixing of 5g of acetic acid and 7.5 g of sodium acetate and making the volume to 500mL (Ka = 1.75 ⨯ 10–5)
Solution.
Illustration 14:
How much volume of 0.1M HAc should be added to 50 mL of 0.2M NaAc solution if we want to prepare a buffer solution of pH 4.91. Given pKa for acetic acid is 4.76.
Solution.
i.e., 4.91 = 4.76 +
of
or
V = 100/1.41 = 70.92 mL
Illustration 15:
50ml of 0.1M sodium acetate, 25 ml of 0.2 M acetic acid were added together to form the buffer solution pKa of CH3COOH is 4.8. Find the pH of the solution.
Solution.
The buffer solution is an acid buffer. Hendersen’s equation for an acid buffer is
llustration 16:
50ml of 0.1M NH4Ohm 25 ml of 2M NH4Cl were used to make a buffer. What is the pH if pKb is 4.8?
Solution.
The given buffer is a basic buffer
For a basic buffer pOH =
Illustration 17:
The pH of buffer prepared by mixing 50 ml of 0.2 M CH3COOH and 25 ml of CH3COONa is 4.8. If the pKa = 4.8, what is the strength of CH3COONa?
Solution.
The given solution is an acid buffer.
The pH of the buffer is given by,
i.e.,
or
[CH3COONa] = 0.4M
Illustration 18:
When 20ml of 0.1M NH4OH are added to 20ml of 1M NH4Cl solution, the pH of the buffer formed is 8.2, what is the pKb of the NH4OH?
Solution.
For basic buffer pH is given by
given the pH of the buffer = 8.2;
14 – 8.2 =
or 5.8 =
or 5.8 = pKb + 1
pKb = 4.8
Exercise 2:
(i) What will be pH of a solution of 0.1M HA (acid) and 0.1M NaA? (Ka = 10–6)
(A) 3 (B) 5 (C) 6 (D) 7
(ii) A 1 lit solution of 0.25 mol NH4Cl and 0.15 mol NH4OH (dissociation constant, 1.98 ´ 10–5) will have a pH of
(A) 9 (B) 9.2 (C) 10 (D) 10.2
Buffer Capacity or Buffer Index:
No. of moles of an acid or a base required to change the pH of one litre of a buffer solution by unity is called as the buffer capacity.
Buffer capacity,
Applications of Buffer Solutions: Buffer solutions are widely used in those solutions where reactions proceeds only in a particular pH range, for example in
– Qualitative analysis – removal of PO43– after IInd group (CH3COOH + CH3 COONa buffer)
– Precipitation of IIIrd group (NH4OH + NH4Cl buffer)
– Blood (H2CO3 + HCO3– buffer)
– Medical formulations
– Preservation of foods, dairy products & fruits
– Industries like dyes, inks, paints, paper
SALT
A salt is a compound formed by the neutralization reactions between an acid and a base. Its +ve part comes from the base while negative part from the acid.
Types of salts:
(a) Simple Salts: Normal : The salts without any replaceable H+ or OH– (complete neutralization) e.g., NaCl, KNO3, CaSO4 Na3BO3 etc.
Acidic : They have replaceable H+, ( incomplete neutralization) e.g., NaHCO3, KHSO4, NaH2PO4 etc.
Basic : They have replaceable OH– (incomplete neutralization) e.g., Zn(OH)Cl, Mg (OH) Br etc.
b) Double Salts: These are formed by reunion of two salts and have properties of each ion of both the constituting salts. g., Potash alum, K2SO4. Al2(SO4)3.24H2O; Mohr salt, (NH4)2SO4.FeSO4. 7H2O etc.
c) Complex Salts: These are formed by coordination of ions to a central metal atom. The species with square bracket is an independent entity and it doesn’t further dissociate into its constituents. e.g.Cl etc.
d) Mixed Salts: These salts furnish more than one cation or anion in solution e.g., Bleaching power, CaOCl2; Rochelle’s salt (CHOHCOONa – CHOHCOOK).
While Mohr salt gives the tests of all of its constituent ions i.e., of NH4+, Fe2+ & SO42– ; K4 [Fe(CN)6] gives tests of only K+ & [Fe(CN)6]–4 i.e., ferrocyanide, but not that of Fe2+ & CN– ions separately.
Salt Hydrolysis:
Reverse to the neutralization reaction and involves the treatment of the salt with the solvent. The cations and anions of a salt interact with the anions and cations of solvent respectively furnishing bases and acids. The process is called salt hydrolysis .
The resulting mixture can be acidic, basic or neutral depending upon the relative strengths of the acid and base formed during the reaction.
Being reverse to neutralization, it’s always endothermic.
Normal salts can further be divided into 4 groups:
Salts of strong acid and strong base e.g., NaCl
Salts of strong acid and weak base e.g., NH4Cl
Salts of weak acid and strong base e.g. CH3COONa
Salts of weak acid and weak base e.g., CH3COONH4
Salts of Strong Acids and Strong Bases: (NaCl, KNO3 CaSO4, Ba(NO3)2 etc.)
These salts don’t hydrolyse. The cations and anions that these salts furnish in solution don’t interact upto appreciable extent with H+ & OH– of H2O, so that the concentration of H+ and OH– remains unchanged, leaving behind a neutral solution (pH = 7). e.g.,
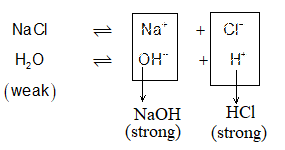
Since NaOH and HCl both are strong, they completely ionize back to produce Na+, OH–, H+ and Cl–. Of these , H+ combines with OH– to give H2O, a weak electrolyte. Hence Na+ and Cl– practically don’t interact with H2O (H+ & OH–).
Salts of strong acidic and weak bases: (NH4Cl, CISO4, FeCl3, AlCl3 etc.)
These salts hydrolyse to give acidic solutions (pH < 7). Cations undergo hydrolysis called cationic hydrolysis. e.g.,
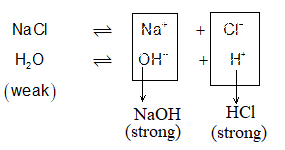
Although equal amounts of ions from H2O (i.e., H+ & OH) are offering the reaction, there should be number change used up in H+ or OH– concentration as per Le Chatelier principle, more H2O will dissociate now to make up the equilibrium condition. However, HCl is strong acid, it dissociates completely into H+ & Cl– while NH4OH being weak base will give only few OH–. Further due to common ion effect, its dissociation is further decreased, making the solution scare of OH– & plenty of H+, hence it will become acidic:
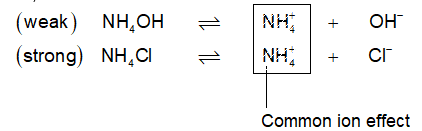
The result:
i.e., equilibrium shifts to left; decreasing OH– concentration
Salts of Weak Acids and Strong Bases: (KCN, Na2S, Na2CO3, CH3COONa etc.)
These salts hydrolyse to give basic solution (pH > 7). Anions are hydrolysed, called anionic hydrolysis.
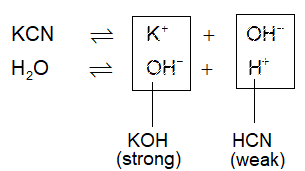
The concentration of H+ is decreased in the solution due to poor dissociation of HCN coupled with common ion effect of KCN. The [OH–] becomes greater due to presence of strong base KOH somewhat like cationic hydrolysis.
Salts of Weak Acids and Weak Bases: (CH3COONH4, Ag2CO3, NH4CN, (CH3COO)2Cu etc.)
These salts hydrolyse to produce almost neutral solution (pH 7). In fact, the nature of solution depends upon the relative strengths of the acids & bases so formed i.e., the resulting solution could be feebly acidic, basic or neutral.
Both the cations and anions undergo hydrolysis, called cationic – anionic hydrolysis.
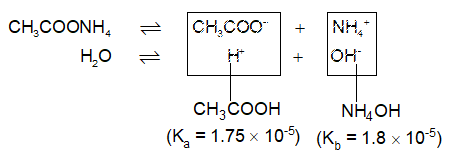
Since strengths of acetic acid & ammonium hydroxide are approximately equal, the resulting solution is almost neutral (actually very little basic)
The aq. solution of Ag2CO3, thus will be basic.
The ammonium carbonate solution will become acidic due to stronger nature of acid (H2C2O4) in comparison to NH4OH.
Degree of hydrolysis (h) and hydrolysis constant (Kh) :
The fraction of a salt that undergoes hydrolysis in comparison to the total number of moles taken, is called degree of hydrolysis (h).
BA + HA + BOH
Moles before hydrolysis 1 … 0 0
Moles after hydrolysis (1-h) h h
Conc. at equilibrium C(1-h) Ch Ch
Applying law of mass action,
Kh for salt of strong acid and weak base (cationic hydrolysis)
Since,
For weak base h << 1; so (1 – h) 1
Kh for salt of weak acid and strong base
Kh for salt of weak acid and weak base
= (multipying by the factor )
= Ka. h
=
=
If
Note : The pKa value and strength of acid are inversely related i.e., higher the pKa value, weaker the acid.
Illustration 19:
A weak acid, HX forms a salt with NaOH. Find the hydrolysis constant? Given Ka = 2 10–6.
Solution.
since the acid is weak
(because at room temperature)
=
Illustration 20:
A weak acid (Ka = 2 10–6) and a weak base (Kb = 5 10–7) form a salt. Calculate the hydrolysis constant.
Solution.
for weak acid – weak base salts.
Illustration 21:
Calculate the degree of hydrolysis of a 0.01M solution of NH4Cl. [Kw = 1.008 10–14, Kb (NH4OH) = 1.81 10–15] 25°C
Solution.
Assuming the degree of hydrolysis to be very small,
= 2.359 10–4
Indicators:
The substances that inform us about the completion of a reaction by (generally change in their colour are called indicators. Generally this completion point of a reaction is called the end point.
Types of indicators:
1) Acid base indicators: Used in neutralization reactions
Change their colour depending on pH of solution. e.g. Phenolphthalein, Methyl red, litmus etc.
2) Redox indicators: Used in oxidation – reduction reactions.
Colour changes with the change of emf e.g., KMnO4, N-phenyl anthranilic acid.
3) Adsorption indicators: Change of colour due adsorption of a substance on its surface.
4) Radioactive indicators: Used in the study of variety of reactions as tracer.
Their presence is detected by suitable devices. e.g., D2, O8, I127etc.
1 g equivalent acid neutralizes 1g equivalent base completely. But in practice, there is no any visible indication that the reaction is complete. Indicators do the job pretty well. They show a variation in their colour between certain pH limits, called indicator range or pH range.
Features of a Good Indicator:
- It should change the colour at a pH which falls in proximity to the end point for a given reaction.
- Colour change should be very sharp.
- Colour should be stable and brilliant.
- The two colours of an indicator should be contrasting so that they can easily be distinguished.
| Indicators | pH range | Colour in | |
| Acidic | Basic | ||
| Methyl orange | 3.1 – 4.5 | Pink | Yellow |
| Methyl red | 4.2 – 6.3 | Red | Yellow |
| Litmus | 5.4 – 7.5 | Red | Blue |
| Phenolphthalein | 8.0 – 9.8 | Colourless | Colourless |
| Thymolphthalein | 9.3 – 10.5 | Colourless | Blue |
Internal indicator: Added into the titration mixture before titration. e.g., Phenolphthalein, N-Phenyl anthranilic acid, Starch + KI etc.
External indicator: Only a small fraction of the reaction mixture is taken out and checked for end point by adding the indicator e.g., .
Self indicator : When any of the titrant itself changes colour during a titration, there is no need to use other external substances. e.g., KMnO4 in redox reactions decolourises at end point.
| Titration | Indicator used |
|
Strong acid Vs strong base Strong acid Vs weak base Weak acid Vs strong base Weak acid Vs weak base |
Phenolphthalein, methyl red Methyl orange, methyl red Phenolphthalein, thymolphthalein No suitable indicator (carried out using conductivity methods) |
Universal Indicator (Multiple Range Indicator)
It is a mixture of certain indicators in a definite proportion, which shows different colour changes over a wide range of pH.
Common universal indicator is prepared by dissolving
| 1 g phenolphthalein |
In 500 mL absolute ethanol and then adding NaOH solution till it turns yellow.
|
| 2 g Methyl orange | |
| 3 g Methyl yellow | |
| 4 g Bromothymol blue | |
| 5 g Thymol blue |
It’s colour change ranges are:
| pH | 2 | 4 | 6 | 8 | 10 |
| Colour | Red | Orange | Yellow | Green | Blue |
Titration: An experiment carried out in order to know the strength/concentration of a substance is called titration.
Theories of indicators: How, it is possible for a same compound to have two colours is explained using following theories:
1) Ostwald Theory
2) Benzenoid–Quinonoid theory
1. Ostwald Theory:
All indicators are weak acids or weak bases. In unionized (moleculer) form and ionized (ionic) form they have different colours. Being weak electrolytes their dissociation into corresponding positive & negative ions is largely affected by other ions present in the solution.
An indicator changes its colour when it is 50% dissociated.
a) Phenolphthalein : It is a weak acid considering it to be HPh, it dissociates into H+ (colourless) and Ph– (pink) ions, but only upto very small extent (as << 1) i.e.,
i) In presence of an acid:
Equilibrium of HPh shifts to the backward direction i.e., dissociation is decreased. Very few Ph– ions are left in the solution hence it remains colorless.
ii) In presence of a base:

Since H+ & OH– are removed, equilibrium shifts to the forward direction. So that more Ph– are produced. Although they may combine with Na+ but NaPh is highly ionized being strong electrolyte, it dissociates almost completely to produce excess of Ph–. These Ph– provide pink colour to the solution.
b) Methyl Orange: The weak basic nature of methyl orange (MeOH) gives very small amount of Me+ (red) and OH– (colourless) ions. Since concentration of Me+ is very less, it remains yellow in neutral solution.
In acidic medium, (H+) increases the dissociation of MeOH and thus produces more Me+ which make the solution red. (Le Chatelier principle)
The basic medium, (OH–), because of common ion effect, decreases the dissociation of MeOH by which only few Me+ are left in the solution. Major portion is the molecular form i.e., MeOH, which appears yellow.
2. Quiuonoid Theory:

According to this theory indicators are aromatic organic compounds. Each indicator has two tautomeric forms i.e., benzenoid & quinonoid. Quinonoid form has deeper colour than benzenoid. Colour transition is observed due to these tautomeric inter-conversions. The tautomeric conversion is pH dependent.
a) Phenolphthalein :
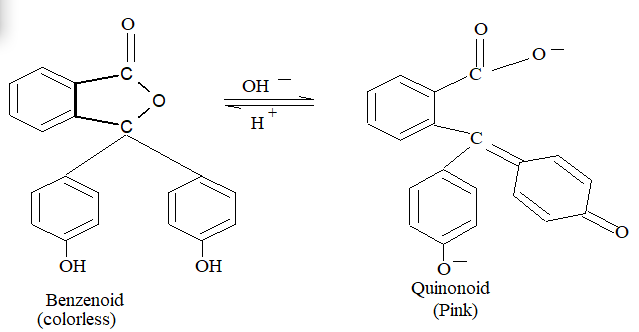
b) 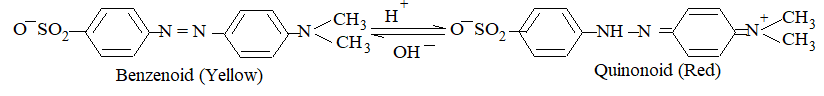
Solubility Product:
For a saturated solution of an electrolyte AxBy:
For saturated solution, = constant
= Solubility product =
Since [AxBy] i.e., solubility of an electrolyte is temperature dependent, Ksp is constant only for given temperature
Ex.
For a solution product of concentration of its cations and anions raised to their stoichiometric coefficients is called ionic product (IP).
If, Ksp < IP solution starts to precipitate.
If, Ksp > IP solution remains as such
and if Ksp = IP solution exists in equilibrium with its precipitate.
Application of solubility product:
a) Predicting the formation of ppt.: A solution of is added to a solution of 10–7 M CaCl2. If Ksp for AgCl is 1.7 ´ 10–10, predict whether precipitate will form.
Ksp = [Ag+] [Cl–]
= 10–3 2 10–7 (For CaCl2 [Cl–] = 2 [CaCl2])
= 2 10–10
Precipitation will take place.
b) Predicting solubility of sparingly soluble salts:
Ex.
c) Purification of common salt : This is achieved by passing dry HCl gas through brine (conc. NaCl) solution. Since excess Cl– are now available, [H+]. [Cl–] i.e., ionic product becomes greater than Ksp; NaCl precipitates out and can be filtered off from the solution.
sufficient Cl– ions to ppt NaCl
d) Salting out of soap: Soaps are Na or K salts of higher fatty acids. They can be made to ppt by adding NaCl or KCl.
effective concentration of Na+ increases
IP of soap i.e., becomes greater than Ksp and hence the soap precipitates out.
e) Inorganic analysis:
The complete analysis of basic radicals excluding zero group (NH4+) is based upon solubility product.
i) 1st group: Pb2+, Ag+ and Hg22+ are precipitated in this group as chlorides. Ksp of the chlorides of above ions are comparatively lower than the other radicals. So when dil HCl is added, sufficient Cl– are produced to precipitate these radicals, while those of all the other radicals/ions being highly soluble (with much higher Ksp values) remain in the solution in dissolved state.
ii) IInd group: IInd group and IV group both are precipitated as sulphides. But to limit the precipitation of only IInd group and not those of Vth or any higher group (III, V, VI etc) we make use of their solubility products. For IInd group radicals HCl is used along with H2S, so as to produce only limited number of S2–.
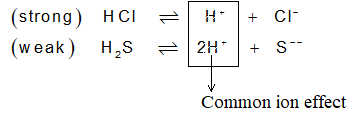
Ksp of IInd group – 10–72 – 10–30
Ksp of IVth group – 10–28 – 10–18
Comparison of the Ksp values of II & IV groups reveals that the Ksp of IInd group members are less than IV group. So, they get precipitated even in the presence of these small number of ions present in the solution.
HNO3 and H2SO4 can’t be used in the place of HCl here to reduce concentration of S2 –; since they are oxidizing agents and oxidize H2S to S.
iii) IIIrd group: Al3+, Fe3+ & Cr3+ are precipitated as hydroxides in presence of NH4OH & NH4Cl. They are selectively precipitated as hydroxides using NH4OH + NH4Cl (called group reagent) ; since more OH– may be produced in the solution otherwise which can precipitate even IV or higher group radicals/ions. Their concentration is controlled (decreased) by using common ion effect, permitting to precipitate only IIIrd group hydroxides.
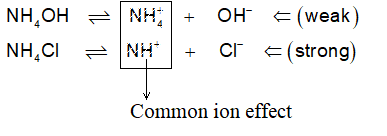
Illustration 22:
The solubility of AgCl in water at 250C is 0.00179g L–1. Calculate its solubility product at the temperature.
Solution.
Solubility =
Ksp [Ag+] [Cl–] = (1.25 10–5)2 mol2 L–2
= 1.56 10–10 mol–2 L–2
Illustration 23:
Calculate the solubility in gL–1 of Al(OH)3, if its solubility product is 8.5 10–32.
Solution.
Let ‘s’ be the solubility,
Illustration 24 :
The solubility product of BaSO4 is 1.5 10-9. Find its solubility in pure water and 0.1M BaCl2 solution.
Solution.
Let solubility be ‘s’ mol L–1
(inpure water)
Let solubility in 0.1 M BaCl2 be s’
Total Ba2+ concentration (S’ + C) mol L–1
concentration (S’ + C) mol L–1
Ksp = (s’ + C)s’
= s’ 2 + s’ C
BaSO4 is sparingly soluble, hence neglecting the s’2
llustration 25 :
The solubility of AgCl is moles2/lit2. What is its solubility?
Solution.
moles/litre
Illustration 26 :
The solubility of Ag2CrO4 is mol/lit. What is the solubility product?
Solution.
Exercise 3:
(i) Solubility product of a sparingly soluble salt AB at a temperature is 1.21 ⨯ 10–6 mol2 L–2. Its molar solubility will be
(A) (B) (C) (D)
(ii) The solubility product of a sparingly soluble salt is 1.08 ⨯ 10–13 mol3 L–3. It its molecular mass is 125, the g L–1 solubility will be
(A) 3 ⨯ 10–5 (B) 3.75 ⨯ 10–7 (C) 3.75 ⨯ 10–5 (D) 3.75 ⨯ 10–5
(iii) The solubility of KAl (SO4)2 is x mol L–1, its Ksp will be
(A) x3 (B) 4x3 (C) x4 (D) 4x4
Answer to Exercises
Exercise – 1
i) C
ii) B
iii) C
iv) B
v) C
Exercise – 2
i) C
ii) A
Exercise – 3
i) C
ii) B
iii) D
FORMULAE AND CONCEPTS AT A GLANCE
1.
2. Degree of Dissociation,
3.
4. Relative strength =
Ka = Dissociation constant for an acid
Kb = Dissociation constant for a base
5. Kw = [H+] [OH–] Kw = Ionic product of water = K [H2O]
6. pH = – log[H+] [H+] = 10-pH; pH=7
7. pKa = – log Ka (for acids)
pKb = – log Kb (for bases)
8. pH of a Buffer solution (Handerson Equation)
For an Acidic Buffer pH =
For a basic Buffer pOH =
9.
10.
11. ()
12. Kh for salt of strong acid and weak base
13. Kh for salt of weak acid and strong base
14. Kh for salt of weak acid and weak base
15. = Solubility product =
16. In general for AmBn type salt Ksp = mnnm (s)m+n
SOLVED PROBLEMS-1
Prob 1.
0.1M aq. solution of a weak acid is ionized upto 2%. What will be the concentration of H+ and OH–? (Kw = 1 ⨯ 10–14)
Sol.
For weak acids,
= 2 ⨯ 10–3
= 5.0 ⨯ 10–12
Prob 2.
What is the initial concentration of a solution of CH3COOH, if its [H+] is 3.4 ⨯ 10–4? (Ka = 1.7⨯10–5)
Sol.
= 6.8 ⨯ 10–3 M
Prob 3.
In a mixture of weak acid and its salt, the ratio of the concentration of acid to salt is increased tenfolds. What will be the change in pH?
Sol.
pH will decrease by 1
Prob 4.
Calculate % hydrolysis of NaCN in M/80 solution when dissociation constant for HCN is 1.3⨯10–9.
Sol.
% hydrolysis = 2.48
Prob 5.
How Ksp of a sparingly soluble salt XY4 is related with its (molar) solubility?
Sol.
At equilibrium 1 – s s 4s
=
= 256s5
or s = (Ksp/256)1/5
Prob 6.
At 250C, Kb for a weak base BOH is 1.0⨯10–12. What will be the [OH–] in 0.01M aqueous soln. of the base?
Sol.
C = concentration, = degree of dissociation
Assuming [OH–] to be practically unchanged
= 1.0⨯10–7
Prob 7.
40 mL of 0.1M NH4OH is mixed with 20 mL of 0.1 M HCl. What is the pH of the mixture? [pKb (NH4OH)) = 4.74]
Sol.
Half of the NH4OH will be neutralized by the acid.
Hence,
[Salt] = [ Base] = 4.74 + log 1 = 4.74
pH = 14 – 4.74 = 9.26
Prob 8.
Ka for ascorbic acid (HAc) is 5⨯10–5. Calculate the [H+] and percent hydrolysis in an aqueous solution in which concentration of ascorbate ions is 0.02 M
Sol.
% hydrolysis = 0.01
= 8.3
[H+] = – antilog 8.3.
Prob 9.
Solubility of calcium phosphate (Mol. mass, M) in water is ‘w’ g per 100 mL at 250C. Calculate its solubility product at 250C. Calculate
Sol.
Solubility,
Ksp = (3s)3 ⨯ (2s)2 = 108 s5 =
Prob 10.
The solubility product of lead iodide is 1.4⨯0–8. Calculate its molar solubility in 0.1 M KI solution.
Sol.
Let the solubility of PbI2 be s
PbI2 Pb2+ + 2I–
s s s
KI is strong electrolyte and hence is completely ionized. Concentration of I– provided by it = 0.1 M.
Ksp = [Pb2+] [I–]2 = s ⨯ (2s + 0.1)2
= s ⨯ (4s2 + 0.4s + 0.01) = 4s3 + 0.4s2 + 0.01s
Since s itself is smaller (PbI is sparingly soluble in water), s2 & s3 can be neglected.
SOLVED PROBLEMS-2
Prob 1.
Ostwald’s dilution law is valid for
(A) strong electrolyte
(B) weak electrolyte
(C) non-electrolyte
(D) strong as well as well electrolytes
Sol.
(B) The degree of dissociation of weak electrolytes increases considerably with dilution (decrease in concentration). While non-electrolytes don’t dissociate at all, strong electrolytes dissociate completely at almost all ranges of concentrations. Hence almost no variation is observed for change in concentration with strong electrolytes.
Prob 2.
Formic acid is 4.5% dissociated in a 0.1N solution at 200C. The ionization constant for the acid is
(A) 10–10 (B) 10–8 (C) 10–6 (D) 10–4
Sol.
(D)
Prob 3.
One litre of water contains 10–7 moles of H+. The degree of dissociation (ionization) of water is
(A) 1.8 ⨯ 10–7% (B) 0.8 ⨯ 10–9% (C) 3.6 ⨯ 10–9% (D) 3.6 ⨯ 10–7%
Sol.
(D) The number of moles in one litre of water =
Out of 1000/18 moles of water, the ionized ones are, 10-7
Out of 1000/18 moles of water, the ionized once will be =
= = 1.8 ⨯ 10–9
% dissociation = 1.8 ⨯ 10–7
Prob 4.
The degree of dissociation of weak electrolytes increases on
(A) increasing dilution
(B) decreasing dilution
(C) increasing concentration
(D) decreasing pressure
Sol.
(A) i.e., 1/C, i.e., is inversely proportional to C that infers the degree of dissociation is directly proportional to dilution (as, concentration 1/dilution)
Prob 5.
When NH4Cl is added to NH4OH solution, the dissociation of NH4OH is reduced due to
(A) oxidation
(B) reduction
(C) common ion effect
(D) hydrolysis
Sol.
(C) NH4Cl as well as NH4OH on dissociation produce NH4+. Since NH4Cl is strong electrolyte, the almost of the NH4+ ions produced in the solution would come from NH4Cl only, thereby reducing the dissociation of NH4OH, a weak electrolyte.
Prob 6.
Cl– ion is the conjugate base of
(A) HCl (B) HOCl (C) HClO3 (D) HClO4
Sol.
(A) Acids after dissociation produce H+ and a conjugate base of the acid.
Prob 7.
Phenolphthalein gives a pink colour in alkaline solution due to the fact that
(A) it is a coloured compound
(B) it ionizes to give coloured ions
(C) it is decomposed by alkali
(D) it forms a complex compound with alkali
Sol.
(B) Phenolphthalein is weak acid. In acidic medium its ionization is suppressed due to common ion effect, but in alkaline medium, OH– produced by the base react with the H+ of the phenolphthalein and
shifts the equilibrium towards right. The Ph– ions thus produced are pink which make the solution coloured.
Prob 8.
What will happen if 1.0M solution of a weak acid is diluted to 0.1M at constant temperature?
(A) [H+] will decrease to 0.01 M
(B) pH will decrease by 2 units
(C) Ka will increase
(D) percentage ionization will increase
Sol.
(D) For strong electrolytes which are 100% ionized, [H+] will decrease to 0.01M and pH will increase. For weak electrolytes, the degree of ionization increases with dilution, Ka being constant for a given temperature.
Prob 9.
The aqueous solution of AlCl3 is acidic due to
(A) cationic hydrolysis
(B) anionic hydrolysis
(C) hydrolysis of both the ions
(D) dissociation
Sol.
(A) AlCl3 dissociates in water in Al3+ ions and Cl–. Since Cl– are very stable being conjugate base of strong acid, they don’t get hydrolysed. Al3+ ion are not very stable in aq. medium, they undergo hydrolysis to form strong Al – OH bonds, thereby releasing H+ which make the solution acidic.
Prob 10.
Which among the following species can act both as an acid as well as a base?
(A) PO43– (B) SO42– (C) HSO4– (D) NH3
Sol.
(C) has an ionizable H atom, i.e.,
Hence it behaves as an acid. At the same time, it is produced by the deprotonation of H2SO4 (an acid), to, it is the conjugate base of the acid.








