Introduction:
Environmental chemistry is that branch of science which deals with the chemical phenomena occurring in the environment. It is a multidisciplinary science which covers many different fields such as chemistry physics, biological sciences agriculture, medical science etc.
A more general term for this branch is environmental science which deals with the study of sources of pollution and the methods of monitoring and controlling the pollutants.
Components of Environment:
Environment comprises of four major components:
1) Atmosphere 2) Hydrosphere 3) Lithosphere 4) Biosphere
1. Atmosphere:
It is a protective cover of gases which is surrounding the earth. It has no well defined upper limits and gradually merges with the outer space.
Constituents of atmosphere are gases, water vapour and aerosols. Pure dry air on an average constitutes 78% nitrogen and 21% oxygen by volume. Remaining 1% accounts for other gases.
(Table for atmospheric composition)
The main constituents which are of great importance for the earth’s climatic conditions are dust particles, carbon dioxide and ozone. The total mass of the atmosphere is about 5 x 1015 tonnes. The air at the surface of earth is compressed by weight of the air above it. Hence, density as well as pressure of the atmosphere decrease as we go higher above the earth surface.Of the total mass of atmosphere about 99% is within a height of 30km from the earth’s surface. On the basis of variation in temperature with increasing altitude, the atmosphere has been divided into the five distinct zones. These zones are troposphere, stratosphere, mesosphere, ionosphere and exosphere.
i) Troposphere: It is the domain of all living organism including plants and animals. It extends roughly to a height of 11km from the earth surface. This is the thinnest layer of the atmosphere, yet it contains about 80% of the total mass of air and all the water vapours and dust particles are concentrated in this zone. This region is greatly affected by air pollution. The temperature of this region decreases with altitude roughly at a rate of 6.4/km and is minimum at about 11 km. The temperature drops upto – 550C at the height of about 11km. The concentration of the water vapours varies from place to place and from time to time at the same place. As the water vapours are carried upward, they cool, condense into cloud particles and return to the earth’s surface by rain (precipitation). Another important constituent of troposphere is the aerosols or suspended particles. Most of the suspended particles act as nuclei around which water vapours condense to form clouds. Smog is also caused by the presence of suspended particles in the atmosphere.
ii) Stratosphere: It extends from 11 to 50 km. This zone is characterised by the absence of changing weather pattern. In the stratosphere temperature begins to increase. The point at which temperature inversion takes place is called tropopause. The ozone present in the stratosphere absorbs the harmful ultraviolet radiation coming from the sun. The rise in temperature is due to absorption of ultraviolet rays which is converted into heat. In the stratosphere, temperature ceases to fall and remains constant upto the height of 20km. Afterwards it gradually increases upto the height of 50km where it reaches to about – 20 Chemical species present in the stratosphere are N2, O2 and O3. The formation and destruction of ozone by the natural process is a dynamic equilibrium between O2 and O3. This equilibrium maintains a concentration of ozone in the stratosphere. Due to the presence of ozone layer, this region is also called ozonosphere. Concentration of ozone in stratosphere ranges between 1 to 5 ppm by volume. Startosphere is comparatively calm and is much less dense than the troposphere. If the pollutants reach the stratosphere, they will stay there for quite a long time and hence will become long term health hazard.
iii) Mesosphere: Above the stratosphere is mesosphere. It extends from 50 to 85km. It contains low concentration of oxygen and nitrogen and posses little capacity to absorb solar radiation. Hence, temperature again decreases with height to an exceedingly low value of about – 1000C. The region of minimum temperature is the limit of mesosphere and is known as the mesopause.
iv) Thermosphere: Above the mesopause, temperature rises very rapidly to very high values. This high temperature region is called thermosphere. It extends from 85 to 500 km. Mesosphere and thermosphere (collectively called ionosphere) contain gases in the ionized form (O2+, NO+, O+). These ions are generated in daytime by UV radiation and have long life times. Some pollutants on reaching this region get converted to free radicals which also have long life time e.g.
The ionized particles in ionosphere are responsible for the reflection of radio waves on earth and enable wireless communication.
v) Exosphere: It is considered to the highest region of the atmosphere. It contains mainly atomic and ionic oxygen, hydrogen and helium. This zone merges with the outer space.
2) Hydrosphere: The total water present on the earth in solid, liquid and gaseous phases constitutes the hydrosphere. About 75% of earth’s surface is covered by hydrosphere. Most of it is in the oceans and contains about 3.5% of dissolved salt. Polar ice caps and glaciers provide 2% water. Fresh water is present in lakes or rivers or ponds which flow into them from rain or melting of snow etc. Sea water is unfit for drinking or agriculture because each kilogram contains 35 kg of dissolved salts.
3) Lithosphere: It is the solid component of the earth consisting of soil, rocks, mountains etc. The outermost thick solid part of the earth is called the crust. Since outer crystal layer comprises of the rocks rich in silica and aluminium, it is called SIAL layer. The upper most part of the earth’s crust contains weathered rocks as well as organic matter and is called soil. The top layer of the soil is the vital component, since all the nutrients required by plants are present in this layer.
4) Biosphere: It is that part of the lithosphere, hydrosphere and atmosphere where living organisms interact with these parts and thus live together. Biosphere extends from 6km above the sea level to about 10km below the sea level. Biosphere and other segments of the environment are interrelated. For example, the levels of O2 and CO2 depend on the plants present in the biosphere.
Environmental Pollution: The addition of any undesirable material to air, water and soil by a natural source or due to human activity to such level of concentration which adversely affects the quality of environment is called environmental pollution.
Pollutants: Any solid, liquid or gaseous substance present in such concentration that may be or may tend to be injurious to the living organisms including human health and environment. In a very simple way, a pollutant can be defined as a substance or an agent which causes pollution.
Contaminant: A substance which is not present in the nature, but released during human or natural activity into environment is called as contaminent. The contaminent which adversely effects life on earth is known as pollutant.
Receptor: The medium which is effected by the pollutant is called receptor. For example, when many vehicles stop at the traffic signal during peak hours, our eyes become red with burning sensation due to the smoke released from the automobiles. The eyes here are the receptors.
Sink: The medium which reacts with pollutants is called sink. For example, micro organisms which eat the dead animals or which convert the dried leaves and garbage into fertilizers act as sink for biodegradable pollutants. Similarly, sea water is a sink for carbon dioxide.
Speciation: The chemical form of the pollutants is known as speciation. The toxicity of the pollutants depends on this form. For example alkylated mercury is more toxic than Hg.
Pollutants can be classified in two different ways as follows:
1) Biodegradable and Non-biodegradable pollutants:
Biodegradable pollutants are domestic wastes which can be rapidly decomposed under natural processes by micro-organisms or by suitable treatment and thus are not harmful. However when accumulation of these occurs i.e., complete degradation does not take place then these pollute the biosphere and create problems.
Non biodegradable: pollutants do not undergo degradation or degrade very slowly in the ecosystem naturally. These are poisonous like mercuric salts, aluminium, lead compounds, DDT etc. These are not recycled in the natural environment.
2) Primary and Secondary pollutants:
Primary pollutants are those which after their formation enter the environment and remain as such. e.g. SO2, NO2, CO etc, are primary pollutant.
Secondary pollutants are those which are formed in the atmosphere by chemical interactions among primary pollutant and normal atmospheric constituents.
e.g., SO3, aldehydes and PAN (Peroxyacyl nitrates) are the secondary pollutants.
The major processes that convert the pollutants from their primary forms to their secondary forms are oxidation, dissociation and dissolution.
Types of pollution:
Pollution can not only be due to addition of undesirable material into the environment but can also be due to factors like noise, ultraviolet rays, etc.
The usual practice is to classify the pollution on the basis of medium (air, water or land) in which significant contamination by pollutants occurs. Sometimes the classification is done according to the type of pollutants.
However following types of pollution have been observed:
a) Atmospheric pollution b) water pollution c) Soil pollution
d) Radioactive pollution e) Thermal pollution f) Noise pollution
g) Industrial pollution h) solid waste pollution
a) Atmospheric pollution: Atmospheric pollution is generally studied as tropospheric and stratospheric pollution.
i) Tropospheric pollution: It occurs due to the presence of undesirable solid or gaseous particles in air. Gaseous air pollutants are oxides of sulphur nitrogen and carbon, hydrogen sulphide, hydrocarbons ozone and other oxidants.
Particulate pollutants are dust, mist, fumes, smoke, smog etc.
i) Gaseous air pollutants:
a) Oxides of sulphur: The two oxides of sulphur i.e., SO2 and SO3 are the most harmful pollutants of the atmosphere. Both are colourless gases with pungent smell. These pollutants are released into the atmosphere through volcanic eruptions and through combustion of sulphur bearing fuels such as coal and oil. SO2 is also released during roasting of sulphide ores such as iron pyrites (FeS2), copper pyrites (CuFeS2), copper glance (Cu2S), zince blende (ZnS), galena (PbS) etc. About 66% of total SO2 is contributed by natural activity and the rest by human activity.
A part of SO2 undergoes photolytic and catalytic oxidation to give SO3. The presence of particulate matter in polluted air catalyses the oxidation of SO2 to SO3.
The reaction can also be promoted by ozone
The SO3(g) formed as above changes to H2SO4 in the presence of moisture.
This acid comes down in the form of sulphuric acid rain.
Harmful effects of SO2 and SO3:
i) SO2 and SO3 are both strongly irritating to the respiratory tract. It has been reported that even a low concentration of SO2 causes respiratory diseases e.g., asthma, bronchitis, emphysema in human beings.
ii) SO2 causes irritation to the eyes, resulting in tears and redness.
iii) High concentration of SO2 leads to stiffness of flower buds which eventually falls off from plants.
iv) High concentration of SO2 and sulphuric acid even over short period produce leaf injuries such as necrotic blotching of broad leaved plants and grasses.
v) Even a very low concentration of SO2 has a very damaging effect on the plants. If plants are exposed to SO2 for days or weeks, it slows down the formation of chlorophyll resulting in the injury to the leaf including loss of green colour. This is called chlorosis.
vi) SO2 as such or after being converted into H2SO4 damage building material especially marble. For example marble of Taj Mahal in Agra is being damaged due to Mathura refinery, thermal power station and a number of foundaries located nearby.
SO2 and SO3 are converted into H2SO4 and this H2SO4 precipitates as acid rain. The acid rain combines with limestone minerals. Thus, limestone minerals act as sink for sulphur oxides.
b) Oxides of Nitrogen: A number of oxides of nitrogen such as NO, N2O, N2O3 and N2O5 are introduced into the atmosphere due to natural sources and due to human activities. Out of these only two oxides i.e., NO and NO2 are considered as pollutants. Nitric oxide (NO) is colourless and odorless gas whereas nitrogen dioxide (NO2) is reddish brown gas having pungent smell and is suffocating in nature.
Sources: At high altitudes when lightning strikes, N2 and O2 combine to form oxides of nitrogen. In an automobile engine (at high temperature) when fossil fuel is burnt N2 and O2 combine to give significant quantities of NO and NO2.
Rate of production of NO2 is faster when nitric oxide reacts with ozone in the stratosphere
These oxide of nitrogen are removed from the atmosphere in the form of nitric acid through following sets of reaction.
Nitric acid comes down from the atmosphere in the form of nitric acid rain. On the earth surface it reacts with bases such as ammonia, lime etc. to form nitrates (NO3–)
Harmful effects:
i) NO2 is a lung irritant that can lead to an acute respiratory disease in children. It is toxic to living tissues also.
ii) The irritant red haze in the traffic and congested places is due to oxides of nitrogen.
iii) Higher concentrations of NO2 damage the leaves of plants and retard the rate of photosynthesis.
vi) NO2 is also harmful to various textile fibres and metals.
v) Nitric oxide alone does not have any adverse effect on humans at concentrations that occur in the atmosphere. Though NO has about 1500 times greater affinity than CO for haemoglobin but it is unable to enter the blood stream from the atmosphere. But its presence in large amount may result in the increase of NO2 by oxidation and thus become harmful.
vi) Acid rain containing HNO3 causes the of the soil to decrease to 4 or 5 and thus reduces the fertility of the soil.
vii) The nitric oxide coming out from supersonic gets directly enters the stratosphere and combine with ozone resulting in the decomposition of O3 to O2 thus decreasing O3 density
viii) The most serious hazard of NO and NO2 is in the formation of an unpleasant mixture of gases and particulates that make up photochemical smog.
To control the pollution due to NO and NO2, catalytic converters are used in automobile exhaust which convert the oxides of nitrogen to free N2 or to a small amount of NH3.
The flue gases coming from power plants or industrial units and containing NO2 and SO2 are freed from these gases by scrubbing them with H2SO4. As oxides of nitrogen and oxides of sulphur are acidic oxides, scrubbing can also be done with alkaline solution such as Ca(OH)2 and Mg(OH)2.
C) Oxides of carbon:
Carbon monoxide:
It is produced as a result of incomplete combustion of carbonaceous fuel. It is mainly released into the air by automobile exhaust. Many vehicles are poorly maintained and several have inadequate pollution control equipments resulting in the release of a greater amount of CO and other polluting gases. Other sources which produce CO involve incompletes combustion of coal, firewood, petrol etc.
Carbon monoxide is a colourless and odourless gas, highly poisonous to living beings because of its ability to block the delivery of oxygen to the organs and tissues. The toxic effect of CO on human beings and animals arise from its reversible combinations with haemoglobin present in RBCs to form carboxy haemoglobin (Hb. CO).
The normal function of the haemoglobin is to combine with oxygen in the lungs to form oxyheaemoglobin reversibly, as follows:
Oxyhaemoglobin travels to the different body cells where it gives up oxygen and takes up CO2, returns to the lungs, then CO2 is exhaled out. However if large amount of CO is present in the surrounding air, it combines more readily with haemoglobin to form carboxyhaemoglobin which is about 300 times more stable than oxygen haemoglobin complex.
In blood when the concentration of carboxyhaemoglobin reaches about 3 – 4 percent, the oxygen carrying capacity of blood is greatly reduced. This oxygen deficiency, results into headache, weak eyesight, nervousness and cardiovascular disorder.
1) Pregnant women who have the habit of smoking, the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies.
It has been found that maximum permissible concentration of CO in the surrounding air is 40ppm for an exposure of 6 – 8 hours. At concentration greater than 100ppm difficulty in breathing starts and there is headache and dizziness.
Concentration of 750ppm or more lead to anoxia or asphyxiation (Acute oxygen starvation). This condition leads to coma and death.
The most effective treatment for CO poisoning is to confined the victim in a chamber having oxygen at 2 to 2.5 atm. Under pressure CO of carboxy haemoglobin is replaced by O2 and thus transport of O2 to different body parts starts. Exposure to oxygen under high pressure also corrects anoxia.
Sinks of CO :
Recent researches have shown that soil is capable of removing large amounts of CO from the atmosphere by the action of some soil microorganisms. Thus soil microorganisms act as a sink for CO.
The main source of CO pollution is the use of internal combustion engines in the automobiles. To prevent CO pollution, catalytic converter is fitted into the exhaust pipe which may convert poisonous gases like CO into harmless products before they are thrown into the air.
Carbon dioxide:
Normally CO2 forms about 0.03%, by volume of the atmosphere. Usually it is not considered as an air pollutant. However release of large amount of CO2 into the atmosphere brings about significant changes in climate. Carbon dioxide is released into the atmosphere by respiration, burning of fossil fuels for energy and by decomposition of limestone during the manufacture of cement. It is also emitted during volcanic eruptions. Excess of CO2 in the air is removed by green plants and this maintains an appropriate level of CO2 in the atmosphere. Deforestation and burning of fossil fuel increase the CO2 level and disturb the balance in the atmosphere. The increased amount of CO2 in the air is mainly responsible for global warming.
d) Hydrocarbons:
Hydrocarbons are formed by incomplete combustion of fuel used in automobiles. Most of these hydrocarbons are of low molecular weight and are gases or voltatile liquids at ordinary temperature. Methane is the most abundant hydrocarbon pollutant. Methane is released into the air in large quantities by the anaerobic decomposition of organic matter in soil, water and sediments.
Harmful effects:
Hydrocarbons are carcinogenic i.e., they cause cancer. They harm plants by causing ageing; breakdown of tissues and shedding of leaves, flowers and twigs.
Due to photochemical reactions of hydrocarbons with oxygen and oxides of nitrogen, they form photochemical oxidants and photochemical smog.
e) Hydrogen sulphide:
H2S is released into atmosphere due to volcanic activity and anaerobic biological decay of protein matter in stagnant water and swamps. Anaerobic reactions occurring in sewage and sanitary landfills also produce H2S. Since H2S is readily oxidized to SO2, the two gases are present together in the atmosphere. H2S has an objectionable smell like that of rotten eggs. Its presence in the atmosphere affects the white lead pigments and silver ware due to conversion of lead and silver to black PbS and Ag2S respectively.
Global warming and Green house Effect:
A green house is a place where plants are grown on the soil but there are walls and roof made of glass. In a green house, visible light passes through the transparent glass and heats up the soil and the plants. The warm soil and plants emit infrared radiations. Glass is opaque to infrared radiation. This mechanism keeps the energy of the sun trapped in the green house. Just as the glass in a green house holds the heat energy of the sun inside, atmosphere traps the sun’s heat near the earth’s surface and keeps it warm. CO2 molecules present in atmosphere also trap heat as they are transparent to visible light but not to the heat radiation. [CO2 present in normal concentration (.03%) in atmosphere is not a pollutant. Rather it helps to maintain the temperature of the earth required for the existence of living organisms. If the amount of CO2 in atmosphere increases, this results in warming of the earth’s surface.]
About 75% of the solar energy reaching the earth is absorbed by the earth surface which increases its temperature. The rest of the heat radiates back to the atmosphere. When the earth cools, the energy is re-emitted from the earth’s surface in the form of infrared radiations. These radiation can be absorbed by CO2 and H2O vapours but can not pass through them. The heat absorbed by CO2 and H2O vapours is radiated back to the surface of the earth. In this way additional heat is kept within the lower atmosphere and warming of the earth occurs. Besides CO2 and H2O vapours other green house gases are methane, nitrous oxide, ozone and chlorofluoro carbons (CFCs).
Methane is produced naturally when vegetation is burnt, digested or rotted in the absence of oxygen. Large amount of CH4 is released in paddy fields, coal mines, from rotting garbage dumps and by fossil fuels.
Chlorofluoro carbons such as Freon – 1 (CFCl3) and Freon – 12 (CF2Cl2) are used as propellants in aerosol sprays, refrigerants, air conditioning and solvents for cleaning electronic components. CFCs are also damaging to the ozone layer.
Consequences of Green house effect:
If the rate at which solar radiation are arriving the earth remains constant but the amount of green house gases increases, the heat radiated back to the earth will increase. If these trends continue, the average global temperature will increase to a level which may lead to melting of polar ice caps and flooding of low lying areas all over the earth.
Increase in the global temperature increases the incidence of infectious diseases like dengue, malaria, yellow fever sleeping sickness etc.
Acid rain:
Normal rain water has a of about 5.6 due to dissolution of CO2 into it
When the of the rain water drops below 5.6 it is called acid rain.
Acid rain is a byproduct of a variety of human activities that emit the oxides of sulphur and nitrogen in the atmosphere.
Due to bacterial action or volcanic eruption or due to human activities, mainly involving burning of fossil fuels (which contain sulphur and nitrogenous matter), such as coal and oil in power stations and furnaces or petrol and diesel in motor engines, SO2 and oxides of nitrogen enter into atmosphere as pollutants.
Oxides of nitrogen i.e., nitric oxide and nitrogen dioxide combine with oxygen and ozone to form higher oxides of nitrogen. These oxides ultimately dissolve in water to form nitric acid.
SO2 reacts with oxygen and water to form sulphuric acid.
The oxidation of SO2 to SO3 is catalysed by aerosol containing metal ions like Cu (II), Fe (II), Mn (II) and Ni (II) or by the presence of NO.
The nitric and sulphuric acid dissolve in rain water and come down to earth as acid rain. This is more in industrial areas. Acid rain have also been reported in some places which are far away from the places where industries are located. This is due to the movement of the rain clouds from industrial areas to other areas due to the flow of wind.
H2SO4 is the main contributor of acid rain (60 – 70%) next is HNO3 (30 – 40%) and least is HCl. Ammonium salts are also formed in atmosphere and can be seen as an atmospheric haze (aerosol of fine particles). Aerosol particles of oxide or ammonium slats in rain drops result in wet deposition. SO2 is also absorbed directly on both solid and liquid ground surfaces and is thus deposited as dry – deposition.
Harmful effect of acid rain:
1) Acid rain is harmful for agriculture, trees and plants as it dissolves and washes away nutrients needed for their growth.
2) Acid rain causes respiratory ailments in human beings and animals.
3) When acid rain falls and flows as ground water to reach rivers lakes etc, it affects plants and animal life in aquatic ecosystem. Acid rain increases the acidity of water in the lakes which is lethal for the fishes. For this reason some of the lakes have become fishless.
4) If corrodes water pipes resulting in the leaching of heavy metals such as iron lead and copper into the drinking water. These metals have toxic effects.
5) Acid rain damages building, statues and other structures made of marble, limestone, state, mortar etc.
For example, Taj Mahal at Agra is made of marble. It is being attacked by acid rain due to high concentration of oxides of sulphur and nitrogen in the air over Agra because of large No. of industries in the surrounding area which are emitting these gases. The acid rain resulting from these gases reacts with marble (CaCO3) of Taj Mahla causing damage to this wonderful monument.
The reaction with marble takes place as follows:
As a result the monument is slowly disfigured and the marble is getting discoloured and lusterless. To save the Taj Mahal from getting disfigured, Government of India announced an action plan in 1995. This plan aims at clearing the air in the Taj Trapezium i.e., an area that includes the towns of Agra, firozabad Mathura and Bharatpur. Under this plan more than 2000 polluting industries in the trapezium would be allowed to use only natural gas on LPG instead of coal or oil. A pipeline would be laid for this purpose. People in these areas would also be advised to use LPG instead of coal, wood and kerosene oil etc. Vehicles in the nearby area would be encouraged to use low sulphur content fuel.
ii) Particulate pollutants:
Small solid particles and liquid droplets suspended in air are collectively called as particulates. Particulates in the atmosphere may be viable or non-viable.
The viable particulates are the minute living organisms that are dispersed in the atmosphere. It includes bacteria, fungi, moulds, algae etc.
Human beings are allergic to some of the fungi found in air. They can also cause plant disease.
The non viable particulates are formed as a result of disintegration of large size materials or by condensation of small size particles or droplets. Non viable particulates may be classified according to their nature and size as follows:
a) Smoke: It contains very small soot particles which can be solid or mixture of solid and liquid. These are produced by combustion of organic matter. Examples are cigarette smoke, smoke from burning of fossil fuel, garbage and dry leaves, oil smoke etc.
b) Dust: It is composed of fine solid particles (over 1 mm in diameter). These particles are produced during crushing, grinding etc. Some typical examples of this type of particulate emission are sand particles, saw dust from wood works, pulverized coal, cement and fly ash from factories, dust storms etc.
c) Mists: These particles are produced by spray liquids and by condensation of vapours in air. Examples of this type are sulphuric acid mist and herbicide and insecticide that miss their targets and travel through air and form mists.
d) Fumes: These are condensed vapours. Generally organic solvents, metals and metallic oxides form fume particles. Fumes are obtained by the condensation of vapours during sublimation, distillation, boiling and several other chemical reactions.
Particulates produced in the atmosphere as a result of chemical reaction are called secondary particulates: These are formed either in gas phase reactions or by heterogenous reactions between gases and already existing primary particulates. The reactions leading to the formation of secondary particulates are largely catalysed by sun light. A few secondary particulates are as follows.
i) Nitrate particulate:
Nitric acid which is formed from NO2, reacts with basic substances to form nitrate aerosols.
ii) Sulphate particulate:
These particulates include sulphuric acid droplets, ammonium sulphate and other metal sulphates. Sulphuric acid itself is formed form SO2 by its oxidation and then reaction with water. H2SO4 combines with atmospheric ammonia to form ammonium sulphate.
Other sulphate salts like lead sulphate are formed by the reaction of sulphuric acid and corresponding metal oxide.
Tetraethyl lead [Pb (C2H5)4] is the source of lead salts in the atmosphere. It is added to gasoline at a rate of 0.5 to 0.8 g per litre to increase its octane number i.e., to improve its antiknock property. Tetraethyl lead is oxidized to PbO which tends to deposit of spark plugs and valves. In order to avoid deposition of PbO suitable amounts of C2H4Cl2 and C2H4Br2 are added to gasoline. These halides convert PbO into PbCl2 and PbBr2. Both of these halides are volatile and get eliminated through the exhaust.
Lead interferes with the development and maturation of red blood cells. This problem has now been overcome by using unleaded petrol in most of the cities in India.
Harmful effects of particulate pollutants:
i) Effect on humans: The effects of particulate pollutants depend on the particle size. Particulate pollutants bigger than 5 micron are likely to lodge in the nasal passage, whereas particles of about 1.0 micron enter into lungs easily. These smaller particles because of larger surface area also provide effective sites for the adsorption of carcinogenic substances such as polynuclear hydrocarbons, asbestos etc, and cause diseases like lung cancer, bronchital asthama, chronic bronchitis etc.
Different types of lung diseases are caused by different types of particulates e.g., asbestos causes asbestosis, dust containing free silica (SiO2) causes silicosis. Beryllium compounds cause beryllosis.
ii) Effect on visibility: Particulates in the atmosphere reduce visibility by scattering and absorption of sunlight. Particulates also reduce visibility by attenuating the light from objects and illuminating the air thereby reducing the contrast between the objects and their background. Reduced visibility is dangerous practically for aircraft and motor vehicles.
iii) Effect on materials: Particulates damage a large number of materials. The adverse effects of particulates include corrosion of metals, erosion, damage to paints, clothes, draperies buildings, soil, sculptures monuments etc. Particulate matter also damages the electronic equipment through chemical or mechanical contacts.
iv) Effect on climate: Particulates scatter and reflect back the heat of the sunlight and thus control the warming up of the earth’s surface. These particulates act as nuclei for cloud formation and hence affect the climate. v) Effect on plants: Particulates deposit on the leaves of plant thereby blocking the stomata and hinders the intake of CO2 from the air. Hence it inhibits photosynthesis.
Control of pollution caused by particulates:
The most effective and efficient method for the removal of particulates is electrostatic precipitation. It is based on the principle that particles of all sizes acquire electrical charge when exposed to a high electric field.
In this method, the air containing the particulates is allowed to enter a tall chamber in which the central electrode is subjected to a negative potential of 30000 – 40000 volts while peripheral electrode is earthed. Because of high potential difference, the air inside the chamber gets ionized into positively charged ion and free electrons. The free electrons get attached to the particles present in flue gases which thus get negatively charged. These negatively charged particles are then attracted towards positive peripheral electrode (grounded) on which they accumulate and are removed by vibrating the electrode. In this way, about 99 per cent of the particulate matter gets removed from the flue gases.
Other methods for the removal of particulate matter from the flue gases are as follows:
i) Gravity settling chamber: In this chamber only larger particles settle down due to gravity however fine particles can not be removed by this method.
ii) Cyclone collector: In this technique, the gas is passed through a circular spiral. Due to the centrifugal forces, particulate move towards the wall and settle down.
iii) Wet scrubbers: In this technique, a suitable liquid (usually water) is introduced in the form of a fine spray which washes away the particulates.
Smog:
The word smog was coined to describe the combination of smoke and fog. The name was so given because for the first time it was found to be formed due to condensation of some kind of fog on the carbon particles present in the smoke. However, now it is given a name depending upon the composition or the method of its formation.
a) Classical smog: It occurs in cool humid climate. It is a mixture of smoke, fog and sulphur dioxide. Many of the chemicals present in the particulates catalyse the conversion of SO2 to SO3 which then combines with H2O of the humidity forming sulphuric acid droplets. These droplets condense on the surface of particulates. This type of smog is formed in the early morning hours of winter month. Shortly after sunrise it increases due to photochemical oxidation of SO2 to SO3 and subsequent combination with moisture to form sulphuric acid aerosol. Chemically it is a reducing mixture, so it is also called as reducing smog. This smog causes bronchitis and other respiratory problems leading to death. This type of smog was first observed in London in December 1952 where about 4000 to 5000 direct and indirect deaths were reported. That is why it is also called London smog.
b) Photochemical smog: This smog occurs in warm, dry and sunny climate. It is formed when the air contains NO2 and hydrocarbons and the mixture is exposed to sunlight. The reaction which leads to the formation of this smog, takes place in the presence of sunlight, hence it is called photochemical smog. This smog has high concentration of oxidizing agents and is therefore called as oxidizing smog. This type of smog was first observed in Los Angeles in 1950 and hence it is also known as ‘Los Angeles smog’.
Mechanism of the formation of photochemical smog:
The main components of the photochemical smog result from the action of sunlight on unsaturated hydrocarbons and nitrogen oxides produced by automobiles and factories. When nitrogen oxides and hydrocarbons are present upto sufficiently high level, a chain reaction occurs from their interaction with sunlight. In presence of sunlight NO2 absorbs energy and breaks up into NO and atomic oxygen.
Oxygen atoms are very reactive and combine with O2 in air to produce ozone.
The ozone thus formed reacts with NO to regenerate NO2 an O2.
Thus NO2 cycle is completed, NO and O3 produced are used up and no extra NO2 is added into the atmosphere. But the trouble arises if hydrocarbons are also present in the atmosphere. Both NO2 and O3 are strong oxidizing agents and can react with the unburnt hydrocarbons in the polluted air to produce chemicals such as formaldehyde, acrolein and peroxyacetyle nitrate (PAN).
Participation of unburnt hydrocarbons may be depicted as:
(Peroxyacylnitrate)
Peroxy acetyl nitrate.
PAN is a powerful tear producer or lachrymator and causes breathing troubles.
Extensive research has shown that CO also plays a very important role in the production of smog.
Effects of photochemical smog:
The main components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN). All these compounds produce irritation in the eyes and also in the respiratory system. Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat. Cough and difficulty in breathing. Photochemical smog leads to cracking of rubber and extensive damage to plant life. It also causes corrosion of metals, stones, building material, rubber and painted surface.
Control of photochemical smog:
If we control the primary precursors of photochemical smog, such as NO2 and hydrocarbons the secondary precursors such as ozone and PAN will automatically be reduced. These primary pollutants (NO, hydrocarbons) can be controlled by fitting efficient catalytic converters in the automobiles. These catalytic converters prevent the release of nitrogen oxide and hydrocarbons to the atmosphere. Certain plants e.g., Pinus, Juniparus, Quercus, Pyrus and Vitis can metabolise nitrogen oxides and therefore, their plantation could help in this matter.
Bhopal gas tragedy:
On December 2, 1984, Bhopal gas tragedy (due to leakage of methyl isocyanate gas) resulted into death of about 3000 people. Methyl isocyanate gas released from a pesticide manufacturing plant of union carbide. This gas caused irritation of eyes and followed by blindness and various lung diseases causing death.
Methyl isocyanate is used in making the insecticide carbaryl (or commercial name sevine). It is obtained by the reaction of methyl amine with phosgene.
Then MIC is treated with 1-naphthol as follows:
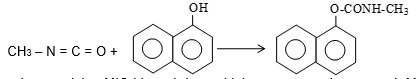
One of the tanks containing MIC blasted due to high pressure and as a result MIC escaped into the atmosphere. The reason for the sudden rise of pressure is believed to be the presence of some moisture into the tank.
2) Stratospheric Pollution:
The upper stratosphere consists of considerable amount of ozone O3. This ozone layer shields the earth from the harmful ultraviolet radiation of the sun. These radiations cause skin cancer (melanoma) in humans. Therefore it is important to maintains the ozone shield.
The formation of ozone in the stratosphere takes place in two steps. In the 1st step, the UV radiations split apart molecular oxygen into free oxygen atoms. In the second step the oxygen atoms react with more dioxygen to form ozone.
The ozone thus formed absorbs the ultraviolet radiation and is again broken into dioxygen and an oxygen atom. Thus a dynamic equilibrium exists between the production and decomposition of ozone molecules. When is broken into dioxygen and an oxygen atom, heat is given out.
For this reason, stratosphere is a zone of increasing temperature. In this way ozone cycle is completed in the stratosphere.
Depletion of ozone layer: In recent years, there have been reports of the depletion of this protective ozone layer because of the presence of certain chemicals in the stratosphere. Two types of compounds have been found to be most responsible for depleting the ozone layer.
i) Chlorofluorocarbons (CFCs): CFCs are synthetic compounds of chlorine, fluorine and carbon and are often referred to by the trade name ‘freons’. The main reason of ozone layer depletion is believed to be the release chlorofluorocarbon compounds. The compounds are non reactive, non flammable nontoxic organic molecules. These compounds are used in refrigerators, air conditioners, in the production of plastic foam and by the electronic industry for cleaning computer parts. Once CFCs are released in the atmosphere, they mix with normal atmospheric gases and eventually reach the stratosphere. In the stratosphere they absorb UV radiation and get photolysed to liberate chlorine free radicals.
The chlorine radicals then react with stratospheric ozone to form chlorine monoxide radicals and molecular oxygen.
Reaction of chlorine monoxide radical with atomic oxygen produces more chlorine radicals.
The chlorine radicals are continuously regenerated and cause the breakdown of ozone. Thus CFCs are transporting agents for continuous generating chlorine radicals into the stratosphere and damaging the ozone layer.
ii) Oxides of Nitrogen: Oxides of nitrogen are produced at the ground level due to human activity or natural sources. Nitrogen oxides are produced in large amounts in the exhaust gases by the engine of supersonic transport planes and introduced directly into the stratosphere. These oxides are also released due to large scale combustion of fossil fuels and enhanced use of nitrogen fertilizers.
Though nitrous oxide is quite inert, in the stratosphere it is photochemically converted into more reactive nitric oxide.
Oxides of nitrogen catalyse the decomposition of ozone and are themselves regenerated to repeat the cycle, which involves the following chain reactions:
Net reaction
Thus in the presence of oxides of nitrogen in the stratosphere, the decomposition rate of O3 increases.
The ozone hole:
Although the chain reactions, initiated by chlorofluorocarbons, leading to depletion of ozone take place in all parts of the stratosphere, yet the ozone hole has mainly been observed in the stratosphere over Antarctica. This is because in other parts of the stratosphere, chlorine monoxide radicals combine away with the oxides of nitrogen present in the stratosphere and chlorine free radicals combine away with the methane present in the stratosphere as follows:
As a result the chain reaction stops. In Antarctica, the climate conditions are quite different. In 1980s atmospheric scientists working in Antarctica reported about depletion of ozone layer commonly known as ozone hole over the south pole. It was found that a unique set of conditions was responsible for the ozone hole. In summer season, NO2 and CH4 react with chlorine monoxide and chlorine atoms, forming chlorine sinks, preventing much ozone depletion, whereas in winter special type of clouds called polar stratospheric clouds are formed over Antarctica. These polar stratospheric clouds provide surface on which chlorine nitrate gets hydrolysed to form hypochlorous acid.
Chlorine nitrate also reacts with hydrogen chloride to give molecular chlorine.
When sunlight returns to the Antarctica in the spring, the sun’s warmth breaks up the clouds and HOCl and Cl2 are photolysed by sunlight.
The chlorine radicals thus formed initiate the chain reaction for ozone depletion. Thus the depletion of ozone over Antarctica takes place during spring i.e. in the months of September and October and is replenished after spring.
Due to presence of polar stratospheric clouds, a tight whirlpool of wind is formed in the stratosphere which surrounds Antarctica. It is called Polar Vortex. It is so rigid that it cuts off Antarctica from the surrounding ozone rich air of the non polar regions. As a result the ozone hole remains unfilled. After the spring, the intensity of sunlight increases and the vortex breaks down. The ozone rich air from surroundings immediately rushes to fill up the ozone hole.
Effects of Depletion of the Ozone Layer:
i) With the depletion of ozone layer, more UV radiation can pass through the stratosphere and reach the surface of the earth. UV radiations lead to ageing of skin, cataract, sunburn, skin cancer, killing of many phytoplanktons, damage to fish productivity etc. ii) It has been reported that plant proteins get easily affected by UV radiations which leads to the harmful mutations of cells.
iii) It also increases evaporation of surface water through the stomata of the leaves and decreases the moisture content of the soil.
iv) Increase in UV radiations damage paints and fibres, causing them to fade faster.
In 1987, an international treaty was signed in Montreal to cut back on the use of chlorofluorocarbons. Production of Freon in the united states was stopped in 1996.
An intense effort is under way to find substitute that are not harmful to the ozone layer. One of the substance is hydrochlorofluoro carbon – 123 (CF3CHCl2). The presence of hydrogen atom makes the compound more susceptible to oxidation in the lower atmosphere, so that it never reaches the stratosphere.
Water pollution:
Water pollution is defined as the contamination of water by foreign substances (organic, inorganic, radioactive or biological) which make it harmful for health of animals or plants or aquatic life and make it unfit for domestic, industrial and agricultural use. Pollution of water originates from human activities. Through different paths, pollution reaches surface or ground water.
Easily identified source or place of pollution is called as point source e.g. municipal and industrial discharge pipes where pollutants enter the water source.
Non point sources of pollution are those where a source of pollution cannot be easily identified e.g., agricultural run off (from farm, animals, and crop-lands), acid rain, storm – water drainage (from streets, parking lots and lawns) etc.
Causes of water pollution
i) Disease causing agents:
The most serious water pollutants are the disease causing agents called pathogens. Pathogens include bacteria and other organism that enter water from domestic sewage and animal excreta. Human excreta contain bacteria such as Escherichia coli and streptococcus faecalis which cause gastrointestinal diseases.
ii) Sewage and other oxygen demanding waste:
Sewage from municipalities, domestic waste, organic wastes from pulp and paper industry and other biodegradable organic compounds are oxygen demanding wastes. Excessive phytoplankton growth within water is also a cause of water pollution.
The large population of bacteria decomposes organic matter present in water. They consume oxygen dissolved in water. The amount of oxygen present in water is called as dissolved oxygen (DO). In cold water dissolved oxygen can reach a concentration upto 10 ppm, whereas oxygen in air is about 200000 ppm. That is why even a moderate amount of organic matter when decomposes in water can deplete the water of its dissolved oxygen. If the concentration of dissolved oxygen of water is below 6 ppm, the growth of fish gets inhibited. The dissolved oxygen is also used by microorganisms to oxidize organic matter. If too much organic matter is added to water, all the available oxygen is used up. Thus anaerobic bacteria begin to break down the organic waste and produce chemicals that have a foul smell and are harmful to human health.
“The amount of oxygen used by suitable microorganisms present in water during five days at 200C is called Biochemical Oxygen Demand (BOD)”. It is empirical in nature. The amount of BOD in the water is a measure of the amount of organic material in the water, in terms of how much oxygen will be required to break it down biologically.
Clean water would have BOD value of less than 5ppm whereas highly polluted water could have BOD value of 17 ppm or more. The untreated municipal sewage has BOD of 100 to 400 ppm. Fish become rare if BOD level is 4 to 5 ppm of water.
The measurement of BOD takes a number of days i.e., generally 5 days, hence another quantity generally measured is called chemical oxygen demand (COD). COD is an index of the waste (organic and inorganic) of water which can be oxidized by a strong oxidizing agent usually K2Cr2O7 in presence of H2SO4. The water sample is treated with a known amount of acidic potassium dichromate solution. The unreacted potassium dichromate is determined by back titration with a suitable reducing agent like Mohr’s salt. From the amount of K2Cr2O7 used, amount of oxygen consumed can be calculated from the following balanced chemical equation.
The amount of oxygen thus consumed by the pollutants is expressed in ppm and is called COD of the given sample of water. COD values do not necessarily match the BOD values. Textile wastes, paper mill wastes etc., with high cellulose content have COD values higher than BOD values as cellulose is not readily attacked by dissolved oxygen.
Biological amplification (magnification) is the process by which pesticides and other substances like heavy metals, non-biodegradable phenolic chemicals become more concentrated in each link of food chain. Increasing concentration of DDT in organisms of a food chain in higher trophic levels is an example of biological amplification.
iii) Inorganic minerals and chemical compounds:
These include various metals and metallic compounds released from human activities or from natural minerals. These pollutants enter the water bodies from municipal and industrial waste water. These pollutants include the following:
a) Toxic metals: Heavy metals such as cadmium, mercury, nickel, lead, arsenic are toxic to humans because our body can not excrete them.
Mercury: Inorganic mercury is converted into methyl mercury by anaerobic bacteria present in bottom muds. Methyl mercury can lead to mercury poisoning in living beings. Mercury poisoning produces a crippling and often fatal disease called Minamata disease. Mercury poisoning occurred in Minamata City, Japan in 1953 when more than 100 persons died or suffered serious nervous damage from eating fish taken from Minamata Bay.
Lead: Compounds of lead which enter into water are PbCl2, PbBr2, PbO, PbS, PbSO4. Lead in the form of Pb(OH)2 gets its entry into water from lead pipes. Excessive intake of lead by humans causes disruption of synthesis of haemoglobin. It also causes anaemia, kidney malfunctioning, nervous disorder and brain damage.
Cadmium: The main source of cadmium is industrial discharge and Ni – Cd batteries. Excessive dose causes kidney malfunctioning, anaemia, disorder of bone marrow etc.
Arsenic: As (III) compounds are highly toxic. The toxicity arises from the complexation of As (III) with the SH groups of enzymes which leads to inhibition of enzymatic action in human body. Higher doses of arsenic result in coagulation of proteins which causes cramps, paralysis and even death.
Minerals containing sulphur on coming in contact with air and water yield sulphuric acid which is carried into lakes and rivers by water draining from the mines.
In cold countries NaCl and CaCl2 are added in large amounts to melt snow from the roads in winter. These salts eventually run off the roads and contaminate lakes and rivers which seriously affect the aquatic life.
iv) Synthetic organic compounds:
These include pesticides, detergents and other industrial chemicals. Organic substances with serious impacts are the pesticides. Various industrial chemicals like polychlorinated biphenyls (PCBs) are highly stable and resistant to oxidation. They are used as fluids in transformers and capacitors. PCBs are suspected to be carcinogenic, and cause skin disorders.
Nowadays most of the detergents available are biodegradable. The bacteria responsible for degrading biodegradable detergent feed on it and grow rapidly while growing, they may use up all the oxygen dissolved in water. The lack of oxygen kills all other forms of aquatic life.
Eutrophication: Nitrogen and phosphorus containing fertilizers are used as plant nutrients. Fertilizers contain phosphates as additives. The addition of phosphates in water enhances algae growth. This profuse growth of algae reduces the oxygen concentration in water. This leads to anaerobic conditions that inhibit the growth of other living organisms in the water body. This process in which nutrients enriched water bodies support a dense plant population, which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity is known as Eutrophication.
v) Oil: Oil pollution results from the discharge of oil from oil tankers and offshore drilling rigs accidentally or intentionally. The most adverse effect of oil pollution is on the sea birds. The oil penetrates their feathers displacing the air which is normally trapped in the feathers and provides buoyancy and insulation against cold. The elimination of entrapped air makes the birds experience difficulty in flying.
Since oil is insoluble in water, it floats and spreads rapidly into a thin layer. This oil layer on the surface of water reduces the DO levels in water as oxygen transfer from atmosphere is prevented.
vi) Thermal pollutants: Thermal power plants, nuclear plants as well as industries use large quantities of water for cooling purposes. Used water is usually discharged directly into water bodies. This results in increase of temperature of water bodies. This brings down the DO level which is harmful for microorganisms. An increase in temperature also increases the toxicity of some chemical pollutants.
International standards for drinking water:
International standards have been laid down for the water to be used for drinking. The chemicals that are allowed to be present and the tolerable limit which must be satisfied are given below:
i) Fluoride: Deficiency of fluoride in drinking water is harmful to man and causes diseases such as tooth decay. Soluble fluoride is often added to drinking water to bring it concentration upto 1ppm or 1mg dm-3. The fluoride ions make the enamel on teeth much harder by converting hydorxyapatite [3 Ca3(PO4)2. Ca(OH2)] into much harder fluorapatite [3Ca3(PO4)2. CaF2].
However F– ion concentration above 2ppm causes brown mottling of teeth. Excess fluoride (over 10 ppm) causes harmful effect to bones and teeth.
In the district of Nalgonda, Guntur, and Prakasam in Andhra Pradesh, the water contains excess of fluorides. It reacts with calcium present in the body to form calcium fluorides.
By this reaction, the colour of teeth turns yellow. For the same reason bones become weak causing the disease fluorosis.
Fluorides can be detected from the reaction of fluorides with zirconium – Alizarin S. dye (The mixture of zirconium – alizarins S is coloured). Fluoride react with zirconium to form zirconium fluoride which is colourless. The colour of the dye becomes weak with the increase in the amount of fluorides.
Deluoridation techniques for drinking water:
The methods and principles of removing fluoride ions are collectively called defluoridation techniques. Because of very small size of fluoride ion, it is difficult to remove this ion from drinking water. The ion exchange methods are attempted for the removal of F– ion but not with much success due to smaller size. Some of the methods in use are as follow:
1) Ion exchange method:
Longtime back defluoron – 1 and defluoron – 2 synthetic resins were used to remove fluoride ions. The filters are packed with resins and water is sent through them. Fluoride ions are removed by ion exchange process. These methods are costly and require lot of skill to handle them. Hence, the use of this method is discontinued.
2) Activated carbon method:
In this method, the water containing high concentration of fluoride is passed through filters packed with activated carbon. Fluoride ions are adsorbed by the carbon and water is freed from fluoride ions. But, continuous use of filters, make activated carbon deactivated and filters lose their adsorption property. In such cases the used filters are first washed with 4% NaOH solution and then with 1% H3PO4 solution. After this treatment filters can be reused. In this method fluoride ion concentration can be reduced from 5 – 12 ppm to 1ppm.
3) Nalgonda Technique:
In 1973, the NEERI at Nagpur introduced this method. In this method bleaching powder, lime and alum are added in the same order and water is stored for some time. The fluoride ions get precipitated as complex calcium aluminium fluoride. This is filtered and pure water is used for drinking. This is a cheap method and it was first introduced in Nalgonda district hence this is called Nalgonda technique.
ii) Lead : The prescribed upper limit concentration of lead in drinking water is about 50 ppb. Lead can damage kidney liver, reproductive system etc.
iii) Sulphate : Excessive sulphate (> 500 ppm) in drinking water causes laxative effect. Otherwise at moderate levels it is harmless.
iv) Nitrate: The maximum limit in drinking water is 50ppm. Excess nitrate in drinking water can cause disease called methemoglobinemia (blue baby syndrome).
Exercise 1:
(i) Which one is the bio-degradable pollutant?
(A) Lead compounds
(B) Mercuric salts
(C) Pesticides
(D) Domestic wastes
(ii) The acid rain possesses
(A) sulphuric acid
(B) nitric acid
(C) sulphurous acid
(D) all of these
(iii) All except which cause pollution
(A) thermal power plant
(B) hydro-electric plant
(C) nuclear power plant
(D) automobiles
Exercise 2:
(i) Lung diseases are four times more in urban areas than rural areas. This is due to the presence of
(A) SO2
(B) CO2
(C) N2
(D) water-vapour
(ii) Which statement is not correct?
(A) CO is the main air pollutant
(B) All pollutants are not wastes
(C) Water is polluted by dissolved oxygen
(D) Lichens are pollution indication
(iii) Which of the following is produced by reaction of ultraviolet light?
(A) CO
(B) SO2
(C) O3
(D) NO2
SOIL POLLUTION:
The soil mainly comprises of inorganic minerals such as silica, quartz, silicates, aluminosilicates, oxides of iron and compounds of many other elements. Minerals in the soil bind to the cations such as Ca2+, Mg2+, K+, NH4+ which are also present in the soil. The soil also contains decayed organic matter to the extent of about 45% which is called humus.
Any factor which deteriorates the quality texture and mineral content of the soil and has a lethal effect on plant growth is called soil pollutant.
Sources of soil pollution:
1) Indiscriminate use of Fertilizers:
Fertilizers act as nutrients for plant but if nitrates and phosphate are present in excess, they have hazardous effects. The fertilizers added to soil get washed by rain into low land lakes. Due to excessive nutrition received from fertilizers, there is overgrowth of water weeds which results in choking of the entire ecosystem of the lakes.
2) Indiscriminate use of Pesticides:
Pesticides are basically synthetic toxic chemicals with ecological repercussions. These chemicals are used to kill or stop the growth of unwanted organisms. Pesticides are further classified into the following different categories.
a) Insecticides: These chemicals are used to kill the insects which destroy the crop. The most common insecticides are the chlorinated hydrocarbons like DDT (dichlorodipheny trichloroethane), BHC (benzene hexachloride), Aldrin, Dieldrin, etc.
During World War II, DDT was found to be of great use in the control of malaria and other insect born diseases. Therefore, after the war, DDT was put to use in agriculture to control the damages caused by insects, rodents, weeds and various crop diseases. However due to adverse effect, its use has been banned in India.
The most common pesticide used in India is BHC, it represents about 50% of total volume of pesticides used in India. Benzene hexachloride is incorrect name from a chemical standpoint, its correct name is Hexachlorocyclohexane. BHC is more toxic to insects than DDT and is used mainly in public heath programs.
Most of the organic toxins are water insoluble and non-biodegradable. These high persistent toxins are transferred from lower trophic level to higher trophic level through food chain. Over the time, the concentration of toxins in higher animals reach a level which causes serious metabolic and physiological disorders. A new series of less persistent or more biodegradable products called organo-phosphate and carbamates have been introduced in the market. But these chemicals are severe nerve toxins and hence more harmful to humans.
b) Herbicides: Herbicides are used to kill weeds. Earlier sodium chlorate (NaClO3) and sodium arsinite (Na3AsO3) were commonly used as herbicides but arsenic compounds being toxic to mammals are no longer preferred. Now organic compounds such as traiazine are used as herbicides to control weeds especially for the corn fields.
c) Fungicides : These chemicals are used to stop the growth of fungi and check plant diseases. Organo-mercury compounds are the most common fungicides. However their dissociation in the soil produces mercury which is highly toxic and proves fatal if it enters into grain.
Another fungicide is Bordeaux mixture, Bordeaux mixture is prepared by dissolving 40g of CuSO4 and 40g of Ca(OH)2 in 5 litres of water. The first pesticide to be used commercially was Bordeaux mixture.
3) Soil conditioners :
The soil conditioners are compounds of toxic metals such as Pb, As, Hg, Cd, Co etc. After the decomposition of these compounds, the resulting metals accumulate in the soil from where they enter the growing crops and from crops to animals. Soil conditioners are used for killing the insects and thus to protect the fertility of the soil.
4) Industrial waste:
About 50% of raw material used by most of the industries becomes waste product which is either thrown into water or dumped into the soil.
Biodegradable wastes are generated by cotton mills, food processing units, paper mills and textile factories.
Non biodegradable wastes are generated by thermal power plants which produce fly ash integrated iron and steel plants produce blast furnace slag and steel melting slag. Hazardous wastes such as inflammable composite explosives or highly reactive substances are produced by industries dealing in metals, chemicals, drugs, pharmaceuticals, dyes, pesticides etc.
The disposal of non-degradable industrial solid wastes, if not done by a proper and suitable method, may cause serious threat to the environment.
Recycling and recovery of material appear to be a reasonable solution for reducing soil pollution.
Now a days, fly ash and slag from the steel industry are utilized by the cement industry.
Fuel obtained from plastic waste has high octane rating. It contains no lead and is known as green fuel.
Technology has now been developed to produce electricity from the garbage. A pilot plant has been set up, where after removing ferrous metals, plastic, glass paper etc., from garbage, it is mixed with water. It is then cultured with bacterial species for producing methane, commonly known as biogas. The remaining product is used as manure and biogas is used to produce electricity.
GREEN CHEMISTRY:
The byproducts generated during a process, if not used gainfully, add to the environmental pollution. Such processes are not only environmental unfriendly but also cost ineffective. The waste generation and its disposal both are economically unsound.
Green chemistry is a way of thinking and is about utilizing the existing knowledge and principles of chemistry and other sciences to reduce the adverse impact on environment.
Extent of any reaction depends upon physical parameters like temperature pressure and catalyst. In a chemical reaction, if reactants are fully converted into useful environment friendly products by using an environment friendly medium, then there would be no chemical pollutants introduced in the environment.
By green chemistry we mean, the production of the chemicals of our daily needs using such reactions and chemical process which neither use toxic chemicals nor emit such chemicals into the atmosphere. Green chemistry does not employ toxic reagents or solvents and severe conditions but use mild and environmental friendly reagents and conditions such as sunlight, microwaves, sound waves and enzymes.
Some examples of green chemistry are given below:
i) Dry cleaning of clothes: Solvent used to dryclean clothes are usually chlorinated compounds such as tetrachloroethylene (Cl2C = CCl2). These compounds contaminate the ground water and are also suspected carcinogen. The process using chlorinated compound is now being replaced by a process, where liquefied CO2 with a suitable detergent is used. Replacement of halogenated solvent by liquid CO2 will result in less harm to ground water.
These days hydrogen peroxide is used for the purpose of bleaching clothes. It gives better results and makes use of lesser amount of water.
ii) Synthesis of chemicals : Ethanal is now commercially prepared by one step oxidation of ethane in the presence of ionic catalyst in aqueous medium with an yield of 90%
iii) Bleaching of paper: These days H2O2 is used for bleaching paper in presence of a catalyst which promotes the bleaching action of H2O2.
iv) Efficiency of Urea as fertilizer: Urea provides nitrogen to soil by decomposing to ammonia and CO2 in presence of an enzyme called urease. This enzyme is present in soil.
But the efficiency of urea as a fertilizer is reduced because 30% ammonia is lost by evaporation before it can be taken up by plant roots.
To overcome this loss, a formulation named Agrotrain has been developed which acts as urease inhibitor. This formulation reduces the rate of decomposition of urease, as a result, NH3 is released more slowly and efficiently.
ANSWERS TO EXERCISES
Exercise 1: (i) D (ii) D (iii) B
Exercise 2: (i) A (ii) C (iii) C
FORMULAE AND CONCEPT AT A GLANCE
1. The term environment means surroundings. It includes (i) atmosphere (ii) hydrosphere (iii) lithosphere and (iv) biosphere.
2. Troposphere is the lowest region of the atmosphere. This is the region of all living organisms including animals and plants.
3. Stratosphere is rich in ozone which absorbs harmful UV-radiations of the sun.
4. Any undesirable change in physical, chemical or biological characteristics in the air, water and land which is harmful to man directly or indirectly through his animals plants industrial units or raw materials is termed environmental pollution.
5. Pollutants are chemical, biological or physical agents that exert undesirable effects on living organisms. These may be (a) non-degradable or (b) bio-degradable.
6. Contaminant is a substance which does not exist in nature but is introduced by human activity into environment. It may or may not be harmful.
7. Primary pollutants are harmful substances which enter directly into the atmosphere as a result of natural and human activities.
8. The oxides of nitrogen and sulphur are responsible for acid rain. Acid rain results from the presence of two strong acids H2SO4 and HNO3. The pH of the acid rain can range between 5.6 to 3.5.
9. The name smog was proposed to describe the phenomenon which occurred due to condensation of some kind of fog on the carbon particles present in the smoke.
10. The atmosphere of the earth acts much like greenhouse. The gases present in atmosphere which are responsible for greenhouse effect are called greenhouse gases. The gases are carbon dioxide, water vapours, oxides of nitrogen, methane, ozone, chlorofluorocarbons, etc.
SOLVED PROBLEMS
Prob 1:
Assertion (A) : Greenhouse effect is responsible for global warming.
Reason (R) : For greenhouse effect, presence of green plants is essential.
(A) Assertion & Reason both are correct and Reason is the correct explanation of Assertion.
(B) Assertion & Reason both are correct but Reason is not the correct explanation of Assertion.
(C) Assertion is correct but Reason is incorrect.
(D) Assertion and Reason both are incorrect.
Sol: (C) Gases such as CO2, H2O vapours, oxides of nitrogen, CH4, O3, chlorofluorocarbons are responsible for green house effect. These gases are called green house gases.
Prob 2:
Assertion (A) : Earth is protected from IR radiations by ozone layer.
Reason (R) : Ozone layer is present in troposphere of the atmosphere.
(A) Assertion & Reason both are correct and Reason is the correct explanation of Assertion.
(B) Assertion & Reason both are correct but Reason is not the correct explanation of Assertion.
(C) Assertion is correct but Reason is incorrect.
(D) Assertion and Reason both are incorrect.
Sol: (D) Ozone layer is present in stratosphere which protects earth from harmful UV radiations.
Prob 3:
Assertion (A) : DDT and BHC are non-biodegradable soil pollutants.
Reason (R) : DDT and BHC are fertilizers.
(A) Assertion & Reason both are correct and Reason is the correct explanation of Assertion.
(B) Assertion & Reason both are correct but Reason is not the correct explanation of Assertion.
(C) Assertion is correct but Reason is incorrect.
(D) Assertion and Reason both are incorrect.
Sol: (C) DDT and BHC are insecticides.
Prob 4: Pollution can be controlled if:
(A) all automobiles must be fitted with exhaust system’s catalytic converters.
(B) use of fossil fuels be minimized and non-conventional energy sources should be developed.
(C) emphasis on green chemistry is given
(D) population is stabilized.
Sol: All are correct.
Prob 5: The gases which is not responsible for photochemical smog
(A) oxides of nitrogen
(B) hydrocarbons
(C) carbon monoxide
(D) inert gases
Sol: (D) Inert gases are not responsible for photochemical smog.
Prob 6: Which of the following is not an air pollutant?
(A) CO
(B) SO2
(C) NO
(D) N2
Sol: (D) N2 is not an air pollutant.
Prob 7: CFCl3 is responsible for the decomposition of ozone to form oxygen. Which of the following reacts with ozone to form oxygen?
(A) Cl2
(B) Cl–
(C) F–
(D) Cl
Sol: (D)
Prob 8: Lead is considered as
(A) water pollutant
(B) soil pollutant
(C) air pollutant
(D) radioactive pollutant
Sol: (C) Lead salts come into atmosphere through exhausts gases from automobiles when is used as antiknocking agent along with C2H4Cl2 and C2H4Br2 in gasoline.
Prob 9: One of the metals used as a catalyst in automobiles catalytic converter is
(A) palladium
(B) copper
(C) radium
(D) iron
Sol: (A) In automobiles the three way catalyst consists of a mixture of platinum, palladium and rhodium deposited on a high surface area of ceramic.
Prob 10: Measurement of the rate of O2 consumption in unit volume of water over a period of time is done to find out.
(A) biogas generation
(B) biological oxygen demand
(C) biosynthetic pathways
(D) fermentation
Sol: (B) Biochemical oxygen demand is a measure of the oxygen utilized by microorganisms during oxidation of organic materials.








