THE BORON FAMILY (Group 13)
Group IIIA (of Mendeleev’s modern table) or 13 (of long form of periodic table) consists of five elements – boron, aluminium, gallium, indium and thallium. They are included in p–block elements since their last (differentiating) electron enter the p–subshell of outermost (nth) shell. They form the first group of p–block elements.
Abundance: Boron occurs in traces in the combined form in the nature, but is well familiar due to its presence in borax and tourmaline crystals. Aluminium is the third most abundant element and first most abundant metal in the earth’s crust. [Oxygen being the first and silicon, the second]. It occurs in the combined state in nature in the form of bauxite, cryolite and feldspar etc. Gallium (twice as abundant as boron), indium and thallium are also trace elements and they occur in the form of sulphides along with zinc and lead ores.
Properties of group 13 elements:
Atomic properties:
1. Electronic Configuration: The general outer shell configuration is ns2np1.
The general configuration is ns2 np1 i.e, they have three electrons in their valence shell (n = no. of period) Table – I: Electronic Configuration of IIIA Group Elements
| Period | Element | Symbol | At.No. | Electronic Configuration |
| 2nd | Boron | B | 5 | [He] 2s22p1 |
| 3rd | Aluminium | Al | 13 | [Ne] 3s23p1 |
| 4th | Gallium | Ga | 31 | [Ar] 3d104s24p1 |
| 5th | Indium | In | 49 | [Kr] 4d105s25p1 |
| 6th | Thallium | Tl | 81 | [Xe] 4f145d106s26p1 |
GEC = [Noble gas] ns2np1
Because of similar configuration, these elements show similar properties. The difference, whenever, is due to the electrons of penultimate shell as observed in their chemical reactivity?
Table – 2: No. of electrons in penultimate and ultimate shells. (The basis of difference in properties)
| Element | Penultimate shell | Ultimate shell |
|
Boron Aluminium Gallium Indium Thallium |
2 8 18 18 18 |
3 3 3 3 3 |
So, boron is quite different from aluminium, which itself has different properties than the rest elements. Only boron is non-metal, in the group due to its smaller size and high ionization energy, the rest are metals. Thallium, the last member is regarded ‘duckbill platypus’ because of its similarities with other elements (i.e. with IA Group – the alkali metals).
2. Atomic and Ionic radii: The radii are less than s–block elements but greater than other p-block elements. Atomic/ionic radii increase from boron to thallium.
| Element | B | Al | Ga | In | Tl |
| Atomic radius () | 0.88* | 1.43 | 1.22* | 1.67 | 1.70 |
| Ionic radius M+ () | – | – | 1.20 | 1.40 | 1.50 |
| Ionic radius M3+ () | 0.27 | 0.53 | 0.62 | 0.80 | 0.88 |
* radii = half of the closest approach The abnormal decrease in atomic radii of Ga can be accounted to
a) unusual crystal structure
b) the electrons have entered the 3d subshell in Ga, which produce poor screening effect than s or p electrons: the result, more the effective nuclear charge, hence greater the pull, smaller the size.
[order of screening or shielding s > p > d > f ]
The sharp increase in the size of Al w.r.t. boron is due to overall more screening of in the penultimate shell of Al, and hence less the attraction of nucleus – larger the size, in comparison to that of in B.
3. Density:
On moving down from boron to thallium density increases because density depends directly on mass and inversely on volume (radii). The effect of mass is greater than that of volume.
| Elements | B | Al | Ga | In | Tl |
| Density | 2.4 | 2.7 | 5.9 | 7.3 | 11.9 |
4. mp, bp & Crystal Structure:
| Elements | B | Al | Ga | In | Tl |
| 2180 | 660 | 30 | 157 | 303 | |
| 3650 | 2467 | 2403 | 2080 | 1457 |
The melting points should decrease on moving from boron to thallium as predicted by the trends. Though there is a normal decrease in mp from boron to aluminium, mp of gallium is exceptionally very low, followed by an increase for indium and thallium.
High mp of boron is due to its giant covalent polymeric structure. Four different allotropic forms of boron have been found. The insufficient number of in the last shell makes it to form a variety of complex allotropic forms in which boron atom tries to achieve the octet. Boron forms icosahedral (20 faces, icosa = 20) units with boron atoms at all 12 corners. It is because other elements can form metallic bond to get stable configuration, but boron can’t do so due to its smaller size and high ionization energy.
Al, In and Tl have close packed metal structure Ga has unusual structure having molecules, which accounts for its very low mp. The increase in mp from In to Tl is explained on the basis of the extent of metallic bonds.
Tl has only slightly greater radius than In, but at the same time it has higher nuclear charge, which leads to formation of a stronger metallic bond.
The boiling points decrease on moving from B to Tl as expected.
The boiling point of gallium is as expected but mp is abruptly low. It is due to unusual crystal structure in solid state which does not exist in liquid.
Because of ‘long liquid range’ (from to ) Ga is used in high temperature thermometry.
Another unusual property of gallium is that when it forms solid, it expands (like water ice; Ge & Bi also share this property with Ga)
5. Ionization Energy:
The outer shell configuration can lose easily due to less nuclear attraction, hence the value of is comparatively less than other p-block elements.
Comparision with s-block elements shows that IE1 of boron and aluminium are greater than Be and Mg but that of Ga, In and Tl are less than their corresponding group IIA elements. The reason is almost the same shielding effect upto Al (two or eight penultimate electrons respectively) which becomes different for following elements due to introduction of d and f penultimate sub-shells in IIIA group elements while for IIA there are p-subshells as penultimate. Removal of second electron (from configuration) is not easier due to more stable configuration and more ever, positively charged cation , which attracts the electron to be removed. Hence, the order of first, second and third ionization energy follows the order:
| Element | ||||
| B | 801 | 2427 | 3659 | 6887 |
| Al | 577 | 1816 | 2744 | 5137 |
| Ga | 579 | 1979 | 2962 | 5520 |
| In | 558 | 1820 | 2704 | 5082 |
| Tl | 589 | 1971 | 2877 | 5437 |
On moving down the group i.e. from boron to thallium the variation is not smooth; actually, it varies in an oscillating manner. There is a decrease in IE from B to Al as usual, descending down increases for Ga, then decreased for In followed by an increase for Tl. This abnormal trend accounts for the poor shielding of 10d electron in Ga, in In there is the same number of but increased atomic size reduces IE. For Tl the much poor shielding of f makes IE to increase.
The order of IE1 :
The boiling point of Ga is as expected but mp is abruptly low. It is due to the unusual crystal structure in solid stage, which doesn’t exist in liquid.
6. Oxidation state & Type of Bonding:
|
Oxidation State |
|
|
Common |
Less Common |
|
III |
|
|
III |
I* |
|
III |
I |
|
III |
I |
|
I |
(III) |
I* doubtful/unstable ( ) less stable
(III) Oxidation state:
All elements exhibit III ox.st. due to ns2 np1 configuration. Tl shows generally I ox.st. due to inert pair effect involving only p electrons of valence shell, the s electron pair remaining inert. The effect is more prominent in the 6th period.
III oxidation is more stable on moving up the group while the I, on moving down i.e.,
Stability:
(I) Oxidation State (INERT PAIR EFFECT):
Because there are 3electron in valence shell, all the elements should exhibit III state, but while going down the group there is an increasing tendency to form univalent compounds. In Ga and In (I) ox.st. is less stable than (III), while in case of Tl the opposite is true i.e., thallous. (I) compounds are more stable than thallic (III).
Monovalency is found to be exist due to presence of single unpaired electrons (p1) which participates in the reaction; the other two electrons (s2) remain paired and chemically inert.
These s2 electron can be unpaired if the energy required to unpair them is greater than the energy evolved while forming a bond.
The mean bond energy for the chlorides is:
| Chloride | GaCl3 | InCl3 | TlCl3 |
| Bond energy* (KJ mol-1) | 242 | 206 | 153 |
Since the energy for Tl is very less, the s electron will not get unpaired, hence remain chemically inert.
*Bond energy: The amount of energy released during the formation of a bond or required to break a bond.
(II) Oxidation State:
Gallium seems to be divalent in some of its compounds like GaCl2, but in fact it contains Ga(I) as well as Ga (III) having the actual structure
Illustration 1: +1 oxidation state is stable only with Tl and not with Al or Ga, why?
Solution: Moving down group the lower oxidation states are more stable for (p-block). This is often observed in 5th and 6th period. The reason is inert pair effect, i.e., the unability of ns2 electrons to participate in a reaction (becoming inert) while only np electron (s) take part.
Type of Bonding:
As per Fajan’s rule,
A compound will have covalence if
a) cations have smaller size, and higher charge (i.e. high charge density)
b) sum of the all ionization energies is large
c) electronegativity values of the atoms are higher
These parameters are well in accordance with boron, which always gives covalent compounds. Many compounds of other elements like AlCl3 and GaCl3 are covalent in anhydrous state; however, in solution they give Al3+ and Ga3+ ions. This change from covalent to ionic is brought about by the hydration energy, the energy evolved during formation of hydrates, which exceeds the ionization energy in this case.
The hydrated metal ions hold 6H2O molecules with strong M – O bonds. This decreases the O – H bond strength, so that H+ (proton) are given into the solution, making it acidic.
Hydrolysis of AlCl3:
The compounds of univalent (I) ions are ionic due to comparatively larger size and low IE than trivalent ions.
7. Electropositive Nature:
The electropositive or metallic nature increase from B to Al then decreases upto Tl, as evidenced by their standard electrode potentials.
| Element | B | Al | Ga | In | Tl | |
| Electrode Potential(volts) | M+/M | +0.55 | -0.79* | -0.18 | -0.34 | |
| M+3/M | (-0.87*) | -1.66 | -0.56 | -0.34 | +1.26 |
* in acidic medium.
Less (more negative) the value of reduction potential, more easily the electrons are lost by it.
From ,
= the free energy change
n = the number of electrons transferred
F = the Faraday constant
G0 standard free energy is the energy available with a substance (or atom) that can be converted into work spontaneously.
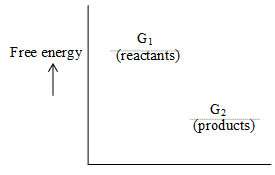
For rection G1 G2
= G2 – G1
If G1 > G2 , = -ve , The process will be spontaneous (no extra energy is required)
If G1 < G2 , = +ve , The process will be nonspontaneous (energy is required for the process to complete)
If G1 = G2 , = 0, The process will be in equilibrium (reactants can freely convert into products and vice versa)
for the reaction,
is +ve; the reaction is not feasible i.e. will not proceed spontaneously.
But for the change is negative;
The reaction is feasible i.e., Al easily loses 3electron.
On moving down the group becomes less negative (decreases of metallic nature) and at Tl it becomes +ve for trivalent ions but –ve for monovalent ions. i.e.,
Hence is less stable than . Electropositive nature decreases from Al to Tl.
Physical properties:
Boron is non-metallic in nature. It is extremely hard and black coloured solid. It exists in many allotropic forms. Due to very strong crystalline lattice, boron has unusually high melting point. Rest of the members are soft metals with low melting point and high electrical conductivity. It is worthwhile to note that gallium with unusually low melting point (303K), could exist in liquid state during summer. Its high boiling point (2676K) makes it a useful material for measuring high temperatures. Density of the elements increases down the group from boron to thallium.
Chemical properties:
1. Action of air:
Pure crystalline boron is very unreactive, but finely divided boron burns in air forming oxide.
Al should react thermodynamically with air but it is actually stable due to formation of a very thin layer of which protects it from further attack. The layer can be removed by amalgamation (formation of an alloy with mercury) then it attacks even cold water, forming and .
The reaction of with Al is highly exothermic, hence used in Aluminothermite process for the source of heat for welding the in situ iron objects, which are too heavier to be moved from their location e.g., rails.
This heat is enough to produce fire. A thermite reaction can be started if a missile hits a ship, made from aluminium alloy. Another use of thermite reaction is extraction of other metals from their oxides because of strong affinity of Al towards oxygen; by the result and metals are obtained.
All other metals of IIIA group are not attacked by at normal temperature but at higher temperature. Ga is only superficially oxidized. Tl gives like Ist group elements, hence stored under oil or in vaseline.
2. Action of water:
Boron does not react with water or steam. However, red hot boron does reacts with steam to form boron trioxide.
Aluminium should react with cold water but due to formation of a layer of it remains passive and reacts only with boiling water. Aluminium decomposes cold water if its coating of is removed.
Gallium and Indium are attached only under oxidizing conditions. Thallium is highly reactive and gives TlOH even with moist air.
3. Action of Nitrogen:
Boron burns in nitrogen to form boron nitride (BN), a slippery white solid with a graphite like layer structure.
Al reacts with at higher temperature to give aluminium nitride (AlN).
Other elements do not react.
4. Reaction with non-metals:
Boron and aluminium form carbides when heated.
All the elements react with halogens to form trihalides at high temperature.
M = B, Al, Ga, In ; X = F, Cl, Br, I
However, thallium reacts with iodine to form thallous iodide, which like KI forms polyhalide with excess of iodine.
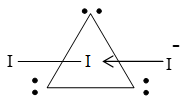
has linear structure involving hybridization in which the equatorial positions are occupied by the three lone pairs.
5. Reaction of metals:
Boron reacts with all metals other than 1st group and forms borides, which are often non stoichiometric.
Other elements of IIIA group being metal, don’t form this type of compounds, instead they form alloys with other metals.
Action of acids:
Non–oxidizing acids like HCl and dilute donot affect boron; the other elements form corresponding trivalent salts.
Oxidizing acids like and conc. attack all the elements.
Al and Ga become passive in conc. due to the formation of a protective layer of their oxides.
6. Action of alkalies:
Boron, aluminium and gallium exhibit amphoteric nature, as they also react with alkalis to form borates, aluminates and gallates respectively.
7. Action of :
Boron reacts with at very higher temperature to form boron nitride (BN).
All other metals form amides.
M = Al, Ga, In, Tl
Nature of the compounds of group 13:
1. Oxides and hydroxides:
All the members of boron family form oxides of the type and hydroxides of respectively.
The nature of their acidic/basic strength:
|
Oxide Hydroxide Nature |
Acidic Amphoteric Basic |
Tl also forms , which is more stable than and behaves like alkali metal oxides.
Acidic nature of can be explained on the basis of small size of boron and its greater nuclear charge which attracts electron form water molecules (H – O – H). The weaking of O – H bond results into easy removal of H+ making the solution acidic.
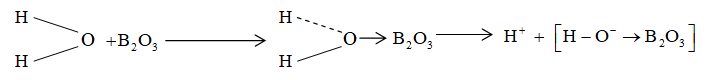
Although is acidic in nature, with strong acids it behaves like a base and forms salts
combines with metallic oxides and gives coloured basic metaborates which are the characteristics of metal oxides hence used in the qualitative analysis under borax bead test.
| Colour of Bead | Green | Brown Yellow | Blue | Violet |
| Metal present | Cu or Cr | Fe | CO | Mn or Ni |
Boric acid is soluble in water; the rest hydroxides are insoluble and form a gelatinous precipitate.
being amphoteric dissolve in acids as well as alkalis.
exists in a crystalline form called corundum, which is very hard and used as abrasive.
Hydroxides also have similar trends. Acidic nature of boric acid is due to coordination of water molecule with it and consequently liberation of a proton from weakened O – H bond.
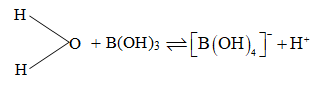
(Orthoboric* acid) is soluble in water and behaves as a weak mono basic acid.
* The term used with oxyacids:
ortho–When the maximum number of – OH group are present i.e., the degree of hydration or hydroxylation is maximum.
meta–When a molecule of an acid dehydrates, eliminating a molecule of the resulting acid is called meta.
pyro–When a water molecule eliminates from dehydration of two or more molecules of an acid, the resulting acid is pyro.
e.g.,
It is important to note that it does not donate proton itself rather accepts , hence is a Lewis acid and should be written as .
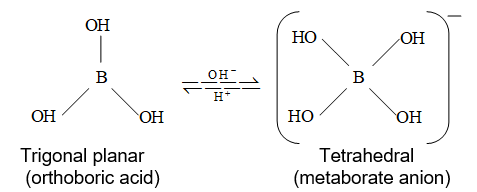
Since reacts partially with water to form metaborate, it behaves as weak acid. The accurate titration of with a NaOH is not possible because no sharp end point is obtained due to presence of free metaborate ions, which being in equilibrium yield back H+ by the hydrolysis.
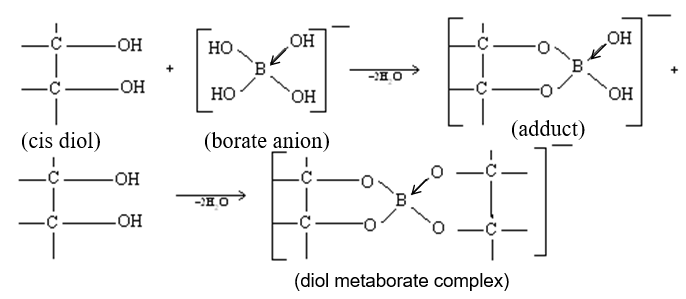
If polyhydroxy organic compounds (like glycerol, mannitol etc.) are added to the titration mixture, behaves like a strong acid, and thereby gives a sharp end point to be easily detected using phenolphthalein.
The added compound must be a cis–diol to increase the acidic properties of . Cis–diols form a stable complex with and thus removing it form equilibrium mixture to enhance the equilibrium reaction towards the right hand side.
In boric acid, boron is hybridized with planar geometry. In solid state units are attached with hydrogen bond which forms a two dimensional sheets of hexamer i.e., . With the increase of size of central atom in , the attraction of O–electrons of O–H group to the metal atom becomes lesser and hence their acidic nature decreases. and are amphoteric in the consequence, followed by basic and .
2. Halides:
a) Trihalides:
All elements give all trihalides except. Boron halides are covalent and Lewis acids due to incomplete octet.
| Halide | ||||
| Physical State | Gas | liquid | liquid(?) | solid |
| Hydrolysis |
Nohydrolysis (addition product) |
|||
| Order of Lewis acid strength | 4 | 3 | 2 | 1 |
The reason behind unability of the to hydrolyse is stronger B–F bond in comparison to B–O bond formed after hydrolysis while all the rest bond are weaker.
is a lewis acid and doesn’t undergo hydrolysis, hence used as a catalyst in organic reactions. (e.g., Friedel – Crafts reaction)
The Anomalous order of acidic strength of Boron halides is explained on the relative ability of back bonding between boron and halogen atoms; as evidence by following data:
B – F bond length [Covalent radii ]
[Expected B-F Bond length = ]
B – F bond energy 646 kJ mol -1 (higher than that of a single B – F bond)
The shortness and strength of the bond is interpreted in terms of a back bonding, leading to somewhat double bond character.
molecular is planar triangular with bond angle involving hybridization. The valence shell has six , hence it is electron deficient.
The unhybridized 2pz – orbital of the boron atom lies perpendicular to the plane of molecule. The three fluorine atoms have pz – orbitals each with pairs of in them.
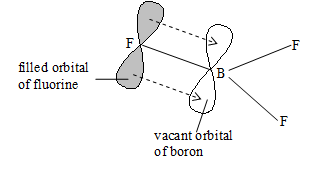
The pz orbital of boron receive B an pair form a F atom and forms a bond or dative bond, which produces double bond character in B–F bond. However, this backbonding may take place between boron and any of the fluorine atoms. The valence bond theory regards to be a resonance hybrid of the three structures:
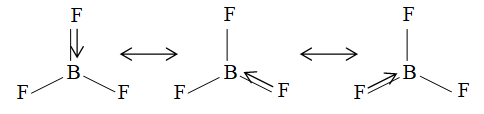
The modern explanation is the delocalization of the bond formed between 4-p orbitals i.e., one form boron and three from fluorine.
has acidic (Lewis acid) properties because of lack of octet. Now, after back bonding the octet is achieved, hence the tendency to accept pair decreases – making it less acidic.
Tendency of back bonding is maximum with F, as we move to I it decrease because of increasing size of halogen atoms (overlapping between (boron) and (iodine) becomes least possible).
Hence remains more acidic, its boron atom being still able to receive electron pair from donors.
The lewis acid character of other elements decrease down the group:
The empty 2pz orbital of boron can also accept a lone pair from other species. e.g., etc. Thereby forming tetrahedral complex with coordination number 4.
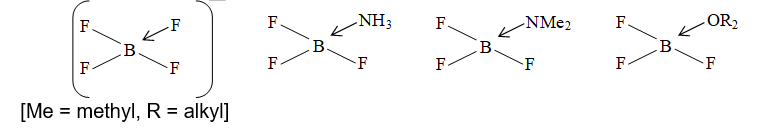
No further bonds can now be formed because no empty orbitals are present. The bond length of B – F bond gets changed with the change of geometry (more the p character, greater the bond length):
|
B – F bond lengths |
|||
|
Hybridization |
Boron can’t extend its coordination no. beyond four because of non-availability of d – orbitals while others do e.g., and .
The trifluorides of Al, Ga, In and Tl are ionic with high mp the other trihalides are largely covalent in anhydrous state. Of these exist as dimmers. The dimmers are stable in non-polar solvents like benzene. When these halide are dissolved in water, they produce and ions. At low temperatures exists as a close packed lattice of with occupying the octahedral holes ( ions are much smaller in comparison to ). On heating is formed with sudden increase of volume due to transition from ionic to covalent structure.
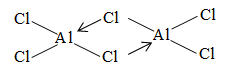
The dimeric structure of exist in vapour phase below , at higher temperature it dissociates into molecules. is used as the ‘catalyst’ in a variety of Friedel Crafts type reactions because of its Lewis acid character and ability to form intermediate complexes with variety of molecules.
b) Dihalides:
Boron forms dihalide with formula . There is a free rotation around B – B bond so that in gaseous and liquid state the molecule adopts a non–eclipsed conformation. However it is planar in solid state because of ease of packing.

Ga & In also form ‘dihalide’
They are more accurately &
c) Monohalides:
Boron forms several stable monohalides which are polymeric i.e.,
These compound are crystalline solid having a closed case structure wherein multicentre bonds cover all the B atoms.
Al, Ga, In all form monohalides only at higher temperature and in gas phase.
These compounds are covalent in nature but very unstable only thallium form stable monohalides of which TlF is ionic.
3. Hydrides:
The IIIA group elements donot directly react with , however, a number of hydrides are known.
| Element | B | Al | Ga | In | Tl |
| General Formulae of Hydride | & | – | |||
| Name of hydride | boranes | allanes | (di) gallanes | (poly) indanes | – |
| Stability | ✓ | ✓ | Unstable | Unstable | does not exist |
| Anionic hydride complex* |
borohydride |
aluminium |
gallium hydride |
– | – |
* Powerful reducing agents Boron forms a number of stable hydrides (boranes) of two series:
| Nido-boranes (nido = nest) | Arachno borane (arachno = web) | ||
| Formula | Name | Formula | Name |
| diborane | |||
| pentaborane – 9 | tetraborane | ||
| hexaborane – 10 | pentaborane – 11 | ||
| octaborane – 12 | hexaborane – 12 | ||
| decaborane | octaborane – 14 | ||
* Afred Stock (1912 – 36) pioneered the boron chemistry and suggested various methods of preparation.
They are electron deficient compounds. The expected boron hydride is however not reported. Because the hydrogen, with which boron is bonded doesnot have free electrons available for back bonding so there is high scarcity or deficiency of an electron pair. , as it would have existed, because of lack of complete octet dimerises to form .
Electron deficient compounds:
The compounds having less than eight electrons in their valence shell are called electron deficient. They are very reactive and tend to stabilize by accepting two or more electrons and making their octet complete e.g. etc.
Anomalous behaviour of boron:
Like Li & Be in s–block, B, the first member of IIIA or 13 group exhibits some properties, which are not in conformity with the rest elements of the group. It is due to high nuclear charge/size ratio (i.e., high charge density), high eletronegativity and non-availability of d-orbitals.
In fact the charge/ratio of B becomes almost comparable to Si that is why it resembles Si in its properties.
Properties of Boron that are different from IIIA Group:
- Boron is bad conductor of electricity; others are good conductor.
- Oxides and hydroxides of boron are acidic; that of others are not.
- Halides exist as dimer and are Lewis acids.
- Hydrides are quite stable.
- HCl has no action on boron; others form chlorides and liberate hydrogen.
- Coordination no. of boron cannot be greater than four, while other members exhibit C.N.6.
- Boron doesn’t decompose steam while other do and form hydroxide and.
- Boron combines with metals to form borides e.g.,; others form alloy.
- nitric acid oxidizes boron to boric acid.
Exercise 1:
(i) Al and Ga have nearly the same covalent radii because of
(A) greater shielding effect of s electrons of Ga atoms
(B) poor shielding effect of s electrons of Ga atoms
(C) poor shielding effect of d electrons of Ga atoms
(D) greater shielding effect of d electrons of Ga atoms
(ii) The liquefied metal expanding on solidification is
(A) Al (B) Ga (C) Zn (D) Cu
(iii) Which of the following is acidic in nature?
(A) Be(OH)2 (B) Mg(OH)2 (C) Al(OH)3 (D) B(OH)3
(iv) Which one of the following is a correct statement?
(A) The hydroxide of aluminium is more acidic than that of boron
(B) The hydroxide of boron is basic while that of aluminium is amphoteric
(C) The hydroxide of boron is acidic while that of aluminium is amphoteric
(D) The hydroxides of both boron and aluminium are amphoteric
Answers
(i) C
(ii) B
(iii) D
(iv) C
Properties of Boron similar to Silicon (diagonal relationship):
- Both, B and Si are typical non-metal, having high mp, bp and approx. same density.
- Both of them donot form cation and give only covalent compounds.
- Both exist in amorphous and crystalline forms and have allotropes.
- Both of them give a number of volatile hydrides, which spontaneously ignite in presence of air.
- Both the non-metals and their oxides are readily soluble in alkalies and form borates and silicates.
- and SiC both the carbides are very hard and abrasive.
- and both are liquid, fume in moist air and readily hydrolyse by water.
Compounds of boron:
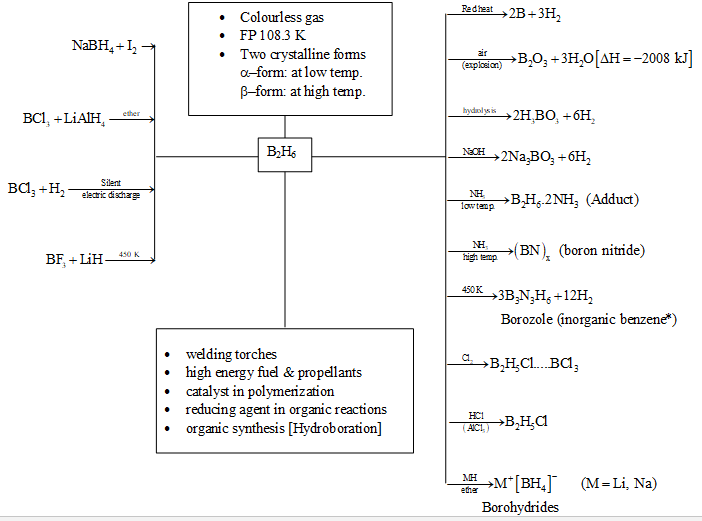
1. Diborane:
Preparation:
1) Stock’s method: Action of dil. acids on magnesium boride give a mixture of volatile boranes (mainly ), which on thermal decomposition give diborane.
2) Reduction of by in presence of aluminium powder.
3) Action of ionic hydrides on [Industrial production]
Ionic hydrides like NaH and on treating with boron trichlorides give diborane.
4) Action of Lithium aluminium hydride on
5) Action of Borohydrides (e.g. ) on or
[Lab method]
[Diglyme : (dimethyl diglycol)
6) By electric discharge of or at low pressure
Structure of diborane:
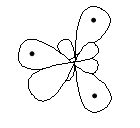
The two boron atoms and six hydrogen atoms if combine to make six or seven (one B–B bond) bond pairs, doesn’t serve the purpose of making the octet complete. So, a new type of bond – a 3 centered – bond called banana bond comes into the picture.
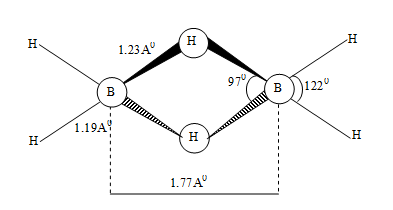
Diethey (1921) proposed the above structure for diborane wherein the terminal hydrogen and boron atoms lie in a plane (let, in the plane of paper), the remaining two i.e., bridging hydrogens lie above and below in a plane perpendicular to the rest molecule.
Hybridization:
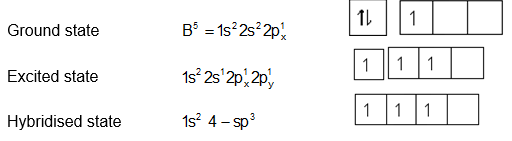
sp3 hybridized boron atom with one empty hybrid orbital.
Formation of a Banana bond:
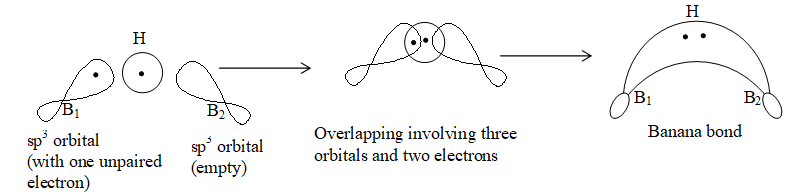
Formation of diborane with banana bond:
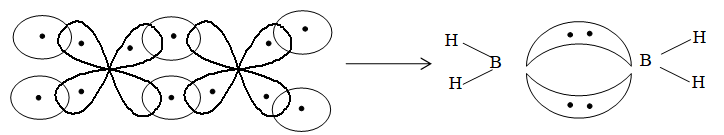
There are two types of H–atoms in diborane – 2 bridging and 4 terminal. Of these, the four hydrogen can be replaced easily by methyl group. However, if the bridging hydrogen is attacked, the molecule ruptures.
Illustration 2: What is Moissan boron?
Solution: 95 – 98% pure boron is obtained on reducing B2O3 with Mg or Na metal at high temperature. It is known as Moissan boron. This is amorphous form of boron.
Properties:
Physical:
Lower boranes are colourless gases and higher are volatile liquids or solids. Diborane has sweet odour and is highly toxic. It is highly reactive, hence handled with utmost care in vaccum frame and mostly prepared in situ, wherever required. It catches fire spontaneously in air and explodes with oxygen. The heat of combustion is very high (i.e.,)
It exists in two allotropic forms : – form at low temperature and another – form at higher temperature.
Chemical:
1) Action of heat:
At red heat the diborane decomposes to boron and .
2) Action of air (dioxygen):
Diborane undergo spontaneous combustion in air because of its high affinity towards oxygen.
3) Hydrolysis:
Lower boranes hydrolyse more easily in presence of water only or aq. sol. of alkali and liberate .
4) Action of :
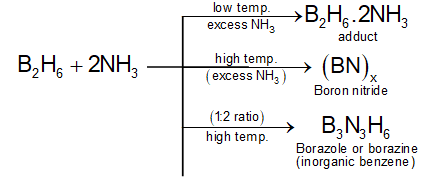
Different products are formed under different conditions of temperature and ratio of reactants.
5) Action of halogens: Chlorine replaces all hydrogens and splits the molecule into two halves with a violent reaction, while substitution with bromine is slower.
(vigorous reaction)
6) Action of HCl: Reaction of HCl with diborane forms bromodiborane in presence of (catalyst).
7) Action of metal hydrides: Ionic hydrides form complex borohydrides with anion units. These borohydrides are good reducing agents.
8) Action of metal alkyls: Alkylation of boron takes place and metal borohydrides are obtained.
9) Action of : Li and Al both are converted into their borohydrides.
10) Formation of addition compounds: Since is electron deficient compound, even the banana bond structure is not very stable due to strain in the molecule. Hence, it wherever possible accepts electron and give addition compound by completing its octet.
(i) With Na or K amalgam:
(i) With Lewis base:
(iii) With CO:
11) Methylation (Action of ): At ordinary temperature four methyl derivatives are formed by the replacement of four terminal hydrogens of borane, called mono, di, tri and tetra methyl diboranes. The two bridging hydrogen (having banana bond) remain undisturbed. The reaction proves that there are two types of B–H bond in diborane.
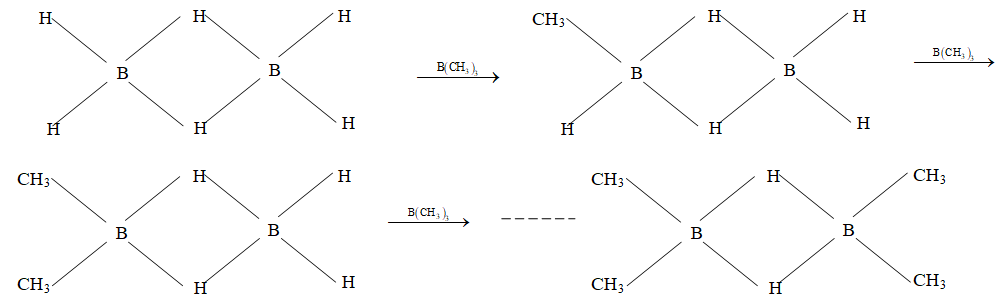
12) Hydroboration reaction: reacts with alkenes at room temperature to give organoboranes. These organoboranes on oxidation with give 10 alcohols – in a way which is overall anti – Markownikov. The reaction is used for obtaining primary alcohols from alkenes.
Exercise 2:
(i) Which of the following compounds is formed when boron trichloride is treated with water?
(A) H3BO3 + HCl (B) B2H6 + HCl (C) B2O3 + HCl (D) None of these
(ii) Boric acid is prepared from borax by the action of
(A) hydrochloric acid
(B) sodium hydroxide
(C) carbon dioxide
(D) sodium carbonate
(iii) Borax bead test is responded by
(A) divalent metals
(B) heavy metals
(C) light metals
(D) metals which form coloured metaborates
Answers
(i) A
(ii) A
(iii) D
Uses of Boron and its compounds:
Boron is used for the following:
- For making boron steel or boron carbide control rods for nuclear reactors. This is because boron has a very high cross-section for capturing neutrons.
- Boron carbide is used as an abrasive.
- For making impact-resistance steel. Boron increases the hardenability of steel. Hardenability means the depth to which the steel gets hardened.
- Borax, , Orthoboric acid and boron sesquioxide are used for making fibre glass for insulation and textiles, and for making borosilicate glass.
- Perborates are used in detergents. Peroxoborates act as brightners.
- Borax is also used in making flame-retardant fabrics and wood, as a flux in soldering, making enamel and in leather tanning.
DIBORANE AT A GLANCE
2. Borax:
Borax occurs naturally and is also called tincal or “suhaga”. Tincal contains about 45% borax. Pure borax is purified by dissolving the natural tincal in hot water and filtering off the insoluble impurities followed by crystallizing the solution left.
Borax exists in three forms:
Prismatic borax :
It contains actually units, hence the actual formula of prismatic borax is . It is less soluble in cold water but highly soluble in hot water. This form of borax is obtained by crystallizing the borax solution at ordinary temperature.
(i) Octahedral borax :
It is obtained by crystalling the solution at . It is called jeweller’s borax.
(ii) Borax glass :
It is obtained by heating the decahydrate above its mp. It is colourless glassy mass and is unstable in moist air; it absorbs moisture and slowly changes into decahydrate. If dehydrate is heated, it losses water and swells up initially, followed by conversion into transparent liquid, which solidifies into glass like material called borax bead.
Properties:
- Aqueous solution of borax is alkaline due to hydrolysis.
- Heating borax with conc. and ethyl alcohol produces triethyl borate which is volatile and burns with green flame.
Uses:
- Making optical and hard glass
- Qualitative analysis in borax bead test of transition metals.
3. Orthoboric acid :
Preparation:
1) By hydrolysis of etc.
2) By decomposition of boron compounds with superheated steam
3) By acidification of aqueous borax solution
Properties:
Physical:
- It is soft peral–white with needle like crystals which have a soapy touch.
- Boric acid is sparingly soluble in cold water but highly in hot. [∵ dissolution is endothermic]
- In solid, it exists in the form of a layer structure of units – giving a hexagonal hydrogen bonded ring structure.
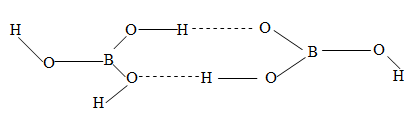
These layers are held with each other by van der Waal’s forces. The slippery touch of the solid boric acid is due to easy sliding of these layers over one another.
- It’s a weak monobasic acid. It doesn’t donate proton, but accepts from water and removes from it, hence is a Lewis acid.
K is the ratio of concentration of products to the reactants (called ionization or dissociation constant) . Since K is far-far less than unity, the concentration of reactants will be much greater than that of products (more the H+ ion, stronger the acid). Obviously, H+ are produced in less amount. Hence, the acid will be a weak monobasic acid.
Chemical:
Action of Alkali:
Aq. KOH, NaOH etc., react with to produce various polymeric metaborates like
etc
Action of Heat:
It loses water molecule to give metaboric acid, tetraboric acid on further heating followed by boron trioxide at red hot.
Action of Sodium Hydroperoxide (NaOOH):
Action of and Conc. or HF:
, if brought to Bunsen burner, a green flame is produced due to volatile.
Illustration 3:
How borax reacts with hot, conc, HCl?
Solution:
This is the preparation of boric acid from borax, the source of boron compounds to us.
5H2O + NaB4O7 + 2HCl 2NaCl + 4H3BO3 (Orthoboric acid).
Illustration 4:
Give the reactions of B with conc. HNO3 and conc. H2SO4.
Solution:
B + 3HNO3 H3BO3 + 3NO2
2B + 3H2SO4 2H3BO3 + 3SO2
Boric acid is formed along with the evolution of NO2 and SO2 gases.
4. Borazine or Borazole or Triboarane triamine :
Colourless liquid
Six memebered ring has partially delocalized – electrons. [Isosteric with benzene]
- First prepared by Afred Stock.
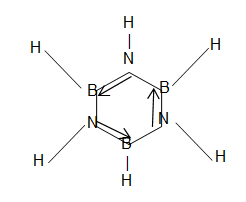
- is stronger Lewis acid than but much weaker Borohydrides than all other boron trihalides.
- Borazole is also called inorganic benzene because it structure and properties resemble benzene.
5. Boron Nitride (Borazon or Inorganic graphite):
- Colourless, good insulator, almost unreactive solid and diamagnetic in nature.
- A hexagonal layer structure similar to graphite is so packed that B–atom of one layer lies above the N–atom of next layer.
- The hexagonal form can be converted into a cubic form like diamond by heating at and 8500 atm. in the presence of an alkali or alkaline earth metal catalyst.
6. Boron carbide: also called NORBIA is the second hardest substance known (after diamond).
Illustration 5:
What is the hardest compound of boron known?
Solution:
Boron carbide is the hardest substance known. Its formula is B4C. It’s so because of strong covalent network structure like that of diamond.
Illustration 6:
What happens when orthoboric acid is strongly heated?
Solution:
Orthoboric acid loses water gradually and finally gets converted into Boron trioxide. The sequence of reactions is as follows.
ALUMINIUM
Ores:
Oxides : Bauxite Al2O3. 2H2O
Corundum Al2O3
Halides : Cryolite Na3AlF6
Extraction of aluminium:
The main source of aluminium is the bauxite ore from which pure aluminium is obtained by converting it into alumina, followed by electrolysis.
Purification of Bauxite:
Bauxite contains impurities of iron oxide (red bauxite), silica (while bauxite), TiO2 etc. The method of purification of bauxite depends on the type of impurity present in it.
i) Baeyer’s process: For the bauxite having iron oxide as the main impurity. The ore having ferric oxide is roasted to convert it into ferric oxide. The roasted ore on digestion with NaOH solution at 200–2500C makes Al2O3 dissolved because of amphoteric nature of Al2O3 and impurities like iron oxide, TiO2 are left undissolved. The filtrate (NaAlO2) when treated with small amounts of Al(OH)3 gets precipitated. The Al(OH)3 obtained is filtered, dried and heated at 11000C to produce pure Al2O3.
ii) Hall’s process: The method is used for purifying the bauxite which has an impurity of iron oxide. The ore in this method is fused with Na2CO3; soluble NaAlO2 is formed which leaves behind the impurity of Fe2O3.
The filtrate is warmed (500C) and CO2 is passed through it to give back Na2CO3 along with precipitate of Al(OH)3, which on filtering, drying and igniting gives alumina.
iii) Serpeck’s process: The bauxite with impurity of SiO2 is purified by heating it with carbon at 10000C in the atmosphere of N2. SiO2 converts into silicon, which volatilizes off. Aluminium nitride on hydrolysis gives Al(OH)3, which after drying and igniting gives Al2O3.
Electrolysis of fused Alumina (Hall Herault process):
Pure aluminium oxide Al2O3 obtained by either of the above process is melted in a steel container, lined with carbon (graphite) in presence of fluxes (cryotite, Na3AlF6 and fluorspar, CaF2) because of poor conductivity and high mp of alumina.
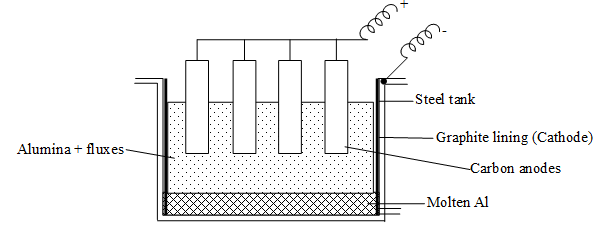
When the electric current is passed through the molten alumina, it is electrolysed and aluminium is liberated at cathode in molten state while oxygen is evolved at anode. At high temperature of the electrolytic cell oxygen reacts with C to form CO and CO2.
At Cathode: Al3+ + 3e– Al
At anode : C + O2– CO + 2e–
C + 2O2– CO2 + 4e–
Exercise 3:
(i) The nature of alumina is
(A) acidic (B) basic (C) neutral (D) amphoteric
(ii) When alumina is heated with carbon in nitrogen atmosphere, the products are
(A) Al + CO (B) Al + CO2 (C) Al + CO + CO2 (D) AlN + CO
(iii) When alumina is electrolysed in presence of cryolite, the gas liberated at graphite anode is
(A) F2 (B) O2 (C) CF4 (D) F2O
(iv) The electrolysis of pure alumina is not feasible because
(A) it is bad conductor of electricity and its fusion temperature is high
(B) it is volatile in nature
(C) it is decomposed when fused
(D) it is amphoteric
Answers
(i) D
(ii) D
(iii) B
(iv) A
Refining of Aluminium (Hoop’s process):
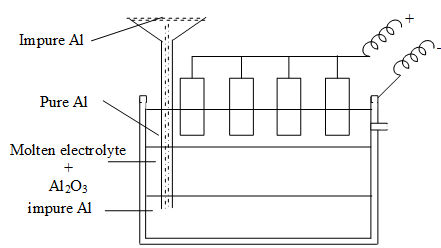
Refining is carried out in a tank having carbon electrodes. The electrolytic cell has 3 layers which differ in density:
i) The bottom layer having impure aluminium (impurities Cu, Si).
ii) The middle layer having molten fluorides of sodium, barium and aluminium along with alumina.
iii) The top layer has pure aluminium. As the current is passed, the Al3+ ions from the middle layer move to the top layer. At the same time equivalent mount of Al passes from bottom to middle layer. By this method 99.98% aluminium is obtained.
Properties of aluminium:
Physical:
- Al is bluish white, light metal (density 2.7g/ml).
- It has high thermal and electrical conductivity, excellent corrosion resistance and non-magnetic behaviour.
- It stands second only to gold for malleability and sixth for ductility.
- mp 6600C, bp 24500C
Chemical:
1. Electropositive nature: Aluminium is high electropositive element. It loses 3e– to form tripositive ion easily because of low reduction potential.
2. Action of air: Although aluminium is quite stable under normal temperature in the presence of moist air, a thin layer of Al2O3 is formed over the surface. When heated to redness, it burns with a brilliant white light and liberates heat.
4Al + 3O2 2Al2O3
3. Action of water: Pure cold water has no action on aluminium but it is decomposed by boiling water.
2Al + 6H2O 2Al (OH)3 + 3H2
4. Action of nitrogen: On heating with N2, nitrides are formed, which produce NH3 on hydrolysis.
5. Action of Chlorine: Anhydrous chlorine on heating with powdered aluminium gives anhydrous AlCl3.
2Al + 3Cl2 2AlCl3
6. Action of acids: Salts of acidic anions are formed:
2Al + 6HCl 2AlCl3 + 3H2
2Al + 6H2SO4(conc.,) Al2(SO4)3 + 3SO2 + 6H2O
Aluminium doesn’t react with nitric acid (dil. or conc), because nitric acid renders it passive due to formation of a protective layer of Al2O3 on its surface.
7. Action of alkalis: Hot NaOH dissolves Al and forms aluminate.
2Al + 2NaOH + 6H2O 2Na [Al (OH)4] + 3H2
8. Action of carbon & sulphur: Carbides and sulphides are produced respectively on heating.
9. Reducing action: Because of strong affinity with oxygen it acts as oxygen withdrawing and hence reducing agent. The property is exploited in metallurgy to convert metallic oxides into metal.
Fe2O3 + 2Al 2Fe + Al2O3
Cr2O3 + 2Al 2Cr + Al2O3
Since, the reaction is exothermic, the heat produced can be used for other purposes also.
An example is thermite welding also called, aluminothermite process, discovered by a German chemist Goldschmidt. Hence, it is also called Goldschmidt aluminothermite process.
Goldschmidt Aluminothermic process:
Uses of aluminium:
i) In electrical transmission lines
ii) In aerospace industries (alloys of aluminium)
iii) In canning of soft–drinks, beer & aerosol cans
iv) In packaging of foods and pharmaceuticals
v) In rolled gold, artificial jewellary, coins, golden paints etc.
Alloys of aluminium:
| Name of alloy | Composition | Uses |
| Magnalium | 95% Al + 5% Mg | Construction of airships, balances, pistons of motor engines |
| Duralumin | 95Al + 4% Cu + 0.5% Mg + 0.5% Mn | Aeroplane, automobiles parts |
| Aluminium bronze | 90% Cu + 9.5% Al + 0.5% Sn | Utensils, photo frames, coins, golden points & artificial jewellary |
| Alnico | 77% steel + 2% Ni + 20% Al + 1 % Co | For making permanent magnets |
| Y – alloy | 93% Al + 4% Cu + 2% Ni + 1% Mg | Piston and machinery parts |
Exercise 4:
(i) In the extraction of aluminium, the function of cryolite is to
(A) lower the melting point of alumina
(B) increase the melting point of alumina
(C) remove impurities from alumina
(D) minimize the anode effect
(ii) In the alumino-thermite process, Al acts as
(A) an oxidizing agent
(B) a flux
(C) solder
(D) a reducing agent
(iii) Which of the following is not an alloy of aluminium?
(A) Duralumin
(B) Spiegeleisen
(C) Magnalium
(D) Nickeloy
(iv) The most covalent aluminium halide is
(A) AlF3 (B) AlCl3 (C) AlBr3 (D) AlI3
Answers
(i) A
(ii) D
(iii) B
(iv) D
Compounds of aluminium:
Aluminium Chloride (AlCl3):
Preparation:
Aluminium or Al(OH)3 is dissolved in HCl and the resulting solution is crystallized to form AlCl3.6H2O.
2Al + 6HCl 2AlCl3 + 3H2
Al (OH)3 + 3HCl AlCl3 + 3H2O
Anhydrous AlCl3 can’t be prepared by heating AlCl3.6H2O, since it undergoes hydrolysis.
Anhydrous AlCl3 is prepared by passing dry Cl2 or dry HCl gas over heated aluminium.
AlCl3 sublimes and is condensed in dry receiver.
Properties:
Pure anhydrous AlCl3 is a white, hygroscopic substance which melts at 1930 under 2 atm. Under normal pressure it sublimes at 1800C. It is a covalent compound as evidenced by its voltatility, solubility in organic solvents (benzene, CS2) and very low conductivity in molten state. It is highly soluble in water and hydrolyses into an acidic solution (a mixture of weak base and strong alkali).
Because of less stable electronic configuration, it forms addition compounds with electron donor molecules like NH3, PH3, COCl2 etc.
Illustration 7:
What do you mean by hydrolysis?
Solution:
The chemical interaction of a compound with water is hydrolysis. Often the equality of [H+] and [OH–] is disturbed.
Uses:
i) In preparation of dyes drugs and perfumes
ii) As catalyst in Friedel–Craft reaction
Illustration 8:
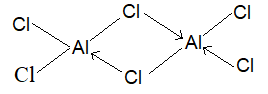
AlCl3 exist only at higher temperature (in gaseous state) while at room temperature it exist in dimeric Al2Cl6 form, why?
Solution:
AlCl3 is a covalent compound. Its octet being incomplete, it tries to accept two electrons, which are provided by the Cl atoms of another AlCl3 molecule with the formation of a co-ordinate bond and hence giving a dimer. This adds stability to it. Since these coordinate bonds are not very strong, at high temperature they break and we have monomer, AlCl3 once again.
Exercise 5:
(i) Aluminium can form
(A) electrovalent compounds only
(B) covalent compounds only
(C) electrovalent and covalent compounds
(D) coordinate compounds only
(ii) Aluminium does not react with
(A) NaOH (B) HCl (C) N2 (D) HNO3
(iii) Which of the following statements about anhydrous aluminium chloride is correct?
(A) It exists as AlCl3 molecule
(B) It is a strong Lewis base
(C) It sublimes at 1000C under vacuum
(D) It is not easily hydrolysed
Answers
(i) C
(ii) D
(iii) C
Alums:
Double salts of mono and trivalent sulphates are called alums. They have similar structures and properties. The general formula corresponds to
or
M univalent ions like Na+, k+, NH4+, Ag+ etc.
trivalent ions like Al3+, Fe3+, Cr3+ etc.
When dissolved in water they exist in hydrated form:
Some common alums are named here under.
Polash alum
Ammonia alum
Ferric alum
Chrome alum
Potash Alum:
Preparation:
1) Lab method: In laboratory potash alum is prepared by crystallizing equimolar mixture of K2SO4 & Al2 (SO4)3.
2) From bauxite: Bauxite on being digested with 62% H2SO4 gets converted into metals aluminium sulphate. The impurity of Fe2(SO)3 is removed by converting it into ppt. of FeSO4 by treating the solution with BaS.
To the filtrate, calculated amount of K2SO4 is added and subjected to crystallize into alum.
Al2O3 + 3H2SO4 Al2(SO4)3 + 3H2O
Al2(SO4)3 + K2SO4 + 24H2O K2SO4. Al2(SO4)3. 24H2O
3) From Alunite (K2SO4. Al2(SO4)3. 4Al (OH)3: The alunite ores are dissolved in H2SO4 to convert Al(OH)3 into Al2(SO4)3. Some more amount of K2SO4 is to be added quantitatively to crystallize it completely.
K2SO4. Al2(SO4)3. 4Al (OH)3 + 6H2SO4 K2SO4 + 3Al2SO4 + 12 H2O
3Al2(SO4)3 + 2KSO4 + 24 H2O K2SO4. Al2(SO4)3. 24H2O
Properties:
1. Alums are colourless, crystalline solid having sour taste.
2. Potash alum are octahedral in shape.
3. They are water soluble and acidic due to hydrolysis of Al3+ ions.
4. When heated, potash alum melts at 92.50C, at higher temperature crystallization water is lost (~2000C) and a white porous mass, burnt alum is formed.
If it is further heated upto redness, the sulphate decomposes into oxide.
Uses:
1. As styptic to stop bleeding for small cuts
2. In purification of water
3. In tanning of leather [Chrome alum]
4. As mordant in dyeing
5. In sizing of paper
Illustration 9:
Identify the alum(s) in the following
(i) Na2SO4.Al2(SO4)3.24H2O
(ii) MnSO4.Al2(SO4)3.24H2O
(iii) FeSO4.Al2(SO4)3.24H2O
(iv) K2SO4.Mn2(SO4)3.24H2O
Solution: (i) & (iv) Because (ii) has first ion Mn2+, while (iii) has Fe2+ which are supposed to be monovalent ions for alum.
Illustration 10:
Lithium does not form any alum. Why?
Solution:
Due to the small size of Li, it does not form alums. The smaller Li+ ions are not retained in the crystal lattice.
Exercise 6:
(i) The nature of the solution of potash alum is
(A) acidic (B) basic (C) neutral (D) uncertain
(ii) Alum is used by dyers of cloth
(A) for fire proofing fabrics
(B) as first aid for cuts
(C) for softening hard water
(D) as mordant
(iii) Which one of the following mixed sulphates is not an alum?
(A)
(B)
(C)
(D)
Answers
(i) A
(ii) D
(iii) D
- Thallium is highly toxic due to resemblance of its chemical properties with alkali metals like Na, K.
- “Red Al” is a reducing agent, which is soluble in benzene having formula Na [AlH (OCH3)2. OC2H5]
- “Red liquor” is aluminium acetate used as mordant in dyeing and calico printing.
- Utramarine [Na3Al3Si3S3O12] is an artificial Lapis–Lazuli having fine blue colour.
- ‘Pseudoalums’ are double sulphates of divalent and trivalent ions
[MSO4 (SO4)3 . 24H2O] M = divalent metal ion e.g. Mg2+, Ca2+, Fe2+.
FORMULAE AND CONCEPTS AT A GLANCE
1. Group 13 elements are known as Boron family.
2. It consists of B, Al, In and Tl.
3. The variation of physical properties like atomic radius, ionic radius (M+3), Electro-negativities, IE’s etc are given an interpretation in terms of electronic structures.
4. The properties of the elements of boron family have many of similar properties; the difference wherever is due to the difference in the number of electrons of penultimate shell.
5. Because of their high IE’s they preferably form covalent compounds.
6. In their compounds these elements show + III or + I oxidation state.
7. + I oxidation state become more stable as one goes down the group from B to TI.
8. The structure of diborane, an example of electron deficient compound is explained on the basis of formation of 3c, 2e bonds or banana bonds.
9. The extraction of aluminium is done principally from its oxide ore i.e., alumina (Al2O3.2H2O)
10. For the electrolysis process CaF2 & Na3AlF6 are added to it which help in reducing its mp & increasing conductivity.
11. Al finds use in construction because of it resistance to corrosion (due to formation of protective layer of Al2O3) and less density (2.7g mL–1).
12. Reaction with O2 or Fe2O3 being highly exothermic, finds use in welding purpose under the name Goldschmit’s aluminothermic (or thermite) process.
13. The double salts with composition M2SO4.M2(SO4)3.24H2O are called alums, and are used for different purposes based on the ions present. [M = univalent ion like, Na+, K+, NH4+ & M = trivalent ion like, Fe3+, Cr3+, Al3+, etc.]
SOLVED PROBLEMS
Prob 1. Alumina on heating with carbon in nitrogen atmosphere gives
(A) Al + CO (B) Al + CO2 (C) AlN + CO (D) Al + CO + N2
Sol: (C)
Prob 2. Which of the following has the minimum heat of dissociation?
(A)
(B)
(C)
(D)
Sol: (D) The tendency of trimethyl boron to act as Lewis acid decreases due to + I.E. of CH3gp, and thus co-ordination becomes weaker.
Prob 3. On adding ammonium hydroxide solution to Al2(SO4)3 (aq.)
(A) A precipitate is formed which does not dissolve in excess of ammonium hydroxide
(B) A precipitate is formed which dissolves in excess of ammonia solution
(C) No precipitate is formed
(D) None of these
Sol: (A)
Insoluble in NH4OH
But soluble in NaOH
Prob 4. Al and Ga have nearly same covalent radii because of
(A) Greater shielding power of s-electrons of Ga atoms
(B) Poor shielding power of s-electrons of Ga atoms
(C) Poor shielding power of d-electrons of Ga atoms
(D) Greater shielding power of d-electrons of Ga atoms
Sol: (C) It is a reason for given fact.
Prob 5. B–F bond order in BF3 is
(A) 1 (B) 2 (C) 3 (D) 4/3
Sol: (D) Due to back bonding (pp – pp), resonance is noticed in BF3 to produce bond order 1.33.
Prob 6. Which of the following reaction will yield crystalline variety of boron
(A)
(B)
(C)
(D) All of these
Sol: (A) Only the action of Al with B2O3 on heating gives crystalline variety of born.
Prob 7. In the reaction and LiH acts as
(A) Lewis acid and Lewis base
(B) Lewis base and Lewis acid
(C) Bronsted base and Bronsted acid
(D) None of these
Sol: (A) LiH has H– ion which donates electron pair (i.e., acts as Lewis base) to AlH3 (a Lewis acid).
Prob 8. Identify the correct statement
(A) BF3, BCl3 are gases
(B) BF3 has partially double bond character due to pp – pp bonding and the molecule shows resonance
(C) BF3 is electron pair donor
(D) BBr3 is solid
Sol: (B) BF3 is gas, BCl3 and BBr3 are colourless fuming liquids, BI3 is white fusible solid. All these are electron deficient and act as Lewis acid or electron pair acceptor.
Prob 9. Among the halides,
1) BCl3 2) AlCl3 3) GaCl3 4) InCl3
The order of decreasing Lewis acid character is
(A) 1, 2, 3, 4 (B) 4, 3, 2, 1 (C) 3, 4, 2, 1 (D) 2, 3, 4, 1
Sol: (B) The acidic character of chlorides increases down the group BCl3 is weak acid to show p-p back bonding hence, remains acidic, while in AlCl3 we can see the coordinate bonding which diminishes the acidic character. Ga & In undergo p-p back-bonding.
Prob 10. Which of the following statement about boron carbide is wrong
(A) Its molecular formula is B4C
(B) It is also called Norbia
(C) It is the hardest substance
(D) It is used for cutting glasses
Sol: (C) B4C is next hardest substance to diamond.








