Organisms require many organic and inorganic substances to complete their life cycle. All such substances which they take from outside constitute their nutrition. On the basis of their nutritional requirements, organisms can be classified into heterotrophs and autotrophs.
All non-green plants and animals, including human beings, are heterotrophs. Autotrophic green plants obtain their nutrition from inorganic substances which are present in soil in the form of minerals, which are known as mineral elements or mineral nutrients and this nutrition is called mineral nutrition.
1. ESSENTIAL MINERAL ELEMENTS
An essential element is defined as ‘one without which the plant cannot complete its life cycle, or one that has a clear physiological role’. Therefore, in 1939 Arnon and Stout proposed the following characters for judging the criteria of essentiality of an element in the plant:
(1) The element must be essential for normal growth and reproduction, which cannot proceed without it.
(2) The requirement of the element must be specific and cannot be replaced by another element.
(3) The requirement must be direct that is, not the result of any indirect effect e.g., for relieving toxicity caused by some other substance.
Essential elements are divided into two broad categories, based on the quantity in which they are required by plants. Macro-elements and micro-elements. Their ionic forms are respectively called macronutrients and micronutrients. Mineral salts dissolved in soil solution are constantly passing downwards along with percolating (gravitational) water. The phenomenon is called leaching. Leaching is more in case of anions.
Macronutrients (Macroelements or major elements)
Which are required by plants in larger amounts (Generally present in the plant tissues in concentrations of 1 to 10 mg per gram of dry matter). Of the non-essential functional elements, silicon and sodium often occur in the range of macroelements. Macroelements are usually involved in the synthesis of organic molecules and development of osmotic potential.
Micronutrients (Microelements or minor elements or trace elements)
Which are required by plants in very small amounts, i.e., in traces (equal to or less than 0.1 mg per gram dry matter). Cobalt, vanadium, aluminium and nickel, may be essential for certain plants. Microelements are mostly involved in the functioning of enzymes, as cofactors or metal activators. The usual concentration of essential elements in higher plants according to D.W. Rains (1976) based on the data of Stout are as follows:
|
Elements |
% of dry weight |
|
Macronutrients |
|
|
Carbon |
45 |
|
Oxygen |
45 |
|
Hydrogen |
6 |
|
Nitrogen |
1.5 |
|
Potassium |
1.0 |
|
Calcium |
0.5 |
|
Magnesium |
0.2 |
|
Phosphorus |
0.2 |
|
Sulphur |
0.1 |
|
Micronutrients |
|
|
Chlorine |
0.01 |
|
Iron |
0.01 |
|
Manganese |
0.005 |
|
Boron |
0.002 |
|
Zinc |
0.002 |
|
Copper |
0.0001 |
|
Molybdenum |
0.0001 |
2. PLANT ANALYSIS
Ash analysis
The plant tissue is subjected to a very high temperature (550-600°C) in an electric muffle furnace and is reduced to ash. The plant ash left behind forms a very small proportion of plants dry weight ranging from 2 to 10% only. Analysis of plant ash shows that about 92 mineral elements are present in different plants. Out of these, 30 elements are present in each and every plants and rest are in one or other plants. Out of these 30 elements, 16 elements are necessary for plants and are called essential elements.
Solution culture (Hydroponics)
In this method plants are grown in nutrient solutions containing only desired elements. To determine the essentiality of an element for a particular plant, it is grown in a nutrient medium that lacks or is deficient in this element.
The growing of plants with their roots in dilute solutions of mineral salts instead of soil led to increased understanding of plant nutrition. This cultivation of plants by placing the roots in nutrient solution is called hydroponics. Probably the first recorded use of soilless culture was by Woodward in 1699. By 1860, the culture solution technique was modernized by Sachs and he showed the essentiality of nitrogen for plant growth.
Another significant worker for studying the essentiality of elements was Knop (1865). The method of growing plants in aqueous nutrient solutions as employed by Sachs and Knop is used experimentally and commercially today and known as hydroponic culture. Now a days a chelating agent Na2-EDTA (Disodium salt of ethylene diamine tetra acetic acid. EDTA (Ethylene diamine tetra-acetic acid) is a buffer which is used in tissue cultures) is added.
Hydroponics or soilless culture helps in knowing
1) The essentiality of mineral element.
2) The deficiency symptoms developed due to non-availability of particular nutrient.
(3) Toxicity to plant when element is present in excess.
(4) Possible interaction among different elements present in plant.
(5) The role of essential element in the metabolism of plant.
Solid medium culture
In this method either sand or crushed quartz is used as a rooting medium and nutrient solution is added to it. The nutrient medium is provided by one of the following methods:
Drip culture: It is done by dripping over the surface.
Slop culture: It is done by having the medium over the surface.
Sub-irrigation: Here the solution is forced up from the bottom of the container.
3. MAJOR ROLE OF NUTRIENTS
Various elements perform the following major roles in the plants
Construction of the plant body
The elements particularly C, H and O construct the plant body by entering into the constitution of cell wall and protoplasm. They are, therefore, referred to as framework elements. Besides, these (C, H and O) N, P and S, Mg and Fe also enter in the constitution of protoplasm. They are described as protoplasmic elements.
Maintenance of osmotic pressure
Various minerals present in the cell sap in organic or inorganic form maintain the osmotic pressure of the cell.
Maintenance of permeability of cytomembranes
The minerals, particularly Ca++, K+ and Na+ maintain the permeability of cytomembranes.
Influence the pH of the cell sap
Different cations and anions influence on the pH of the cell sap.
Catalysis of biochemical reaction
Several elements particularly Fe, Ca, Mg, Mn, Zn, Cu, Cl act as metallic catalyst in biochemical reactions.
Toxic effects
Minerals like Cu, As, etc. impart toxic effect on the protoplasm under specific conditions.
Balancing function
Some minerals or their salts act against the harmful effect of the other nutrients, thus balancing each other.
4. SPECIFIC ROLE OF MACRONUTRIENTS
The role of different elements is described below
Carbon, hydrogen and oxygen
These three elements, though cannot be categorised as mineral elements, are indispensible for plant growth. Carbon, hydrogen and oxygen together constitute about 94% of the total dry weight of the plant. Carbon is obtained from the carbon dioxide present in the atmosphere. It is essential for carbohydrate and fat synthesis. Hydrogen and oxygen would be obtained from water which is absorbed by the plants from the soil. Some amount of oxygen is also absorbed from the atmosphere.
Nitrogen
Source: The chief source of nitrogen for green plants is the soil. It is absorbed mainly in the form of nitrate ions . The major sources of nitrate for the plants are sodium nitrate, potassium nitrate, ammonium nitrate and calcium nitrate.
Functions: Nitrogen is an essential constituent of proteins, nucleic acids, vitamins and many other organic molecules as chlorophyll. Nitrogen is also present in various hormones, coenzymes and ATP etc. It plays an important role in protein synthesis, respiration, growth and in almost all metabolic reactions.
Deficiency symptoms
(i) Impaired growth
(ii) Yellowing of leaves due to loss of chlorophyll, i.e., chlorosis.
(iii) Development of anthocyanins pigmentation in veins, sometimes in petioles and stems.
(iv) Delayed or complete suppression of flowering and fruiting.
Excessive supply of nitrogen produces following symptoms:
(i) Increased formation of dark green leaves.
(ii) Poor development of root system.
(iii) Delayed flowering and seed formation.
Phosphorus
Source: Phosphorus is present in the soil in two general forms, organic and inorganic. Plants do not absorb organic phosphorus, either from the solid or solution phase of soil. However, organic compounds are decomposed and phosphorus is made available to plants in inorganic form. Soil solution contains phosphorus in inorganic forms as the phosphate ions obtained as and . When pH is low phosphate ions are present in the form of . When pH is high, phosphate ions are represented in .
Functions
(i) Phosphorous is present abundantly in the growing and storage organs such as fruits and seeds. It promotes healthy root growth and fruit ripening by helping translocation of carbohydrates.
(ii) It is present in plasma membrane, nucleic acid, nucleotides, many coenzymes and organic molecules as ATP.
(iii) Phosphorus plays an indispensable role in energy metabolism i.e., hydrolysis of pyrophosphate. Thus it is required for all phosphorylation reactions.
Deficiency symptoms
(i) Leaves become dark green or purplish.
(ii) Sometimes development of anthocyanin pigmentation occurs in veins which may become necrotic (Necrosis is defined as localized death of cells).
(iii) Premature fall of leaves.
(iv) Decreased cambial activity resulting in poor development of vascular bundles.
(v) Sickle-leaf disease.
Sulphur
Source: Sulphur is present as sulphate in mineral fraction of soil. In industrialized areas, atmospheric sulphur dioxide and sulphur trioxide (; in low concentration) may be important sources of sulphur nutrition.
Functions
(i) Sulphur is a constituent of amino-acids like cystine, cysteine and methionine; vitamins like biotin and thiamine, and coenzyme A.
(ii) It increases the nodule formation in the roots of leguminous plants. It favours soluble organic nitrogen and there is decrease in the quantity of soluble nitrogen with its increase.
(iii) The characteristic smell of mustard, onion and garlic is due to the presence of sulphur in their volatile oils.
(iv) Sulphur in plants is required in stem and root tips and young leaves. It is remobilised during senescence.
Deficiency symptoms
(i) Leaves remain small and turn pale green i.e., symptoms of chlorosis. Chlorosis affects young leaves more because of immobile property of the sulphur. The young leaves develop orange, red or purple pigment.
(ii) Leaf tips and margins roll downwards and inwards e.g., tobacco, tea and tomato.
(iii) Delayed flowering and fruiting.
(iv) Apical growth is retarded whereas premature development of lateral buds starts.
(v) The tea yellow disease is caused in tea plants.
(vi) Decrease in stroma lamellae and increase in grana stacking.
(vii) Increase in starch and sucrose accumulation, and decrease in reducing sugars.
Potassium
Source: Source of K+ to the plants is inorganic compounds like potassium sulphate, potassium nitrate, etc. Potassium is usually present in sufficient amount in clay soils. It contains approximately 0.3 to 6.0 percent of whole plant. In seeds, it is found in less amount.
Functions
(i) It differs from all other macronutrients in not being a constituent of any metabolically important compound.
(ii) It is the only monovalent cation essential for the plants.
(iii) It acts as an activator of several enzymes including DNA polymerase.
(iv) It is essential for the translocation of photosynthates, opening and closing of stomata, phosphorylation, synthesis of nucleic acid and chlorophyll.
It takes part in the formation of cell membrane and it is also responsible for maintenance of turgidity of cells.
Deficiency symptoms
(i) Mottled chlorosis followed by the development of necrotic areas at the tips and margins of the leaves.
(ii) K+ deficiency inhibits proteins synthesis and photosynthesis. At the same time, it increases the rate of respiration.
(iii) The internodes become shorter and root system is adversely affected.
(iv) The colour of leaves may turn bluish green.
(v) Widespread blackening or scorching of leaves may occur as a result of increased tyrosinase activity.
(vi) Rosette or bushy habit of growth may be seen in plants.
Destruction of pith cells of tomato and increased differentiation of phloem elements.
Calcium
Source: It is absorbed by the plants in the form of from calcium carbonate etc. It occurs abundantly in a non-exchangeable form such as anorthite . Much of the exchangeable calcium of the soil is absorbed on to the surface of clay micelle.
Functions
(i) It is necessary for formation of middle lamella of plants where it occurs as calcium pectate.
(ii) It is necessary for the growth of apical meristem and root hair formation.
(iii) It acts as activator of several enzymes, e.g., ATPase, succinic dehydrogenase, adenylate kinase, etc.
(iv) Along with Na+ and K+ it maintains the permeability of plasma membrane.
(v) It is involved in the organisation of spindle fibres during mitosis.
(vi) It antagonises the toxic effects of Na+ and Mg++.
It is essential for fat metabolism, carbohydrate metabolism, nitrate assimilation and binding of nucleic acids with proteins.
Deficiency symptoms
(i) Ultimate death of meristems which are found in shoot, leaf and root tips.
(ii) Chlorosis along the margins of young leaves, later on they become necrotic.
(iii) Distortion in leaf shape.
(iv) Roots poorly developed or may become gelatinous.
(v) Young leaves show malformation and leaf tips becomes hooked.
(vi) Its deficiency checks flowering and causes the flowers to fall early.
(vii) Potato tubers become small and malformed.
Magnesium
Source: Magnesium occurs in the soil in the form of magnesite (MgCO3), dolomite (MgCO3, CaCO3), magnesium sulphate (MgSO4) and as silicates. It is absorbed from the soil in the form of (Exchangeable cation) ions (Mg++). It is easily leached and thus become deficient in sandy soils during rainy season.
Functions
(i) It is an important constituent of chlorophyll.
(ii) It is present in the middle lamella in the form of magnesium pectate.
(iii) It plays an important role in the metabolism of carbohydrates, lipids and phosphorus.
(iv) It acts as activator of several enzymes.
(v) It is required for binding the larger and smaller subunits of ribosomes during protein synthesis.
Deficiency symptoms
(i) Interveinal chlorosis followed by anthocyanin pigmentation, eventually necrotic spots appear on the leaves. As magnesium is easily transported within the plant body, the deficiency symptoms first appear in the mature leaves followed by the younger leaves at a later stage.
(ii) Stems become hard and woody, and turn yellowish green.
(iii) Depression of internal phloem and extensive development of chlorenchyma.
5. SPECIFIC ROLE OF MICRONUTRIETNS
Iron
Source: It is present in the form of oxides in the soil. It is absorbed by the plants in ferric as well as ferrous state but metabolically it is active in ferrous state. Its requirement is intermediate between macro and micro-nutrients.
Functions
(i) Iron is a structural component of ferredoxin, flavoproteins, iron prophyrin proteins (Cytochromes, peroxidases, catalases, etc.)
(ii) It plays important roles in energy conversion reactions of photosynthesis (phosphorylation) and respiration.
(iii) It acts as activator of nitrate reductase and aconitase.
(iv) It is essential for the synthesis of chlorophyll.
Deficiency symptoms
(i) Chlorosis particularly in younger leaves, the mature leaves remain unaffected.
(ii) It inhibits chloroplast formation due to inhibition of protein synthesis.
(iii) Stalks remain short and slender.
(iv) Extensive interveinal white chlorosis in leaves.
(v) It may develop necrosis aerobic respiration severely affected.
(vi) In extreme deficiency scorching of leaf margins and tips may occur.
Manganese
Source: Like iron, the oxide forms of manganese are common in soil. However, manganese dioxide (highly oxidised form) is not easily available to plants. It is absorbed from the soil in bivalent form (Mn++). Increased acidity leads to increase in solubility of manganese. In strong acidic soils, manganese may be present in toxic concentrations. Oxidising bacteria in soils render manganese unavailable to plants at pH ranging from 6.5 to 7.8.
Functions
(i) It acts as activator of enzymes of respiration (malic dehydrogenase and oxalosuccinic decarboxylase) and nitrogen metabolism (nitrite reductase).
(ii) It is essential for the synthesis of chlorophyll.
(iii) It is required in photosynthesis during photolysis of water.
(iv) It decreases the solubility of iron by oxidation. Hence, abundance of manganese can lead to iron deficiency in plants.
Deficiency symptoms
(i) Chlorosis (interveinal) and necrosis of leaves.
(ii) Chloroplasts lose chlorophyll, turn yellow green, vacuolated and finally perish.
(iii) ‘Grey spot disease’ in oat appears due to the deficiency of manganese, which leads to total failure of crop.
(iv) ‘Marsh spot’s in seeds of pea.
(v) Deficiency symptoms develop in older leaves.
Copper
Source: Copper occurs in almost every type of soil in the form of complex organic compounds. A very small amount of copper is found dissolved in the soil solution. It is found in natural deposits of chalcopyrite (CuFeS2).
Functions
(i) It activates many enzymes and is a component of phenolases, ascorbic acid oxidase, tyrosinase, cytochrome oxidase.
(ii) Copper is a constituent of plastocyanin, hence plays a role in photophosphorylation.
(iii) It also maintains carbohydrate nitrogen balance.
Deficiency symptoms
(i) Both vegetative and reproductive growth are reduced.
(ii) The most common symptoms of copper deficiency include a disease of fruit trees called ‘exanthema’ in which trees start yielding gums on bark and ‘reclamation of crop plants’, found in cereals and legumes.
(iii) It also causes necrosis of the tip of the young leaves (e.g., Citrus). The disease is called ‘die back’.
(iv) Carbon dioxide absorption is decreased in copper deficient trees.
(v) Wilting of entire plant occurs under acute shortage.
(vi) Grain formation is more severely restricted than vegetative growth.
Molybdenum
Source: It is available to the plants mostly as molybdate ions. It is required in extremely small quantities by plants. It is found relatively in higher concentration in mineral oil and coal ashes.
Functions
(i) Its most important function is in nitrogen fixation because it is an activator of nitrate reductase.
(ii) It is required for the synthesis of ascorbic acid.
(iii) It acts as activator of some dehydrogenases and phosphatases.
Deficiency symptoms
(i) Mottled chlorosis is caused in the older leaves as in nitrogen deficiency, but unlike nitrogen-deficient plants, the cotyledons stay healthy and green.
(ii) It is also known to inhibit flowering, if they develop, they fall before fruit setting.
(iii) It leads to drop in concentration of ascorbic acid.
(iv) Its deficiency causes ‘whiptail disease’ in cauliflower and cabbage. The leaves first show an interveinal mottling and the leaf margins may become gray and flaccid and finally brown.
Zinc
Source: Zinc occurs in the soil in the form of ferromagnesian minerals like magnetite, biotite and hornblende. Increase in soil pH decreases the availability of zinc.
Bivalent form of zinc (Zn++) is exchangeable and is readily available in the soil. Plants require this mineral only in traces and its higher concentrations are highly toxic.
Functions
(i) It is required for the synthesis of tryptophan which is a precursor of indole acetic acid-an auxin.
(ii) It is a constituent of enzymes like carbonic anhydrase, hexokinase, alcohol dehydroge-nase, lactic dehydrogenase and carboxypeptidase.
(iii) It is required for metabolism of phosphorus and carbohydrates.
(iv) Zinc also appears to play an important role in protein synthesis because in its absence there is substantial increase in soluble nitrogenous compounds.
Deficiency symptoms
(i) The first symptom appears in the form of interveinal chlorosis of the older leaves, starting at the tips and the margins.
(ii) Growth becomes stunted due to formation of smaller leaves and shortened internodes. Reduced stem growth is due to less synthesis of auxin.
(iii) The leaves become distorted and sickle shaped and get clustered to form rosettes. This effect is known as ‘little leaf disease’.
(iv) In maize, zinc deficiency produces ‘white bud disease’ which leads to greatly reduced flowering and fruiting as well as poorly differentiated root growth.
(v) Its deficiency causes khaira disease of rice and mottled leaf of apple, Citrus and walnut.
Boron
Source: Boron is present in the soil in very small amounts. It appears in exchangeable soluble and nonexchangeable forms in the soil or . It is absorbed from the soil as boric acid and tetraborate anions. Its calcium and magnesium salts are soluble. Its availability to plant decreases with increase in pH.
Functions
(i) It facilitates the translocation of sugars.
(ii) It is involved in the formation of pectin.
(iii) It is also required for flowering, fruiting, photosynthesis and nitrogen metabolism.
(iv) Boron is required for uptake and utilisation of Ca2+, pollen germination, seed germination and cell differentiation.
(v) It regulates cellular differentiation and development.
Deficiency symptoms
(i) The first major symptom of boron deficiency is the death of shoot tip because boron is needed for DNA synthesis.
(ii) Generally flowers are not formed and the root growth is stunted.
(iii) The leaves develop a thick coppery texture, they curve and become brittle.
(iv) Some of the physiological diseases caused due to boron deficiency are internal cork of apple, top rot of tobacco, cracked stem of celery, browning of cauliflower, water core of turnip, hard fruit of Citrus and heart rot of sugar beets and marigold. These diseases can be cured by application of small doses of sodium tetraborate in the soil.
(v) Its deficiency checks the cells division of cambium but continues cell elongation.
Chlorine
Source: It is absorbed from the soil as chloride ions. Hence, it is rarely supplied as fertilizer.
Functions
(i) It is required for photolysis of water during photosynthesis in photosystem-II.
(ii) In tobacco, it increases water volume inside the cell and also regulates carbohydrate metabolism.
(iii) With Na+ and K+, chlorine helps in determining solute concentration and anion cation balance in the cells.
(iv) It is essential for oxygen evolution in photosynthesis.
Deficiency symptoms
(i) The deficiency symptoms of chlorine consist of wilted leaves which later become chlorotic and finally attain a bronze colour.
(ii) Roots become stunted or thickened and club shaped and fruiting is reduced.
(iii) Photosynthesis is also inhibited.
Critical elements: Macroelements which become commonly deficient in the soils are called critical elements. They are in number–N, P and K most fertilisers contain critical elements. They are called complete fertilisers.
6. MECHANISM OF ABSORPTION OF MINERAL ELEMENTS
Plants absorb the minerals from the soil and translocate them to other parts of the body. Soil serves as a main source of mineral salts in which clay crystals with a central nucleus is called micelle. The micelles are negatively charged. To maintain the balance, they hold positively charged ions on their surface. When this balance is disturbed by salt absorption, the equilibrium is again restored by transferring some of the absorbed ions into the solution. The movement of ions is called as flux. The movement of ions into the cell is called influx and outward migration of ions is known as efflux. Various theories have been proposed to explain the mechanism of mineral salt absorption and can be placed under the following two categories.
Passive absorption
Absorption of ions without the use of metabolic energy is known as passive absorption. This type of absorption is carried out by purely physical forces.
In most of the cases, the movement of mineral ions into root occurs by diffusion. Diffusion of molecules is their net movement down a free energy or chemical potential gradient. The rate of diffusion varies with the chemical potential gradient or the difference in activity (essentially equivalent to concentration) across the diffusion distance.
Briggs and Robertson (1957) demonstrated the passive absorption of ions by root system. They showed:
(i) Mineral salt absorption is not affected by temperature and metabolic inhibitors.
(ii) Rapid uptake of ions occurs when plant tissues are transferred from a medium of low concentration to high concentration.
Some of the important theories explaining the mechanism of passive absorption of minerals are given below:
Mass flow hypothesis
According to Hylmo (1953, 1955), the ion absorption increases with increase in transpiration. The ions have been considered to move in a mass flow with water from the soil solution through the root and eventually to the shoot. The theory was supported by Kramer (1956), Russel and Barber (1960), etc. Later, Lopushinsky (1960) using radioactive P32 and Ca45, has supported this experiment.
Simple diffusion hypothesis
According to this hypothesis, if the concentration of solutes inside the plant is lower than the soil, the mineral ions are thought to migrate into the root by simple diffusion. As a result, a state of equilibrium is reached. The part of plant cell or tissue that permits free diffusion is sometimes called outer space. The apparent volume that accomodates these ions has been referred to by some workers as apparent free space. The accumulation of ions in the cell against concentration gradient can not be explained by this concept.
Facilitated diffusion hypothesis
According to this concept, the ions are transported across the membrane by a carrier protein. When the ions enter the cell through protein channels and not through the lipid layer the phenomenon is called facilitated diffusion.
Ion exchange hypothesis
According to this view the ions adsorbed to the cell surface are exchanged from the external medium. A cation is exchanged for a cation and anion for anion. If a particular ion is absorbed by the plant, in exchange it offers H+ or OH– ions which are made available by the dissociation of water molecule.
There are two theories to explain the mechanism of ion exchange.
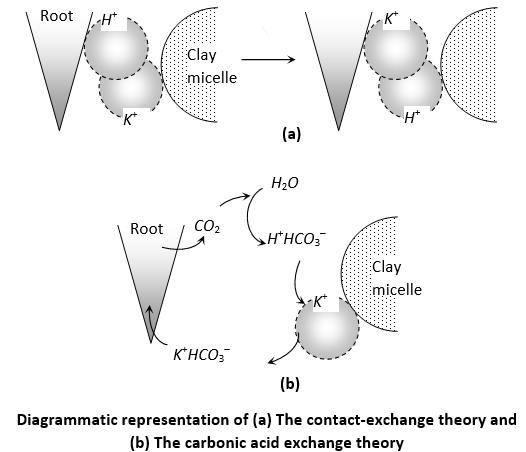
(i) Contact exchange theory: According to this theory, ions are not completely static, they are always oscillating around their absorption surface and when the oscillation volume of the ions on the roots and on the colloidal particles overlap each other, ion exchange occurs. An equilibrium is maintained between the dissolved fractions as any depletion in the soil solution is covered by movement of ions.
(ii) Carbonic acid exchange theory: In this case, CO2 released by roots during respiration reacts with water to produce carbonic acid which dissociates into hydrogen ions and bicarbonate ions. Hydrogen ion exchanges itself with the cations adsorbed on the colloidal particles and the bicarbonate ions release the adsorbed anions to supply both anions and cations nearby.
Donnan equilibrium: This mechanism, given by F.G. Donnan (1927), takes into account the effect of non-diffusible ions, which may be present on one side of the membrane. Unlike diffusible ions, the membrane is not permeable to non-diffusible ions. Such ions are termed as fixed ions. They may be anions or cations. In a system, in which there are no fixed ions, there are equal number of anions and cations on both sides of the membrane at equilibrium. But in Donnan equilibrium, in order to balance the charge of the fixed ions (say anions), more ions of the other charge (say cations) would be required.
Mathematically, the Donnan equilibrium may be represented by following equation:
Here: = Cations inside; = Cations outside
= Anions inside; = Anions outside
Active absorption
Generally, the lipid-protein membrane of a cell is largely permeable to free ions. The energy is considered to be involved in the transport of such free ions across the membrane. The absorption of ions, involving use of metabolic energy, is called active absorption. Energy used in these mechanisms comes from metabolic activities, especially respiration. Mineral absorption is mainly active process. Hoagland (1944) indicated active ion absorption and their (ions) accumulation against concentration gradient in green algae Nitella and Valonia.
Following evidences show the involvement of metabolic energy in the absorption of mineral salts:
(i) Higher rate of respiration increases the salt accumulation inside the cell.
(ii) Respiratory inhibitors check the process of salt uptake.
(iii) By decreasing oxygen content in the medium, the salt absorption is also decreased.
Active transport in necessary for living cells because certain substances must be concentrated and others must be excluded. Active uptake of minerals by roots mainly depends on availability of oxygen. Some of these are discussed below:
Carrier concept: This concept was proposed by Van den Honert (1937). The space in a cell or tissue where mineral ions enter by the usage of metabolic energy is called inner space. According to this concept there are separate carriers for cations and anions. A carrier forms an ion-carrier complex on the outer surface of the membrane. This complex breaks up and releases the ion into the inner space and this release is perhaps mediated by the enzyme phosphatase. The inactivated carrier is again activated by the enzyme kinase and in this process an ATP is used up. ATP molecule combine with carrier molecules and allow passage of substances against concentration gradient. The activated carrier again accepts new ions and the entire cycle is repeated.
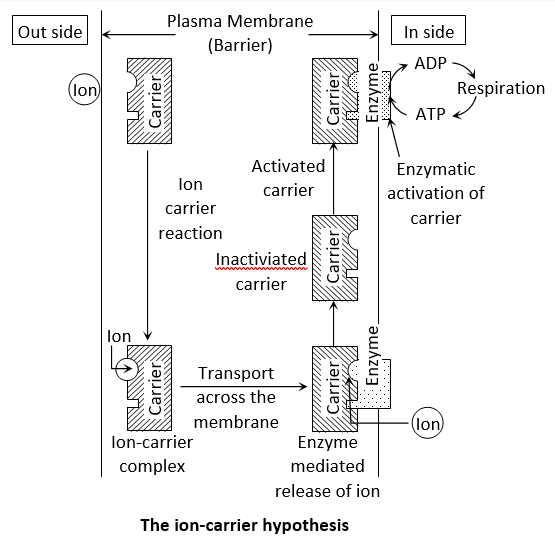
Carrier* + Ion (+/–) → Ion – Carrier complex*
Ion – Carrier complex* Carrier + Ion
Cytochrome – pump hypothesis: This theory was proposed by Lundegardh (1950, 1954). According to this explanation only anions are absorbed actively, i.e., anion uptake requires energy and the absorption of cations does not require energy, (i.e., they are absorbed passively). At the outer surface of the membrane, the cytochrome undergoes oxidation and loses one electron and in exchange picks up an anion. This is then transported to the inner side of the membrane through to the cytochrome chain and on the inner surface of the membrane the anion is released and the cytochrome gets reduced by the action of dehydrogenase involved in respiration.
The cations move passively along the electrical gradient created by the accumulation of anions at the inner surface of the membrane.
The evidence in favour of Lundegardh’s hypothesis is that the respiration increases when a plant is transferred from water to salt solution. The increased respiration is called salt respiration or anion respiration.
Criticism:
(i) It is applicable to absorption of anions only.
(ii) It fails to explain selective absorption of ions.
(iii) It has been observed that even cations can stimulate respiration.
(iv) ETS is poorly developed in anaerobically respiring forms.
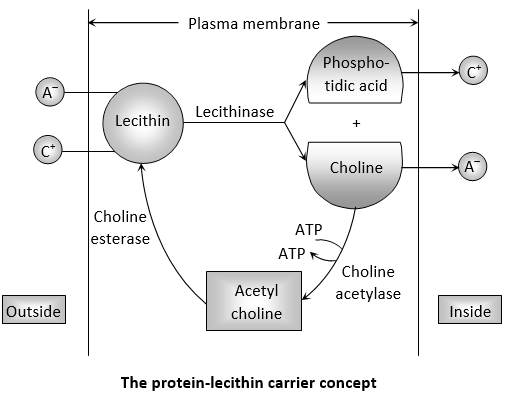
Protein-lecithin carrier concept: Bennet-Clark (1956) proposed that the carrier could be some amphoteric molecule which can carry anions as well as cations. He suggested it to be a membrane-bound protein which is conjugated with a phosphatide called as lecithin. Lecithin functions as a carrier. According to this theory, the phosphate group in the phosphatide acts as the cation binding site and choline acts as the anion binding site. During transport, ions are picked up by lecithin to form an ion-lecithin-complex. The ions are released on the inner surface of the membrane due to hydrolysis of lecithin by the enzyme lecithinase into phosphatidic acid and choline.
Lecithin is resynthesised from these components in the presence of enzyme choline acetylase and choline esterase which requires ATP.
Goldacre, 1952 proposed a mechanism of ion transport where contractile proteins act as ion carrier. They bind ions in unfolded condition on the outer face of the membrane and then contract releasing the ion into the cell and again become unfolded. The energy for this folding and unfolding is provided by ATP. In hydrophytic plants, water and salts are absorbed by outer layer of plants.
7. FACTORS AFFECTING MINERAL ABSORPTION
The process of mineral absorption is influenced by the following factors:
Temperature
The rate of absorption of salts and minerals is directly proportional to temperature.
The absorption of mineral ions is inhibited when the temperature has reached its maximum limit, perhaps due to denaturation of enzymes.
Light
When there is sufficient light, more photosynthesis occurs. As a result more food energy becomes available and salt uptake increases.
Oxygen
A deficiency of O2 always causes a corresponding decrease in the rate of mineral absorption. It is probably due to unavailability of ATP. The increased oxygen tension helps in increased uptake of salts.
pH
It affects the rate of mineral absorption by regulating the availability of ions in the medium. At normal physiological pH monovalent ions are absorbed more rapidly whereas alkaline pH favours the absorption of bivalent and trivalent ions.
Interaction with other minerals
The absorption of one type of ions is affected by other type. The absorption of K+ is affected by Ca++, Mg++ and other polyvalent ions. It is probably due to competition for binding sites on the carrier. However, the uptake of K+ and Br– becomes possible in presence of Ca++ ions. There is mutual competition in the absorption of K, Rb and Cs ions.
Growth
A proper growth causes increase in surface area, number of cells and in the number of binding sites for the mineral ion. As a result, mineral absorption is enhanced.
8. MINERAL TRANSLOCATION
P.R. Stout and D.R. Hoagland (1939) proved that mineral salts are translocated through xylem. After absorption of minerals by root, ions are able to reach xylem by two pathways.
Apoplast pathway
In this pathway inflow of water takes place from the cell to cell through spaces between cell wall polysaccharides. Ions thus are able to move from cell wall of epidermis to cell walls of various cells in cortex, cytoplasm of endodermis, cell wall of pericycle and finally into xylem.
Symplast pathway
In this pathway ions move through cytoplasm of epidermis and finally move through cytoplasm of cortex, endodermis, pericycle through plasmodesmata and finally into xylem.
Minerals in xylem are carried along with water to other parts of the plant along transpiration stream. Minerals reaching leaves take part in assimilation of organic compounds and then transported to other parts of the plant through phloem.
9. NITROGEN NUTRITION IN PLANTS
Higher plants generally utilize the oxidized forms such as nitrate and nitrite or the reduced form of nitrogen which is made available by a variety of nitrogen fixers. Nitrogen can be fixed by three methods:
Process of Nitrogen fixation
On the basis of agency through which the nitrogen is fixed the process is divided into two types abiological and biological.
(1) Abiological: They are two types:
(i) Natural or Atmospheric nitrogen fixation: By photochemical and electrochemical reactions, oxygen combines with nitrogen to form oxides of nitrogen. Now they get dissolved in water and combine with other salts to produce nitrates.
Physical nitrogen fixation out of total nitrogen fixed by natural agencies approximately 10% of this occurs due to physical processes such as lightening (i.e., electric discharge), thunder storms and atmospheric pollution.
Due to lightening and thundering of clouds, N2 and O2 of the air react to form nitric oxide (NO). The nitric oxide is further oxidised with the help of O2 to form nitrogen dioxide (NO2).
NO2 combines with H2O to form nitrous acid (HNO2) and nitric acid (HNO3). The acid falls along with rain water. Now it acts with alkaline radicals to form water soluble (nitrates) and (nitrites).
The nitrates are soluble in water and are directly absorbed by the plants.
(ii) Industrial nitrogen fixation: Nitrogen and hydrogen combines to form ammonia industrially, under pressure and temperature.
(2) Biological nitrogen fixation: The conversion of atmospheric nitrogen into inorganic or organic usable forms through the agency of living organisms is called biological nitrogen fixation. The process is carried by two main types of microorganisms, those which are “free living” or asymbiotic and those which live in close symbiotic association of with other plants.
(i) Asymbiotic biological nitrogen fixation: This is done by many aerobic and anaerobic bacteria, cyanobacteria (blue green algae) and some fungi e.g.:
Free living bacteria: Free living N2 fixing bacteria add 10–25 kg of nitrogen /ha/annum.
Aerobic – Azotobacter
Anaerobic – Clostridium
Photosynthetic – Chlorobium
Chemosynthetic – Thiobacillus
Cyanobacteria (blue-green algae) e.g., Anabaena, Nostoc, Tolypothrix cylindrospermum, Calotherix and Aulosira etc. They add 20–30 kg of N2 per hactare of soil and water bodies.
Free living fungi e.g., Yeast cells and Pullularia.
(ii) Symbiotic biological nitrogen fixation: Symbiotic bacteria are found in the root nodules of the members of family Leguminosae. The best known nitrogen fixing symbiotic bacterium is Rhizobium leguminosarum (Bacillus radicicola).
Rhizobium penetrates to the cortex of root through infection thread. Simultaneously cortical cells or root are stimulated to divide more vigorously to form nodules on the root. Neither bacterium nor plant alone can fix nitrogen in such cases. Nitrogen fixation is actually the outcome of symbiotic relationship between the two. When a section of root nodules is observed the presence of a pigment, leghaemoglobin is seen to impart pinkish colour to it. This pigment is closely related to haemoglobin and helpful in creating optimal condition for nitrogen fixation. Like haemoglobin, leghaemoglobin is an oxygen scavenger. Fixation of nitrogen is done with the help of enzyme nitrogenase, which functions under anaerobic conditions. Leghaemoglobin combines with oxygen and protects nitrogenase.
Symbiotic bacteria (Frankia) have also been found to occur in root nodules of Casuarina, Cycas, Alnus, etc. Leaf nodules develop in some members of family Rubiaceae, the bacteria being Mycobacterium. Some cyanobacteria also have symbiotic association with plants e.g., lichens; Anthoceros (a liverwort) and Azolla (a water fern).
Mechanism of biological nitrogen fixation: Several schemes incorporating such idea have been proposed and Burris (1966) accepts that the total reduction of nitrogen occurs on an enzyme complex (Nitrogenase) without release of intermediates less reduced than ammonia.
The enzyme complex nitrogenase consists of two sub-units
(i) A non-heme iron protein commonly called Fe protein (or dinitrogen reductase, component I).
(ii) An iron molybdenum protein called MoFe protein (or dinitrogenase, component II).
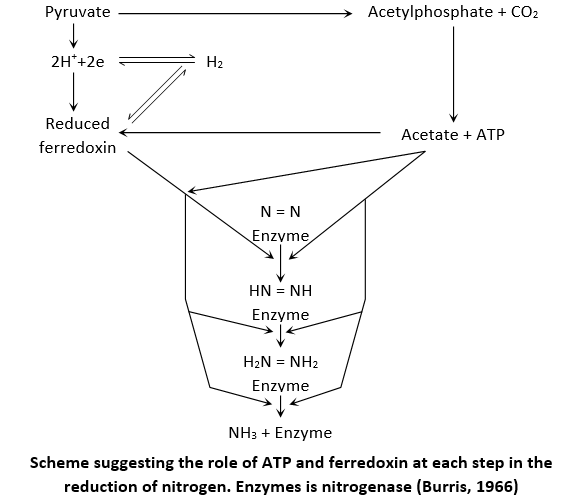
According to Burris (1966) hypothesis for nitrogen fixation suggesting the function of ATP and ferredoxin at each step in the reduction of nitrogen. The pretty function of ATP donor is furnished by pyruvate which also acts as electron donor for N2 reduction as well.
Pyruvate on one hand acts as ATP donor while on other hand it supplies hydrogen ions and electrons for nitrogen reduction via NADH2 and ferredoxin. The nitrogenase enzyme require 16 ATP molecules, 8 hydrogen ions and 8 electrons to reduce one molecule of nitrogen to 2NH3 molecules.
|
Flavodoxin or |
|
|
|
|
Ferredoxin or Dithimite |
Fe-protein (reduced) 2(Mg2+ ADP) |
Mo-Fe protein (reduced) |
|
|
Flavodoxin or Ferredoxin |
Fe protein (oxidised) 2(Mg2+ ADP) |
Mo-Fe protein (oxidised) |
|
Explaining the mechanism of nitrogenase activity, its now believed that electrons are transferred from the reducing agent (Ferredoxin, Flavoprotein or Dithionite) to complex of Mg-ATP and Fe-protein (component II). From here electrons flow to Mo-Fe protein (component I) and then to substrate (nitrogen) which is finally reduced (to NH3).
The ammonia formed in biological nitrogen fixation is not liberated. It is highly toxic and is immediately converted into amino acids.
Ammonia + α-ketoglutarate + NADH Glutamate + NAD+ + H2O.
The amino acids are transported through phloem to other parts of the plant.
Ammonification and nitrification
The free living nonsymbiotic nitrogen fixing organisms do not enrich the soil immediately. It is only after organism death that the fixed nitrogen enters the cyclic pool by the two steps namely the ammonification and nitrification.
Ammonification: The nitrogenous organic compounds in the dead bodies of plants and animals are converted into ammonia or ammonium ions in the soil. This is carried out by ammonifying bacteria. Ammonia is toxic to the plants but ammonium ions can be safely absorbed by the higher plants.
Nitrification: Once ammonia has been produced it is converted into nitrates by nitrifying activities and process is called nitrification. Soil bacteria such as Nitrosomonas and Nitrosococcus convert ammonia into nitrite ions.
Nitrites are then oxidised to nitrates by Nitrobacter.
The nitrifying bacteria are chemoautotrophs and are benefited by utilising energy released in oxidation, which is used in chemosynthesis. At soil temperatures 30°C – 35°C in alkaline soils and with sufficient moisture and aeration, the activity of ammonifying and nitrifying bacteria is found to be maximum.
Some bacteria such as Thiobacillus denitrificans, Pseudomonas aoruginosa and Micrococcus denitrificans also occur in the soil which convert the nitrate and ammonia into atmospheric free elemental nitrogen. Such bacteria are called denitrifying bacteria and the process is called denitrification. These bacteria act very well in soil where there is more water and less oxygen and there are high level of the carbohydrate.
Nitrate assimilation in plants
It is first reduced to ammonia and then incorporated into organic compounds.
The process of nitrate reduction to ammonia occurs in the following steps:
Nitrate → Nitrite →Hyponitrite →Hydroxylamine → Ammonia
(1) Reduction of nitrate to nitrites: First the nitrate is reduced to nitrite by an enzyme called nitrate reductase. The reductase enzyme is a flavoprotein and contains FAD (Flavin adenine dinucleotide) as prosthetic group which receives hydrogen from reduced NADP or NAD. Molybdenum in enzyme serves as electron carrier.
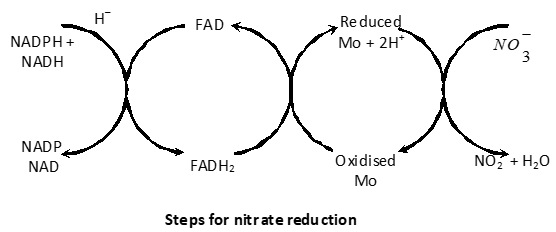
(2) Reduction of nitrites: The nitrite ions are reduced to ammonia by an enzyme called nitrite reductase. This change occurs in leaves in the presence of light more rapidly and in dark with lesser speed. This is due to the reducing power of reaction from photochemical splitting of water.
Nitrite reductase does not need molybdenum but may require the presence of iron and copper. NADH and NADPH act as hydrogen donors.
Application of fertilizers: Most of the soil usually contain sufficient amounts of essential mineral elements for the better crop production. Some of them are, however, deficient in certain elements. These elements are required to be supplemented externally by adding the appropriate fertilizers. Moreover, constant agricultural cultivation in field may also cause depletion of certain elements which must be replenished in order to improve the fertility of soil. The important elements need to be replenished in crop fields are nitrogen, phosphorus and potassium. These are grouped as nitrogenous fertilizers, phosphate fertilizers and potash fertilizers. These are abbreviated as NPK. Common sources of NPK are ammonium chloride, ammonium sulphate, ammonium nitrate, bone meal, calcium magnesium phosphate and nitrate of soda.
10. SPECIAL MODES OF NUTRITION
The method of taking in and synthesis of various types of foods by different plants and animals is called nutrition.
Generally plants are autotrophic in their mode of nutrition, but there are some examples which are heterotrophic in their mode of nutrition. These plants are unable to manufacture their own food due to lack of chlorophyll or some other reasons.
Parasites
These plants obtain either their organic food prepared by other organisms or depend upon other plants only for water and minerals with the help of which they can synthesize their own food. The living organism from which the parasite obtains its organic food or water and minerals is called host. Any part of the body of parasite is modified into a special organ called haustorium which enters into the cells of host and absorbs food or water and minerals from the host.
Parasites can be classified into two categories:
(i) Total parasites. (ii) Semiparasites or partial parasites.
(i) Total parasites: These plants never possess chlorophyll, hence they always obtain their food from the host. They may be attached to branches, stem (stem parasites) or roots (root parasites) of the host plants.
Total stem parasite: Cuscuta is a rootless, yellow coloured, slender stem with small scale leaves, which twines around the host. The parasite develops haustoria (Small adventitious sucking roots) which enter the host plant forming contact with xylem and phloem of the host. It absorbs prepared food, water and minerals from the host plant.
Total root parasite: Total root parasites are common in the families like Orobanchaceae, Rafflesiaceae, Balanophoraceae, etc. Orobanche, Rafflesia and Balanophora are some of the common root parasites.
Orobanche is commonly known as broom rape. It has scale leaves and pinkish or bluish flowers. The tip of the root of parasite makes haustorial contact with the root of host and absorbs food from the host. Orobanche is usually parasitic upon brinjal, tobacco. In Rafflesia (stinking corpse lily) another root parasite, vegetative parts of the plant are highly reduced and represented by cellular filaments resembling fungal mycelium. These filaments get embedded in the soft tissue of the host while the flowers emerge out in the forms of buds.
Balanophora occurs as a total stem parasite in the roots of forest trees.
(ii) Semiparasite or partial parasite: Such parasitic plants have chlorophyll and, therefore, synthesize their organic food themselves. But they fulfill their mineral and water requirements from their host plants. These are two types:
Partial stem parasites: The well known example of partial stem parasite is Viscum album (mistletoe) which parasitizes a number of shrubs and trees. The mature plant of Viscum is dichotomously branched with green leaves born in pairs attached on each node of stem. The shoots are attached to the host by means of haustoria. The primary haustoria reaches up to cortex of the host which runs longitudinally. It sends secondary haustoria which make connection with the xylem of the host and absorb water and minerals Loranthus is another partial stem parasite.
Partial root parasites: The common example of partial (semi-parasite) root parasite is Santalum album (Sandal wood tree) which is an evergreen partial root parasite which grows in South India. It grows on the roots of Dalbergia sisso, Eucalyptus. Like other partial parasites, it also has green leaves and absorbs only minerals and water from the host plants.
Similarly, Striga on roots of sugarcane and Thesium on the roots of grasses are other partial root parasites.
Saprophyte
These plants live upon dead organic matter and are responsible for conversion of complex organic substances into simple inorganic substances (minerals), e.g., some bacteria, some fungi (Yeast, Mucors, Penicillium, Agaricus), few algae (Polytoma), few bryophytes (Buxbaumia, Hypnum and Splanchnum), few pteridophytes (like Botrychium) and some angiosperms (Monotropa and Neottia) also.
Monotropa, commonly known as Indian pipe, lacks chlorophyll and is colourless or ivory white. It is found in Khasi hills and in the dense forests of Shimla. Monotropa, though usually referred to as a saprophyte, actually gets its nourishment from fungal mycelium which surround its roots. Such association between roots of higher plants and fungi is known as mycorrhiza. Neottia (Bird’s nest orchid) grows in the humus rich soil of the forests. It has very few reduced leaves and thick pale yellow stem. The roots lack root hairs and the nutrients are absorbed by mycorrhiza.
Symbiotic plant
Sometimes two different species of organisms spend much or all of their lives in close physical association, deriving mutual benefit. Such an association is known as symbiosis and each organism is known as symbiont. Symbiotic association is so close that symbionts appear to be different parts of the same plant.
Symbiotic association may be between two higher plants or between a higher plant and a lower plant. Some common examples of symbiosis are described below.
Lichens: Lichens is a special group of plants, when an alga and fungus live together and are mutually benefitted (alga provides food and fungus provides water minerals and protection of alga).
The fungus component of the lichens, called mycobiont, is generally a member of Ascomycetae or occasionally a Basidiomycetae. The algal component of the lichen is known as phycobiont and is generally a member of Chlorophyceae (e.g., Trebouxia) or Cyanophyceae (e.g., Nostoc, Gloeocapsa).
Mycorrhiza: It is a mutually beneficial association between a fungus and the root of higher plant. In such association the fungal mycelium forms a mantle over the root surface and some of the hyphae penetrate between cortical cells and metabolites are transferred in both directions (i.e., from fungus to the root cells and vice-versa).
Root nodules of leguminosae: Members of the sub-family Papilionaceae of the Leguminosae (e.g., pea, beans, trifolium) harbour species of Rhizobium, a nitrogen fixing bacteria. The bacteria form nodules in the roots. They fix elemental nitrogen of the atmosphere and make it available to the plant in forms that can be utilized. In turn they derive food and shelter from the leguminous plant.
Myrmecophily: It is the symbiotic relationship between ants and some higher plants. The ants obtain food and shelter from the plant. They protect the plant (e.g., Mango) from other animals. In Acacia sphaerocephala the stipules are hollowed to function as ant shelter. Leaflet tips (Belt’s corpuscles) and rachis (extrafloral nectaries) possess feeding materials. A higher plant which is benefitted by association with ants is called myrmecophyte. The term myrmecophily is also used for pollination by ants.
Insectivorous plants
These plants are autotrophic in their mode of nutrition but they grow in marshy or muddy soils, which are generally deficient in nitrogen and in order to fulfil their nitrogen requirement, these plants catch small insects. The organs and specially leaves of these plants are modified variously to catch the insects. These plants have glands secreting proteolytic enzymes which breakdown complex proteins into simple nitrogenous substances, which in turn are absorbed by these plants. Some of these plants are as follows:
Drosera (Sundew): It is a herbaceous plant having spathulate or lunate leaves. The leaves are covered by glandular hair with a swollen tip. The glands secretes a sticky purple juice which shines like a dew drop in bright light sunshine, hence the name sundew. These long special hair are generally referred to as ‘tentacles’. When an insect alights on the leaf, the tentacles curve due to thigmonasty. The insect is killed and its proteins are digested by pepsin hydrochloride. Similar tentacles are also found in Drosophyllum.
Utricularia (Bladderwort): It is submerged floating aquatic herb which lacks roots. Some of the species of Utricularia also occur in moist soil. The leaves are dissected into fine segments and appear like roots. Some of the leaf segments are modified into pear-shaped sacs called bladders or utricles.
The bladders are triangular or semicircular structures having a single opening guarded by a valve. There are numerous bristles near the mouth and digestive glands inside. The bladders show special trap mechanism. The valve of the bladder opens on the inner side. When small aquatic animalcules enter the bladder along with water current, they get trapped inside. Their proteins are digested enzymatically. When a bladder is full of undigested matter, it degenerasis.
Nepenthes (Pitcher plant): They are commonly found in tropical areas like Assam and Meghalaya (i.e., N. Khasiana). In this plant the leaf base is winged, petiole is tendrillar and the lamina is modified into pitcher. The pitcher has a distinct collar at the mouth and the apex is modified into the lid. The undersurface of the lid has alluring glands whereas the inner surface of pitcher is lined by numerous digestive glands and several downward directed hair. The lid attracts insects which slide down into the pitcher. The downward directed hair check their escape. The insect is killed and its proteins are digested by pepsin hydrochloride. Other insectivorous plants having leaf pitchers are Sarracenia, Cephalotus, Heliamphora, etc.
Dionaea (Venus fly trap): It is a small herbaceous plant found mainly in America. The plant has a rosette of radiating leaves. The petiole is winged and photosynthetic. The lamina is bilobed and the midrib acts like a hinge between the two lobes of the lamina. Each lobe has 15-20 trigger hairs or bristles. These hairs are very sensitive to nitrogenous substances. When an insect alights on the leaf and touches the sensitive hairs, the two lobes of lamina fold along the midrib. Thus the insect is trapped in between the lobes. Pepsin hydrochloride secreted by the digestive glands, present in the upper part of the lobes digests the insect.
Sarracenia (Pitcher plant; Devil’s boot): This pitcher plant is found in the temperate regions. It has a very reduced stem which bears a rosette of leaves. The leaves are modified into pitchers. It can easily be distinguished from Nepenthes on the basis of its trumpet-shaped sessile pitchers. The pitchers of Sarracenia lack digestive enzymes and here the insects are decomposed by bacteria.
Pinguicula (Butterwort): It is a herbaceous plant having a basal rosette of ovate leaves. The leaf margins are slightly curved in upward direction. The dorsal (upper) surface of leaf has two types of glands stalked and sessile. The stalked glands secrete mucilage while the sessile glands secrete digestive enzymes.
Aldrovanda (Water flea trap): It is also a rootless, submerged aquatic plant (bog plant) recalling the habit of Utricularia. The leaves are bilobed with long petioles. There are five bristle like outgrowths associated with the lamina. The leaf surface is covered by visid stalked glands. The proteins of the insect are digested enzymatically.








