1. TEMPERATURE
Mr. Raj was on his way home after office. The sun was beating down and he was sweaty and uncomfortable. As soon as he reached home, he sat down for a while. Then he went straight to the refrigerator for a bottle of cool water.

Why did the bottle of water feel cool while it was so hot outside? The answer lies in the concept of temperature.
Temperature indicates the degree of ‘hotness’ or ‘coldness’ of a body or a place.
An Experiment – Hot & Cold are relative
Aim: To show that the terms ‘hot’ and ‘cold’ as we feel them are only relative terms.
Materials needed: Three glasses, hot water (as hot as you can bear to dip your finger in), ice-cold water, and water at room temperature.
Method
(i) Pour hot water, water at room temperature, and the ice-cold water into three separate glasses.
(ii) Keep the three glasses side by side on a table with the glass containing water at room temperature in the middle.
(iii) Dip the index finger of one hand in the ice-cold water and that of the other hand in the hot water. Hold the fingers in the water for some time (for about a minute).
(iv) Then dip both the index fingers into the water at room temperature.
Observation: You will find that to one finger, the water feels hot and to the other it feels cold.
Conclusion: This is because the finger that was in ice-cold water has become cold, and when it was placed in the water at room temperature, it felt that the water was warm, relative to the earlier experience of ice-cold water.
Similarly, the finger that was first in hot water will feel the water at room temperature to be relatively cold. So you see the terms ‘hot’ and ‘cold’ are only relative terms. There always has to be a reference body with respect to which we can say whether a given body is hot or cold. Therefore, it is important that we use an instrument, rather than our own judgment to measure temperature.
2. MEASUREMENT OF TEMPERATURE
We measure temperature with an instrument called the thermometer. All thermometers measure temperature by making use of some property of a substance that varies with temperature. One such property is the change in volume (expansion and contraction) with temperature. The substance which is generally used in a conventional thermometer is either mercury or alcohol.

Temperature Scales
Just like we use different units to measure length (like inch, centimetre, etc.), we use different units to measure temperature. These different units are represented by different temperature scales. Here is a simplified description of how a temperature scale is defined. Two reference temperatures are chosen and the difference between these two temperatures are divided into a certain number of divisions. Each division is called one degree. The most commonly used reference temperatures are the melting point of ice and the boiling point of water. The two commonly used scales are the Celsius and the Fahrenheit scales. There is a third scale, the Kelvin scale which you will learn in higher classes.
I. Celsius Scale
This scale is indicated by °C [read as degree Celsius (in honor of Anders Celsius) or degree 1 centigrade]. On this scale the melting point of ice is taken as 0 °C and the boiling point of water as 100 °C. The difference between these two points is divided into 100 degrees.
II. Fahrenheit Scale
This scale is indicated by °F (read as degree Fahrenheit). On this scale, 32 °F is taken as the melting point of ice and 212 °F as the boiling point of water. The difference between these two points is divided into 180 degrees 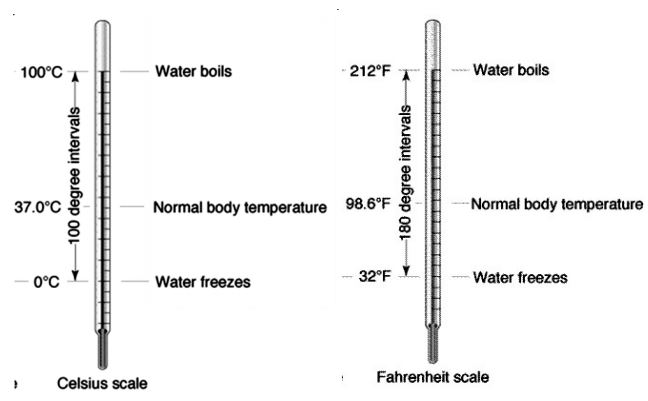 Conversion Formula
Conversion Formula
Two simple equations given below will help us to scale convert one temperature scale to another
where F is for Fahrenheit and C is for Celsius.
3 THERMOMETERS
I Mercury Thermometer
In 1714, Gabriel Fahrenheit invented the first mercury thermometer. Mercury is used in thermometers because of the following reasons:
• Mercury remains in the liquid state for a wide range of temperature.
• It melts at – 38.87 °C and boils only at 356.58°C.
• It is relatively easy to see because of its silvery grey colour.
• It does not stick to glass.
• It has a fairly uniform rate of expansion for a wide range of temperatures. 
II. Alcohol Thermometer
Thermometers in which alcohol is used have some advantage over mercury thermometers. Alcohol is cheaper and less harmful than mercury. Alcohol thermometers can measure much lower temperatures (up to -115 °C) than mercury. However, the main disadvantage of using an alcohol thermometer is that these thermometers can measure temperature only up to 78 °C while mercury thermometers can measure much higher temperatures. 
Reading a Conventional Thermometer
A laboratory thermometer is shown in. It consists of a thin glass tube which is sealed at one end and has a bulb at the other end. The bulb is generally filled with mercury or alcohol. Mercury appears as a silvery grey line and alcohol looks like a red line in the thermometer. To read the temperature on this thermometer, you just read the number on the scale at the tip of the red or silvery grey line. Remember to mention the unit used (Celsius or Fahrenheit). The range of temperature which a laboratory thermometer can measure is from -10°C to 110°C.

The thermometer used to measure our body temperature is called a clinical thermometer. It is generally a mercury thermometer, though nowadays digital thermometers are becoming very popular.
Characteristic feature of Mercury Thermometer
• There is a little arrow (at 98.4 or 98.6 °F) showing the normal body temperature.
• There is a constriction or ‘kink’ in the tube near the bulb. This kink has been made to ensure that the mercury in the thermometer does not contract (and flow back into the bulb) before the temperature has been read.
Precaution in handling Thermometer
• Wash the thermometer well and dip it in an antiseptic liquid before and after using it.
• Be careful while using a thermometer. It can break easily. Remember, mercury is a toxic substance.
Digital Thermometer
These days digital thermometers are used to measure temperature. These thermometers do not use mercury. The temperature is displayed as a number.

4. HEAT
Have you stirred hot tea or milk with a stainless steel spoon? Next time you do this, leave the spoon in the hot liquid for a little while. Now, touch the handle of the spoon. What do you notice? The handle of the spoon would have got a little warm. Why did the handle of the spoon become warm? When there is a difference in temperature between two bodies, a certain type of energy flows between them. This energy is called heat energy. When this heat energy flows into a body, it warms the body while if it flows out of a body, it cools the body. In other words, the hot body loses heat energy while the cold body gains heat energy.
Flow of Heat energy
Heat energy flows from a region of higher temperature to a region of lower temperature and never in the other direction. Heat flow continues till the temperatures of both the hot and the cold body become the same (whose value will be somewhere in between the temperatures of the hot body and the cold body). 
Activity – Direction of Flow of Heat
Aim: To show that heat energy flows from a hot body to a cold body.
Materials needed: A coin, tongs, boiling water, cold water, and two glasses.
Method:
1. Take two glasses and fill them to three-fourths of the capacity with cold water. Boil water in a vessel and drop a coin in the boiling water. Leave it for some time so that the coin gets really hot.
2. Ask an adult to use the pair of tongs to pick up the hot coin and drop it in one of the glasses filled with water.
3. After about 2 minutes, dip your finger in the two glasses, first in the glass without the coin and then in the glass with the coin.
Observation: You will observe that the water in the glass in which you dropped the hot coin will be warmer.
Conclusion: Heat energy has been transferred from the hot body (coin) to the cold body (water).
5. MEASURING HEAT Just as we measure length in centimetres and metres, heat energy (and any other form of energy for that matter) is measured in calories or joules. In the SI system (which you know is the international standard for the system of units), heat energy is measured in joules. The symbol used to represent ‘joule’ is ‘J’.
Note Temperature is not energy. However, if we know the temperature of a substance, we can calculate the amount of heat energy it contains.
6. CONDUCTION OF HEAT
We now know that heat is a form of energy and that it flows from a region of higher temperature to a region of lower temperature. But how does this happen? The methods by which heat is transferred from one region to another are called the modes of heat transfer. There are three modes of heat transfer, namely, conduction, convection and radiation. We know that the metal objects heat up when they come in contact with hot solids or liquids. Just like the stainless steel spoon becoming hot when left in a hot liquid. How do you think this happens? The heat from the hot liquid moves to the stainless steel spoon by a process called conduction.
How does Conduction Occur?
We know that matter is made up of tiny particles called atoms and molecules. We know that in solids, the particles are very tightly packed and they cannot move around freely. However, they can vibrate about a fixed point. Let us take the example of a metal rod being heated at one end by a flame. Heat increases the vibrations of the particles at that end of the rod which is in contact with the flame. These particles collide (bump) and transfer their energy to their more slowly moving neighbors further away from the flame, and make them vibrate faster. They in turn pass the vibration on to their neighbors even further away from the flame, and so on. In this manner, heat energy is transferred from one particle to the next, although each individual particle remains in its original location. Conduction is the primary mode of heat transfer through a solid. Conduction of heat energy can occur within a body or between two bodies when they are in contact with each other. However, the rate of conduction of heat is different in different materials. Also some materials allow heat energy to flow through them easily while others do not.
Conductors & Insulators
The substances that conduct heat easily are called conductors. For example, metals are good conductors of heat. Substances such as wood, straw, clay, rubber, glass, and Bakelite (a kind of plastic) do not conduct heat very well and are called insulators. Air and water are also poor conductors of heat. Some materials such as wool, fur, and bird feathers are good insulators because they trap air between their fibres.
Activity – Rate of Conduction
Aim: To demonstrate that the rate of conduction of heat is different for different materials.
Materials needed: Rods of equal diameters and equal lengths made of aluminium, copper, and iron (you will find these in your school lab), two wooden slabs, small metal balls, wax, and flame
Method:
1. Take two rods at a time, say, first the copper and aluminium rods.
2. Stick the metal balls to the rods at equal distances using wax.
3. Light the burner and make your observations.
4. Repeat the experiment with iron and aluminium.
Observation: You will see that the balls drop fastest for copper, then aluminium and slowest for iron.
Conclusion: Of the three metals, the rate of conduction is highest for copper and the lowest for iron.
Practical Applications of Conduction
1. Both good and bad conductors are useful to us, depending on our needs. We use good conductors (metals) for making cooking utensils, and heat-resistant plastic for making the handles for these vessels.
2. We wear woolen clothes in winter because wool is a bad conductor of heat. Thus, it helps in retaining body warmth. The wool fibre has a series of curls and these are called ‘crimps’. These crimps create small air pockets. The greater the number of crimps, the greater is the number of air pockets which can hold and trap air.
Thus, a thin layer of air is created which insulates us from the cold weather and also prevents body heat from escaping into the surroundings.
7. CONVECTION OF HEAT
This mode of heat transfer is seen in liquids and gases in which the molecules are less densely packed and are free to move. Consider the following example . When a vessel containing water is heated, after sometime, the water at the top of the vessel also becomes hot. How does this happen? The water at the bottom of the vessel gets hot, and since hot water is lighter than cold water, it rises up, carrying the heat energy with it. The colder and denser water on the top falls to the bottom of the vessel and gets heated in its turn. This process continues and results in a circulating stream of hot and cold water. As a result, the “whole water in the vessel gets heated. This method by which heat is transferred by the mass movement of the liquid or gas itself is called convection.
Activity – Convection Transfers Heat
Aim: To show that heat transfer in water is due to convection.
Materials needed: A glass beaker made of heat-resistant glass/glass, test tube, candle, match box, potassium permanganate crystals/artificial food colouring, and a spoon.
Method
1. Fill the beaker to the half way mark with clear water.
2. Take a pinch of the potassium permanganate crystals/food colouring and drop it gently to the bottom of the beaker.
3. Let it settle for a few minutes.
Observation: You will see that the water at the bottom of the beaker is coloured, but the water at the top is quite clear. Light the candle and pick up the beaker very gently and hold it on top of the candle flame. You will see columns of coloured liquid rise up in the beaker.
Conclusion: This is due to convection. Water molecules at the bottom get heated up and rise, carrying the heat energy with them.
Practical Applications of Convection
1. The principle of convection of heat can be used in many applications.
2. When you want to warm a fluid, you should warm it from below. If a room has to be warmed with a room heater, the heater should be placed at a lower level, so that the room gets warmed evenly.
3. Similarly, an air conditioner used for cooling a room should be placed at a higher level.
4. In olden times, buildings were designed where windows and ventilators were cleverly placed so that the buildings were kept cool in summers.
Sea Breeze and Land Breeze
Sea Breeze: Convection plays a major role in maintaining a moderate temperature in places near the sea. Land masses (beach, coastal town, or city) heat up much faster than water bodies (sea, ocean) during daytime, and cool down much faster during the night. This difference in temperature sets up a wind pattern. During the day, the air above land rises since it is warm and cooler air from over the sea flows in to take its place. This gives rise to a sea breeze (sometime in the afternoon) which cools the land.
Land Breeze: In the night, since land cools down much faster than the sea, the cooler air over land flows out to take the place of warmer air over the sea which rises, setting up a land breeze.
8. RADIATION OF HEAT
Radiation is the form of heat transfer that can occur in the absence of a medium. Thus, it is the mode of heat transfer through vacuum. While heat radiations can travel through vacuum, they can also travel through media such as air, water, etc. All bodies give out energy that travels in the form of radiation (much like light) through space. Energy from the sun reaches us through radiation. Bodies that absorb this radiation become hot.
Radiation of Heat and Colour
The amount of heat energy that is absorbed by a body depends on its colour. Bodies that are black absorb more of radiated heat than white bodies. We feel more comfortable wearing white and light – coloured clothes in summer. This is because white clothes absorb comparatively less amount of heat than dark clothes, and therefore keep us relatively cooler. The reverse applies in winter; we are more comfortable in dark clothes. This is because we need to absorb as much heat as possible from our surroundings to keep ourselves warm, and dark clothes absorb more heat than light – coloured ones. The converse is also true. Black and dark bodies also radiate more heat than light – coloured bodies.
Activity – Radiation of Heat
Aim: To show radiation of heat.
Materials needed: A source of heat, like a room heater or an electric bulb, and adult supervision.
Method:
1. Switch on the room heater or the electric bulb.
2. Wait for a few minutes, and put your hand close to it without touching.
Observation: You will observe that you can feel the heat of the heater/bulb even when you do not touch it.
Conclusion: Heat energy is reaching your hand from the heater/bulb in the form of radiation. In the above activity, you can confirm that the heat energy reaching your hand is being carried by radiation and not convection.
Hold your hand a little below the bulb. The heat which you feel below the bulb is mostly due to radiation because hot air always rises up.
Practical Applications of Radiation
1. Heat radiations travel in vacuum and in air just like light rays. That is why electric room heaters have mirrors behind the heating coil. This helps in focussing the heat from the heating coil in front of the heater.
2. Solar panels used in households for heating water, are designed to maximize the absorption of heat from the sun. A black metal sheet is used to increase heat absorption.
3. A thermometer called the black bulb thermometer uses the principle of heating by radiation to measure the amount of radiation received by sources such as the sun.
Preventing Loss of heat
A thermos flask is a very good example of how heat loss by all three modes of heat transfer, conduction, convection, and radiation, is minimized. Let us see how this is done. Heat loss due to conduction is minimized by using insulating materials (like plastics) for the outer casing and the cap of the thermos flask. The inner jar is a double-walled bottle made of glass or stainless steel. The space between the two walls is a vacuum, so that heat loss due to conduction is minimized. This also reduces heat loss due to convection since there are no air molecules to carry-away the heat. Heat loss due to radiation is minimized by making the surface of the jar highly reflective, so that heat radiations are reflected back into the jar…








